Abstract
In the present research, the removal of high concentration of ammonia, nitrite and nitrate was determined by using the efficient ammonia-oxidizing bacterial isolates (AOB AMS 1, AOB AMS 2, AOB AMS 5 and AOB AMSS 1) along with the consortium of all four bacterial isolates from the aquaculture both in lab-scale as well as in-situ at bioreactor level. It was found that the highest percentage of ammonia was degrade by AOB AMS 2 (99.64%± 5) followed by AOB AMS 5 (99.51%± 4.6), AOB AMSS 1 (99.38%± 5) and AOB AMS 1 (89.66%± 3.5) from an initial concentration of 30 mg/L whereas from an initial concentration of 60 mg/L, the removal rate of each strain was AOB AMS 5 (99.36%± 5), AOB AMSS 1 (99.15%± 5.60), AOB AMS 2 (98.78%± 5.3) and AOB AMS 1 (88.36%± 3.2) and their consortium removes (99.97%± 5.20)at 60 mg/L. Further, in-situ transformation was carried out in the river water with 5% of inoculum. Their growth was monitored (initial and final) by observing the colony forming unit (CFU) and transformation of ammonia and nitrite was also observed and it was found that (100 ± 2%)of ammonia was removed. The growth of consortium was 3 × 102CFU/ml on 1st day, 2.5 × 104 CFU/ml on 5th day and 4.2 × 105 CFU/ml on 10th day. This study proved that the bacterial consortium of AOB AMS 1, AOB AMS 2, AOB AMS 5 and AOB AMSS 1 was highly efficient for the removal of ammonia, nitrite and nitrate from the aquatic system to reduce the toxic level from aquaculture environment.Currently we observed the transformation efficiency at lab-scaleand in-situ level.
Similar content being viewed by others
Introduction
Water pollution is the major environmental issue.Currently, 70% of the earth’s surface is covered with water. Each and every day, garbage like food, paper, glass, rubber, plastic, aluminium etc. get deposited into the river, lakes and ocean and it take weeks to hundreds of years to get decompose and hence they are the major cause of water pollution and causes harmful effect on the aquatic ecosystem. The contamination of stream, lakes, ocean, river or any other water system depleting the quality of water whereas toxic chemical changesthe colour of water and it also elevatesthe amount of minerals that makes it toxic for the environment and water organisms1.
Ammonia is one of the natural by-productand is produced and released by fish which is very muchdangerous and highly toxic compound to fish and other aquatic animals thateven its small concentration can leadthe fish to die. Ammoniaand nitrite can also be produced by the breakdown of fish waste, decaying plant matterand uneaten fish and also by dead fish. The safe level of ammonia is 0 mg/L. Even the concentration increases to 2 mg/Lcan cause fish to die in the aquatic system2. According to World Health Organisation (WHO), surface water contains up to 12 mg/L of ammonia3. United States Environmental Protection Agency (USEPA) recommends a limit of 0.02 mg/Las ammonia in the marine environments or freshwater whereas the safe concentration of ammonium ion in the marine environment is between 0.02 and 0.44. If the concentration of ammonia gets marginally higher, it can be disastrous fortheaquatic system5. In fish, ammonia formed as a principal end product from the metabolism of protein. Due to the diffusion of branchial, fish expel out the NH3 through their gills, additionally, in freshwater fish, for monovalent cation, ammonium may also be branchially exchanged. When ammonia and ammonium ion arises from the gills, it contains 60–90% of total nitrogenthat excreted out by fish6.
Further, ammonia broken down intothe nitrite by some beneficial bacteria that present in the aquatic system and this nitrite is also the most dangerous compound present in the natural aquatic system. Nitrite should be kept below 0.25 mg/L2. The level of 0 mg/Lcan be ideal.According to USEPA, in the United States of America, naturally occurring levelof nitrite shouldnot exceed 0.3 mg/L7. Next, if the concentration of nitrite gets elevated, it reducesthe ability of fish to obtain oxygen. Due to the high concentration of nitrite, fish get suffocated and die eventually. In aquatic system, nitrite occurs naturally by denitrification process. If the level of ammonia get reduces, it also affects the nitrite2,8. In farming water management, nitrite is one of themost essential key because of their toxicity which directly impact on theproductivity of aquaculture9,10 as well as the survival of aquatic animals11,12. Consequently, in farming water, the maintenance of low concentration of nitrite is a key target for successful culture13,14. In aquaculture systems, the formation of nitrite occurs from the ammonia-oxidation by chemolithoautotrophic ammonium-oxidizing bacteria (AOB) but it can also be formed in the biofilter in anoxic zones as a consequence of denitrification activity15.
The accumulation of nitrite is toxic to fish, as nitrite reacts with haemoglobin and formsmethaemoglobin by inhibiting the oxygen transport resulting in methemoglobinemia or brown blood disease16. There aredifferent concentration of nitrite mentioned in the literature ranging from below 0.2 mg/L NO217 to 12 mg/L NO218,19 targeted the level that is safe in aquaculture systems. AOB and nitrite-oxidizing bacteria (NOB) plays an importantrole in the aquaculture system for the removal of nitrite and ammoniafrom the water.
Unluckily, large amounts of wastewater have been generated from many aquaculture systems that are rich in total nitrogen, total phosphorus, suspended solids and organic matters which discharged into the oceans, rivers and lakes20. Water quality non-conservative parameters such as nitrate, nitrite, chemical oxygen demand, total ammonia nitrogen, biological oxygen demand and phosphate are very much essential butif their level elevates it causes excessive harm to the aquatic life21.
Globally, in most regions, water shortage has becoming a critical issue that derives people to find another source of drinking water such as rivers, reservoirs and lakes because of their abundance and availability. They are used as a reliable water supply in many of the countries specifically in rural area of developing countries22,23. Nevertheless, the presence of natural level of nitrate is not a concern but when the level of nitrate elevates in water due to the excessive use of fertilizers in intensive agriculture and irrigation with domestic wastewater, they reduces the nitrate to toxic nitrite and causes potential health risks to humans and live stocks. It can also causesserious illness such as cancer, methemoglobinemia and because of blue-baby syndrome, an infant less than six months of age can be fatal sometimes if not treated timely24,25,26after the transformation of nitrate to nitrite in the oesophagus and forms a carcinogenic N-nitroso compounds in the digestion system27,28. Consequently, the contamination of nitrate is diffused both in developed as well as in developing countries. The concentration of nitrate amongst many of the European Union (EU) ground water quality monitoring station showed 20% and 40% over 50 mg NO3/L and 25 mg NO3/Lcorrespondingly29whereas high concentration of nitrate also observed in many developing countries30,31,32,33,34,35. For drinking water, the concentration of nitrate is at or near the accepted standard i.e., 40–50 mg/L in many regions36.
The effective management of disposals and wastewater treatment strategy for the nitrogenous compounds are compulsory for any water resources and sustainable aquaculture system. There are variousphysical, chemical and biological methods that are used for the treatment of wastewater. Among all these methods, biological methods are preferred due to its low capital and operating costs, environmental friendliness, acceptance by public and low consumption of energy37. The one of the most viable strategyto achieve a feasible aquaculture is the microbial-based farming systems which represents as the beneficial microbe that can improve the quality of water and also reducesthe discharge of farming wastes and disease occurrences38,39,40. There are techniques that are used for the removal of ammonia and nitrite from the water like electrodialysis, reverse osmosis, chemical denitrification and ion exchange etc. but these techniques are very expensive for pilot scale operation and have limited potential applications41,36. Currently, several bio-remediators continuously searching indigenous organism for aquaculture wastewater to driven the necessity of ammonia and nitrite removal by potential bacterial strain. Therefore, in the current work we looked the potential strain for ammoniaand nitrite oxidation/transformationby four bacterial strains as well as by microbial consortia. Further, thiswork was exploited for in-situ transformational studies.
Materials and methods
Sample collection
Eleven different soil and water samples were collected from the different nitrogen polluted sites of Maldevta, Kesharwala [latitude (30.3199264) and longitude (78.103986)], Assan Barrage [latitude (30.4367° N) and longitude (77.6751° E)], Chhattisgarh (Raipur) [latitude (21.2514° N) and longitude (81.6296° E)], Rishikesh [latitude (30.0869° N) and longitude (78.2676° E)] and Slurry from various places in a different sterile polybags and stored under refrigerated condition.
Isolation and screening of organism
10 gram of soil was weighed out for the isolation of ammonia-oxidizing bacteria (AOB). Further, samples was suspended in the Erlenmeyer flasks having 100 ml of liquid mineral medium, then shaken at 120 rpm for 2–3 days at room temperature in an orbital shaker. Next, to ensure the complete homogenization of soil, they had been shaken again in a voter shaker for 30 s at room temperature.
Further, serial dilutions was made to ammonia-oxidizing bacteria (AOB) from the homogenized soil in a liquid mineral medium ranging from 10− 1 to 10− 5. Next, 0.1 ml of each dilution was inoculated into the petri plates for ammonia-oxidizing bacteria. Further, media was incubated into the incubator at room temperature (37 ºC) until the growth was observed in most of the plates. Further, resistant colonies was selected and purified by repeated sub-culture.
From eleven samples, 23 isolates of ammonia-oxidizing bacteria was isolated, out of which 4 isolates had shown the transformation of ammonia to nitrate at two different concentrations (30 mg/L and 60 mg/L) by using the liquid medium of (NH4)2SO4, anhydrous Fecl3, Na2HPO4, MgSO4.7H2O, KH2PO4, NaHCO3, Cacl2.2H2O and mineral agar medium MgSO4.7H2O, NaSO4, K2HPO4.3H2O along with the sucrose. Table 1 represents the location of the sample, sample name, number of organisms found thatshown the transformation.
Transformation of ammonia by using bacteria (AOB)
Inoculum preparation
Selected strain was grown into the specific liquid medium mentioned above containing ammonia concentration of 1 mg/L. Further, kept the inoculum into the incubator shaker at 37℃ for overnight incubation. Next, optical density was monitored at 0.412 by using UV-Vis Perkin Elmer Spectrophotometer (600 nm). Further, culture was inoculated for the transformation experiment having observation of 0.412 to 0.52542.
Ammonia oxidation
50 ml of liquid medium was prepared containing ammonium-sulphate concentration of 30 mg/L and 60 mg/L. Further, added 5 ml of inoculum in each Erlenmeyer flask. Next, kept the flasks on incubator shaker for at least one-week of incubation. Further, determined the optical density and transformation of ammonia-oxidizing bacteria at different time intervals (12, 24, 48, 72, 96, 120 h).
Quantitative analysis of ammonia, nitrite and nitrate
3 ml of sample was added in an Erlenmeyer flask (50 ml) for the analysis of ammonia along with 0.12 ml of phenol solution, 0.12 ml of sodium nitroprusside solution and 0.3 ml of oxidizing solution with thorough mixing after each addition. Next, covered the samples with paraffin wrapper or plastic wrap film. Let the colour to develop at room temperature (22 to 27 ºC) in a subdued light for at least 1 h. Colour was stable for 24 h. Further, measured the absorbance at 640 nm. Simultaneously, prepared a blank and two other standards by diluting the stock ammonia solution into the sample concentration range. Later, treat the standards same as the samples43.
Further, filtered the sample for nitrite analysis through 0.45 μm-pore-diam membrane filter. Next, adjusted the pH range with NH4OH or 1 N HCL when its range not between 5 and 9. Further, 2 ml of color reagent (85% phosphoric acid (100 ml), sulfanilamide (10 g), N-(1-naphthyl)-ethylenediamine dihydrochloride (1 g) in 1 L of distilled water) was added in 50 ml of sample along with thorough mixing. Further, after the addition of color reagent to sample and standard, absorbance was measured at 543 nm between 10 min and 2 h44.
Next, for nitrate analysis, 3 ml of sample were taken in a test tube andadded 0.06 ml of hydrochloride solution in it. Mix them thoroughly. Further, read the absorbance by using a wavelength of 220 nm and 275 nm to determine the interference due to dissolved organic matter45.
Qualitative analysis of ammonia
In a test tube, taken 2 ml of sample and added Nessler’s reagent in it. After the addition of Nessler’s reagent, dissolved it completely, if brown colour precipitate forms at the bottom signifies the presence of ammonia. Simultaneously, prepared standard by taking pinch of ammonium chloride salt in a test tube and makes their aqueous solution with distilled water. Further, dissolved it completely, after the addition of Nessler’s reagent, brown colour precipitation was formed46.
Qualitative analysis of nitrate
In a test tube, 2 ml of sample were taken and added concentrated H2SO4 at the edges of test tubes by keeping the angle of test tube at 45 ℃ and the preparation of ring starts at the junction of two liquids. If brown color ring started floating in the liquid signifies the presence of nitrate. Simultaneously, prepared the standard by taken pinch of potassium nitrate in a test tube and make aqueous solution with distilled water as well as taken pinch of ferrous-sulphate in another test tube and prepared aqueous solution with distilled water. Further, added freshly prepared FeSO4.7H2O aqueous solution into the potassium nitrate solution. Next, added the concentration H2SO4, brown color ring started floating into the liquid47.
Rate determination of ammonia-oxidizing bacteria
The optical density of ammonia containing concentration of 30 mg/L and 60 mg/L was observed at different time intervals (12, 24, 48, 72, 96 and 120 h) to compare the transformation/oxidation of bacterial growth.
In-Situ Remediation of Ammonia and Nitrate by Using Microbial Consortium
Preparation of microbial consortium
Polluted water sample was collected from the river or canal in a sterile plastic bottle. Sub-culturing was done of each culture (AOB AMS 1, AMS 2, AMS 5 and AMSS 1) into the specific liquid mediumplates(concentration of ammonia 60 mg/L). Further, inoculum was prepared for each strain and kept it in the incubator shaker at 37℃ for overnight incubation. Next, optical density of each inoculum was monitored at 0.412 to 0.525 by using UV-Vis Perkin Elmer Spectrophotometer at 600 nm. Further, strains which shown the optical density 0.412 to 0.525 was selected for the preparation of consortium.Next, microbial consortium was prepared by the addition of AOB AMS 1, AMS 2, AMS 5 and AMSS 1.
In-Situ Transformation of Ammonia by Microbial Consortium
One liter of polluted water was taken in two liter flask. Next, 60 mg/L concentration of ammonia was added in the polluted water because the concentration of ammonia was very less in this water. Further, consortium of AOB AMS 1, AMS 2, AMS 5 and AMSS 1 (5%) was added into the polluted water for the transformation of ammonia to nitrate. Incubated the flasks at room temperature for 15 min. Next, monitored the growth of consortium in terms of colony forming unit (CFU).
Statistical analysis
All experiments were performed in three replicates and statistical analysis was performed using Graph Pad Prism (version 4.03) software (GraphPad, CA, USA).
Results
Isolation and selection of potential organisms
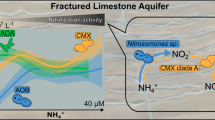
23 isolates of ammonia-oxidizing bacteria (AOB) were isolated from eleven samples and out of which four potential isolates had shown the transformation of ammonia to nitrite and nitrite to nitrate at two different concentrations (30 mg/L and 60 mg/L) in the liquid medium at above mentioned concentration (organisms denoted as AOB AMS 1, AOB AMS 2, AOB AMS 5 and AOB AMSS 1). Next, this bacterium used for the transformation of ammonia and nitrate at 30 mg/L and 60 mg/L. Figure 1 represents the unveiling microbial processes: from sampling to transformation.
Transformation of ammonia by using potential organisms
Inoculum preparation and ammonia oxidation
Selected strain was grown into the specific medium containing 1 mg/L concentration of ammonia. Further, optical density was monitored at 540 –600 nm. Next, inoculated the culture for the transformation experiment having optical density of 0.412 to 0.525.
In an Erlenmeyer flask, 50 ml of liquid medium was prepared containing 30 mg/L and 60 mg/L concentration of ammonium-sulphate.Incubated the flasks on incubator shaker at 37℃ for 24 h. Further, 5% of inoculum was added into the liquid medium and kept the flasks on incubator shaker for at least one-week. Next, transformation and optical density of ammonia-oxidizing bacteria was determined at different time intervals (12, 24, 48, 72, 96, 120 h) to observethe bacterial growth and transformation.
Growth was observed in six days by ammonia-oxidizing bacteria (AOB). AOB AMS 1 represents (0.751) growth on 1st day and (2.238) on 6th day whereas AOB AMS 2 shown (0.769) growth on 1st day and (2.157) on 6th day. Next, AOB AMS 5 shown (0.782) growth on 1st day and (2.206) on 6th day. Further, AOB AMSS 1 shown (0.735) on 1st day and (2.098) on 6th day at 30 mg/L which shows that all the strains are growing efficiently at this concentration whereas at the concentration of 60 mg/L, AOB AMS 1 shown (0.538) growth on 1st day and (1.590) on 6th day whereas AOB AMS 2 shown (0.542) on 1st day and (1.498) on 6th day. Further, AOB AMS 5 shown the growth (0.531) on 1st day and (1.530) on 6th day whereas AOB AMSS 1 shown (0.511) growth on 1st day and (1.648) on 6th day. At 60 mg/L the growth is comparatively less than 30 mg/L but the growth is optimum for the transformation. Tables 2 and 3 represents the growth observation of ammonia-oxidizing bacteria at 30 mg/L and 60 mg/L respectively.
Next, qualitative analysis of ammonia and nitrate was analyzed by all four strains at different time intervals (12, 24, 48, 72, 96 and 120 h). It was observed that by using the strain AOB AMS 1, AOB AMS 2, AOB AMS 5 and AOB AMSS 1, there was no transformation of ammonia to nitrate in 12 to 48 h but at 72 h, slightly conversion of ammonia to nitrate was found and further at 96 and 120 h there was complete transformation of ammonia and nitrate was observed at 30 mg/L and 60 mg/L. Table 4 mentioned the qualitative analysis of ammonia and nitrate at different time intervals of AOB AMS 1, AMS 2, AMS 5 and AMSS 1 at 30 mg/L and 60 mg/L.
The rate of transformation of each isolates was also determined and shows that all four isolates were able to degrade the ammonia and nitrate and the highest percentage of degradation was given by AOB AMS 2 (16.57%± 0.03) on 1st day and (99.64%± 5) on 6th day followed by AOB AMS 5 (17.62%± 0.05) on 1st day and (99.51%± 4.6) on 6th day, AOB AMSS 1 (18.07% ± 0.03) on 1st day and (99.38%± 5) on 6th day and AOB AMS 1 (10.88% ± 0.02) on 1st day and (89.66% ± 3.5) on 6th day at 30 mg/L whereas at 60 mg/L, AOB AMS 5 degrade (17.93%± 0.03) of ammonia on 1st day and (99.36%± 5) on 6th day followed by AOB AMSS 1 (18.05%± 0.03) on 1st day and (99.15%± 5.60) on 6th day, AOB AMS 2 (15.30%± 0.05) on 1st day and (98.78%± 5.3) on 6th day and AOB AMS 1 (10.63%± 0.03) on 1st day and (88.36%± 3.2) on 6th day. Further, the consortium of all four isolates were used at lab-scale for the transformation of ammonia and found that the microbial consortium degrade (21.64%± 0.06) on 1st day, (76.09%± 1.89) on 3rd day and (99.97%± 5.20) on 6th day at 60 mg/L concentration. In this correspondence, nitrate formation is shown in Tables 5 and 6 on different days and Table 7 represents the transformation of ammonia and nitrate by consortium from aquatic system.
Figures 2 and 3 represents the transformation % and rate of transformation of AOB AMS 1, AOB AMS 2, AOB AMS5 and AOB AMSS 1 at 30 mg/L and 60 mg/L.
In-situ remediation
Next, for in-situ remediation, prepared the microbial consortium of all four isolates AOB AMS 1, AOB AMS 2, AOB AMS 5 and AOB AMSS 1. This consortium shown the growth of 3 × 102 CFU/ml on 1st day and 4.2 × 105 CFU/ml on 10th day of transformation. Microbial consortium were able to degrade the ammonia (10 ± 2%) on 1st day, (68 ± 3%) on 5th day and (100 ± 2%) on 10th day whereas observed nitrate (90 ± 3%) on 1st day, (32 ± 4%) on 5th day and (0 ± 2) on 10th day. Table 8 represents the CFU count and transformation of ammonia and nitrate by in-situ remediation.
Discussion
In the aquaculture industry, pollution of nitrogenthat occurs from ammonia and nitrite becomes an important environmental concern48,49as their toxicity level directly impacts the aquaculture productivity9,10 as well as the survival of aquatic animals11,12. Aquaculture plays an essential role in improving the living standards of humans50. Bioremediation is an eco-friendly approach for a sustainable aquaculture production where certain concentration of microorganisms were added to improve the quality of water. In this aspect, it was found that the microbial consortium is more advantageous over single-cultures due to their co-operative interactions between the co-cultivated microorganisms which enhances the removal rate of nutrients51. In the current study, four potential isolates were found that shown the highest percentage of transformation of ammonia, nitrite and nitrate at two different concentrations (30 mg/Land 60 mg/L) as mentioned in results. The transformation of ammonia, nitrite and nitrate by these four isolates (AOB AMS 1, AOB AMS 2, AOB AMS 5 and AOB AMSS 1) were observed at different time intervals (12, 24, 48, 72, 96 and 120 h). It has been observed that the highest percentage of ammonia was transform by AOB AMS 2 (99.64%± 5) followed by AOB AMS 5 (99.51%± 4.6), AOB AMSS 1 (99.38%± 5) and AOB AMS 1 (89.66%± 3.5) from an initial concentration of 30 mg/Lto 29.892 ± 0.05 mg/L; 29.853 ± 0.03 mg/L; 29.816 ± 0.01 mg/Land 26.900 ± 0.02 mg/Lcorrespondingly whereas AOB AMS 5 shown (99.36%± 5) followed by AOB AMSS 1 (99.15%± 5.60), AOB AMS 2 (98.78%± 5.3) and AOB AMS 1 (88.36%± 3.2) from an initial concentration of 60 mg/L to 59.621 ± 0.02 mg/L; 59.495 ± 0.03 mg/L; 59.268 ± 0.01 mg/L and 53.018 ± 0.05 mg/L correspondingly in 120 h. Similarly, Gogoi et al. found that Bacillus albus strain reduces the concentration of ammonia from 100 mg/L to 5 mg/Lin 98 h but under the immobilized conditions52 whereas Huang et al. found that by using co-cultural heterotrophic nitrification and aerobic denitrification (HNAD) strains of Marinomonas communis and Halomonas titanicae, Marinomonas aquimarina and Halomonas titanicae and Marinomonas aquimarina and Cobetia marina rapidly reduced ammonia-nitrogen (65.2%) from 20.2 mg/Lto 7.0 mg/Lin 8 h48. Barik et al. determined that the concentration of ammonia-nitrogen reduces from 10 mg/Lto 0.3 mg/Lwithin 3 days after the inoculation of microbial inoculums along with the aeration in water53whereas Yun et al. found that the bacterial strain of Sphingomonas sp. efficiently removes ammonia nitrogen (96%) from an initial concentration of 8 mg/Lto 0.3 mg/Lin 48 h50 and John et al. investigated the three bacterial isolates Bacillus cereus, Bacillus amyloliquefaciens and Pseudomonas stutzeri for ammonia and nitrate transformation51whereas Zhang et al. shows the removal efficiency of ammonia-nitrogen 53.4% by Bacillus methylotrophicus L7 at low C/N ratios of 4–8 in saline media54.Chen et al.demonstrated that heterotrophic nitrification and aerobic denitrification (HN-AD) bacteria Klebsiella aerogenes strain shows maximum removal rates for ammonia, nitrate and nitrite 96.48%; 93.48% and 90.92% at an initial concentration of 6.18 mg/L/h, 4.45 mg/L/h and 2.38 mg/L/h simultaneously55 whereas Hoang et al. screened two denitrifying Bacillus sp. ST20 and Bacillus sp. ST26 from shrimp ponds in Vietnam and found that the removal efficiency of nitrite reached 68.5–82.5% from an initial concentration of 20 mgN-NO2/Lunder the anoxic conditions after 72 h56. Similarly, Yi et al.found that Pseudomonas chengduensis BF6 strain effectively removes the ammonia, nitrite and nitrate 97%, 79.10% and 61.40% within 24 h through assimilation in recirculating aquaculture systems57 whereas Shao et al. found the removal efficiency of ammonia-nitrogen (> 73%) and total nitrogen (> 68%) from aquaculture wastewater58. Sun et al.demonstrated that the degradation rate of ammonia and nitrite was 98.03% and 93.62% based on the synthesis of poly-γ-glutamic acid (γ-PGA) by Bacillus subtilis NX-2 at the inoculum level of 104 CFU/ml59. So, therefore the current strain has ability to transform ammonia and nitrate at high concentration with less time interval.
Further, microbial consortium of all four isolates were prepared for the transformation of ammonia at an initial concentration of 60 mg/L and observed that by using the consortium of all four isolates, the removal concentration of ammonia was (99.97%± 5.20) in 120 h from 60 mg/L to 59.985 ± 0.05 mg/L correspondingly. Similarly, Yang et al. determined that the consortium of ammonia-oxidizing bacteria removes around 100% of ammonia-nitrogen from an initial concentration of 262.28 ± 8.21 mg/L within 10 days and only 16.54 ± 0.52% of ammonia-nitrogen was able to convert nitrite-nitrogen by (HNAD) without N2O emission60whereas John et al. found that by using the consortium of three bacterial isolates, the residual concentration of ammonia in the tank was lower (4.8 ± 0.068 µML[-1) as compares to the control tank without using any bacteria (7.29 ± 0.292 µML[-1)51 and Yang et al. observed that the removal efficiency of ammonia-nitrogen was higher (90.4%) by using the bacterial consortium of Pseudomonas sp. and Acinetobacter sp61. as compares to single strain of Pseudomonas stutzeri YG-24 (73.2%) and Acinetobacter Junii YB (82.1%)62 at low C/N ratios of 4–5.Simon et al. developed mixed bacterial consortium and exhibits the efficient NH4+-N and NO3-N removal efficiency of 93.4 ± 1% and 90 ± 0.75% correspondingly63 whereas Geng et al.demonstrated that 94.67% of NH3-N reduced by the treatment with Bacillus subtilis, Bacillus licheniformis, and Chlorella vulgaris after 6 days of treatment64which reassures that the microbial consortium have higher efficiency to convert ammonia to nitrite and then to nitrate which is less toxic.
For in-situ remediation, microbial consortium of all four isolates (AOB AMS 1, AOB AMS 2, AOB AMS 5 and AOB AMSS 1) were prepared with an initial concentration of 60 mg/L and found that microbial consortium transforms the ammonia (10 ± 2%) on 1st day, (45 ± 5%) on 3rd day, (68 ± 3%) on 5th day, (90 ± 4%) on 7th day, (100 ± 2%) on 9th day and (100 ± 2%) on 10th day whereas nitrate was observed (90 ± 3%) on 1st day, (55 ± 5%) on 3rd day, (32 ± 4%) on 5th day, (10 ± 3%) on 7th day, (0 ± 2%) on 9th day and (0 ± 2%) on 10th day. Similarly, Yang et al. found that in the bio augmentation treatment of eutrophic wastewater, the removal efficiency of ammonia-nitrogen reached 77.88 ± 1.86% from an initial concentrations of 40.31 ± 0.57 mg/L by using the microbial consortium60.
This microbial consortium shown the growth of (3 × 102) CFU/ml on 1st day, (3.8 × 103) CFU/ml on 3rd day, (2.5 × 104) CFU/ml on 5th day, (3.9 × 104) CFU/ml on 7th day, (4 × 105) CFU/ml on 9th day and (4.2 × 105) CFU/ml on 10th day. Similarly, Huang et al. shown that the diluted cell suspension of each HNAD strain was around 1 × 105 CFU/ml48 whereas John et al. determined that the suspended inoculum density (4 × 108) of consortium was sprinkled uniformly over the surface51. It has been found that the removal efficiency of consortium was higher than by using single-isolate.
Conclusion
Currently this work is novel because this microbial consortium has the ability to transform the ammonia and nitrate (99.97%± 5.20)at lab-scale and (100 ± 2%) at in-situ remediation at higher concentration. Till now only (90.4%) has proven but there is no paper which shows the degradation of ammonia and nitrate at 30 mg/L and 60 mg/L both in lab-scale as well as in in-situ remediation up to 100 ± 2%.
This consortium is highly potential for the transformation of ammonia upto 60 mg/L. Currently this result obtained at lab-scale up to 60 mg/L for the transformation of ammonia and nitrite. Now we conducting factor responsible at in-situ level at field study. This potential consortium will be applied at field level at ammonia and nitrite polluted sites.
Data availability
No sequence data. No clinical trial data. All data is provided within the manuscript. No other data is available.
References
Bhambri, A., Karn, S. K. & Kumar, A. Application of Algae and Bacteria in Aquaculture. In book- Next-Generation Algae. Wiley Scrivener. (2023). https://doi.org/10.1002/9781119857839.ch6
Elliott, P. How to lower ammonia levels in a fish tank if they are not very high. MRCVs https://www.csh.rit.edu/~tonyl/goldfish/testarea/advcare.htm (2020).
Geneva, W. H. O. Ammonia. (Environmental Health Criteria, No. 54). (1986).
Boyd, C. E. Ph.D. Ammonia nitrogen dynamics in aquaculture. Global Aquaculture Advocate (2019).
Wang, S., Hu, J., He, S. & Wang, J. Removal of ammonia and phenol from saline chemical wastewater by ionizing radiation: performance, mechanism and toxicity. J. Hazard. Mater. 433, 128727. https://doi.org/10.1016/j.jhazmat.2022.128727 (2022).
Rychly, J. Nitrogen- balance in trout: 2 Nitrogen- excretion and retention after feeding diets with varying protein and carbohydrate- levels. Aquaculture 20, 343–350 (1980).
USEPA. Estimated National Occurrence and Exposure to Nitrate and Nitrite in Public Drinking Water Supplies (United States Environmental Protection Agency, Office of Drinking Water, 1987).
Bhambri, A. & Karn, S. K. Nitrate problems and its remediation. In The book- An Innovative Approach of Advanced Oxidation Process in Wastewater Treatment. Nova Science, USA. (2020b).
Ebeling, J. M., Timmons, M. B. & Bisogni, J. J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic and heterotrophic removal of ammonia- nitrogen in aquaculture systems. Aquaculture 257, 346–358 (2006).
Muthukrishnan, A., Sabaratnam, S. & Chong, V. C. Ammonia nitrogen removal by an aerobic heterotrophic bacterium, Microbacterium sp. VCM11. Malays. J. Sci. 31, 76–82 (2012).
Lin, C. Y. & Chen, J. C. Acute toxicity of nitrite on Litopenaeus vannamei (Boone) juveniles at different salinity levels. Aquaculture 224, 193–201 (2003).
Xian, J. A. et al. Cytotoxicity of nitrite on haemocytes of the tiger shrimp, Penaeus monodon, using flow cytometric analysis. Aquaculture 317, 240–244 (2011).
Gross, A., Abutbul, S. & Zilberg, D. Acute and chronic effects of nitrite on white shrimp, Litopenaeus Vannamei, cultured in low- salinity brackish water. J. World Aquacult. Soc. 35, 315–321 (2004).
Bhambri, A. & Karn, S. K. Strategic approach for characterization of bacterial community in enhanced biological phosphate removal process. In the book Wastewater Treatment Reactors. Maulin P. Shah and Susana Rodriguez-Couto (Editor). ISBN: 978-0-12-823991-9. Elsevier, Netherland. 28–45. (2021). https://doi.org/10.1016/B978-0-12-823991-9.00021-6
Bhambri, A., Karn, S. K. & Kumar, A. Regulation and Measurement of Nitrification in Terrestrial Systems. In book: Anaerobic Ammonium Oxidation. De Gruyter. (2023). https://doi.org/10.1515/9783110780093-004
Frances, J., Allan, G. L. & Nowak, B. F. The effects of nitrite on the short-term growth of silver perch (Bidyanus bidyanus). Aquaculture 163, 63–72 (1998).
Blancheton, J. P. Developments in recirculation systems for mediterranean fish species. Aquacult. Eng. 22, 17–31 (2000).
Mazik, P. M. et al. Influence of nitrite and chloride concentrations on survival and hematological profiles of striped bass. Trans. Am. Fish. Soc. 120, 247–254 (1991).
Chen, J. C. & Lee, Y. Effects of Nitrite on mortality, ion regulation, and acid-base balance of Macrobrachium rosenbergii at different external chloride concentrations. Aquat. Toxicol. 39, 291–305 (1997).
Turcios, A. & Papenbrock, J. Sustainable treatment of aquaculture effluents- what can we learn from the past for the future? Sustainability 6 (2), 836–856 (2014).
Lananan, F. et al. Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing effective microorganisms (EM- 1) and microalga (Chlorella sp). Int. Biodeterior. Biodegradation. 95, 127–134 (2014).
Li, Y., Liu, Y., Feng, L. & Zhang, L. Coupled mixotrophic denitrification and utilization of refractory organics driven by Mn redox circulation for significantly enhanced nitrogen removal. J. Hazard. Mater. 445, 130595. https://doi.org/10.1016/j.jhazmat.2022.130595 (2023b).
Li, X. Y. & Chu, H. P. Membrane bioreactor for the drinking water treatment of polluted surface water supplies. Water Res. 37 (19), 4781–4791 (2003).
Shen, Y. et al. The nitrogen reduction in eutrophic water column driven by Microcystis blooms. J. Hazard. Mater. 385, 121578 (2020).
Buttiglieri, G. et al. Denitrification of drinking water sources by advanced biological treatment using a membrane bioreactor. Desalination 178 (1–3), 211–218 (2005).
Rocca, D. C., Belgiorno, V. & Meric, S. Overview of in-situ applicable nitrate removal processes. Desalination 204 (1–3), 46–62 (2007).
WHO. Guidelines for Drinking Water Quality, 3rd ed., World Health Organization. Geneva. (2003).
Tannebaum, S. R. & Green, L. C. Selected Abstract on the role of Dietary Nitrate and Nitrite in Human Carcinogenesis (International Cancer Research Data Bank Program, National Cancer Institute, 1985).
ECC. Implementation of council Directive 91/676/ECC concerning the protection of waters against pollution caused by nitrates from agricultural sources, Report from the Commission. COM. 407 Final, Brussels. (2002). (2002).
Bhambri, A. & Karn, S. K. Bio- technique for Nitrogen and Phosphorus Removal: A Possible Insight. Chemistry and Ecology (Taylor & Francis Group). London. 36, 785–809 (2020). https://doi.org/10.1080/02757540.2020.1777991
Datta, P. S. et al. Groundwater NO3 and F contamination processes in Pushkar valley, Rajasthan as reflected from 18O isotopic signature and 3H recharge studies. Environ. Monit. Assess. 56, 209–219 (1999).
Tandia, A. A., Diop, E. S. & Gaye, C. B. Nitrate groundwater pollution in suburban areas: example of groundwater from Yeumbeul, Senegal. J.A Fr. Earth Sci. 29 (4), 809–822 (1999).
Costa, J. L. et al. Nitrate contamination of a rural aquifer and accumulation in the unsaturated zone. Agr Water Manage. 57, 33–47 (2002).
Mourabit, F., Ouassini, A., Azmania, A. & Muellerc, R. Nitrate occurrence in the groundwater of the Loukkos perimeter. J. Environ. Monit. 4, 127–130 (2002).
Li, Y. & Zhang, J. Agriculture diffuse pollution from fertilisers and pesticides in China. Water Sci. Technol. 39 (3), 25–32 (1999).
Kesseru, P., Kiss, I., Bihari, Z. & Polyak, B. Investigation of the denitrification activity of immobilized Pseudomonas butanouora cells in the presence of different organic substrates. Water Res. 36, 1565–1571 (2002).
Bhambri, A., Karn, S. K. & Singh, R. K. In-situ remediation of nitrogen and phosphorus of beverage industry by potential strains Bacillus sp. (BK1) and Aspergillus sp. (BK2). Scientific Reports, (Nature Publishers), London, UK. 11, 12243. (2021). https://doi.org/10.1038/s41598-021-91539-y
Zhou, Q., Li, K., Xie, J. & Bo, L. Role and functions of beneficial microorganisms in sustainable aquaculture. Bioresour Technol. 100, 3780–3786 (2009).
Crab, R., Defoirdt, T., Bossier, P. & Verstraete, W. Biofloc technology in aquaculture beneficial effects and future challenges. Aquaculture 356–357, 351–356 (2012).
Martinez-Cordova, L. R., Emerenciano, M., Miranda-Baeza, A. & Martinez Porchas, M. Microbial-based systems for aquaculture of fish and shrimp: an updated review. Rev. Aquac. 7, 131–148 (2015).
Kapoor, A. & Viraraghavan, T. Nitrate removal from drinking water review. J. Environ. Eng. 123, 371–380 (1997).
Huang, F., Pan, L., He, Z., Zhang, M. & Zhang, M. Culturable heterotrophic nitrification-aerobic denitrification bacterial consortia with cooperative interactions for removing ammonia and nitrite nitrogen in mariculture effluents. Aquaculture 523, 735211. https://doi.org/10.1016/j.aquaculture.2020.735211 (2020).
Parsons, T. R., Maita, Y. & Lalli, C. M. A Manual of Chemical and Biological Methods for Seawater Analysis (Pergamon Press. Elmsford, 1984).
Boltz, D. F. (ed) Colorimetric Determination of Nonmetals (Interscience, 1958).
Armstrong, F. A. J. Determination of nitrate in water by ultraviolet spectrophotometry. Anal. Chem. 35, 1292 (1963).
O’Neil, C. & Renfrow, W. B. A qualitative test for ammonia in aqueous solution. J. Chem. Educ. 29 (2), 94. https://doi.org/10.1021/ed029p94 (1952).
Wiberg, E. & Holleman, A. F. Inorganic Chemistry. Elsevier. ISBN-0-12-352651-5. (2001).
Huang, F., Pan, L., He, Z., Zhang, M. & Zhang, M. Culturable heterotrophic nitrification-aerobic denitrification bacterial consortia with cooperative interactions for removing ammonia and nitrite nitrogen in marinculture effluents. Aquaculture 523, 735211. https://doi.org/10.1016/j.aquaculture.2020.735211 (2020).
Rajakumar, S. et al. Nitrate removal efficiency of bacterial consortium (Pseudomonas sp. KW1 and Bacillus sp. YW4) in synthetic nitrate-rich water. J. Hazard. Mater. 157, 553–563. https://doi.org/10.1016/j.jhazmat.2008.01.020 (2008).
Yun, L. et al. Ammonia nitrogen and nitrite removal by a heterotrophic Sphingomonas sp. Strain LPN080 and its potential application in aquaculture. Aquaculture 500, 477–484. https://doi.org/10.1016/j.aquaculture.2018.10.054 (2019).
John, E. M., Krishnapriya, K. & Sanskar, T. V. Treatment of ammonia and nitrite in aquaculture wastewater by an assembled bacterial consortium. Aquaculture 526, 735390 (2020).
Gogoi, M. et al. Aquaculture effluent treatment with ammonia remover Bacillus albus (ASSF01). J. Environ. Eng. 9 (4), 105697. https://doi.org/10.1016/j.jece.2021.105697 (2021).
Barik, P., Ram, R., Haldar, C. & Vardia, H. K. Study on nitrifying bacteria as bioremediator of ammonia in simulated aquaculture system. J. Entomol. Zool. Stud. 6 (3), 1200–1206 (2018).
Zhang, Q. L. et al. The characteristics of a novel heterotrophic nitrification-aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresour Technol. 108, 35–44 (2012).
Chen, Y. et al. Exploring the nitrogen removal capacity of Klebsiella aerogenes B23 isolated from shrimp farm wastewater: heterotrophic nitrification and aerobic denitrification. Aquacult. Int. https://doi.org/10.1007/s10499-023-01224-2 (2023).
Hoang, P. H. et al. Characterization of isolated aerobic denitrifying Bacteria and their potential use in the treatment of Nitrogen-Polluted Aquaculture Water. Curr. Microbiol. 79, 209. https://doi.org/10.1007/s00284-022-02898-2 (2022).
Yi, M. et al. Efficient nitrogen removal of a novel Pseudomonas chengduensis strain BF6 mainly through assimilation in the recirculating aquaculture systems. Bioresour. Technol. 379, 129036. https://doi.org/10.1016/j.biortech.2023.129036 (2023).
Shao, S., Wang, M., Zhong, J. & Wu, X. Characteristics of tetracycline degradation coupled simultaneous nitrification-denitrification and phosphorus removal in Aquaculture Wastewater. Geomicrobiol J. 40 (4), 399–412 (2023).
Sun, L. et al. A strategy for nitrogen conversion in aquaculture water based on poly-γ-glutamic acid synthesis. Int. J. Biol. Macromol. 229, 1036–1043 (2023).
Yang, X., Liu, L. & Wang, S. A strategy of high-efficient nitrogen removal by an ammonia-oxidizing bacterium consortium. Bioresour. Technol. 275, 216–224. https://doi.org/10.1016/j.biortech.2018.12.057 (2019).
Yang, Y., Liu, Y., Yang, T. & Lu, Y. Characterization of a microbial consortium capable of heterotrophic nitrifying under wide C/N range and its potential application in phenolic and coking wastewater. Biochem. Eng. J. 120, 33–40. https://doi.org/10.1016/j.bej.2016.12.008 (2017).
Ren, Y. X., Yang, L. & Liang, X. The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour Technol. 171, 1–9. https://doi.org/10.1016/j.biortech.2014.08.058 (2014).
Simon, M., Joshi, H., Yadav, A. K. & &Hopke, P. K. Simultaneous nitrogen and phosphorus removal from polluted water using a novel bacterial consortium (BACON-38): promising application for wastewater remediation. J. Water Process. Eng. 66, 106044 (2024).
Geng, B., Li, Y., Liu, X., Ye, J. & Guo, W. Effective treatment of aquaculture wastewater with mussel/microalgae/bacteria complex ecosystem: a pilot study. Sci. Rep. 12, 2263 (2022).
Acknowledgements
I like to thanks SKK for giving space in this project which not only help me learn but also helped me understand and resolve the problem and develop scientific temperament to work honestly. Authors acknowledge SBS University and SGRR University for providing space and facility to carry out this work.
Author information
Authors and Affiliations
Contributions
SKK framed the idea of the manuscript AB conducted the experiment AK help in conduction of the experiment. Overall all the authors are aggred and checked the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bhambri, A., Karn, S.K. & Kumar, A. Ammonia transformation by potential consortium in the aquatic system. Sci Rep 15, 6066 (2025). https://doi.org/10.1038/s41598-025-90206-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90206-w
Keywords
This article is cited by
-
Development of a psychrotolerant composite microbial agent for nitrogen removal and its nitrogen metabolism pathways
Bioprocess and Biosystems Engineering (2026)
-
Enrichment of nitrifying microbial communities in aquaculture: current trends and prospects
Aquaculture International (2025)