Abstract
MRI is the preferred method for follow-up imaging of post-treatment WHO grade 3 or 4 gliomas. While positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine) (18F-FET PET) offers higher diagnostic accuracy, its use is limited due to low availability. We propose a sequential, threshold-based workflow to triage patients for additional 18F-FET PET scans based on MRI dynamic susceptibility contrast (DSC) perfusion-derived rCBV values, to optimize 18F-FET PET resource allocation. Patients with high-grade gliomas who had undergone standard-of-care treatment and developed new or enlarging contrast-enhancing post-treatment lesions on MRI were included, with a 18F-FET PET study performed within 4 months of the MRI. Patients were excluded if there were significant changes in lesion size or treatment between the MRI and 18F-FET PET scan. An rCBV threshold was determined and the performance of a threshold-based imaging workflow was evaluated compared to the gold standard defined here as surgical verification or long-term imaging follow-up without further intervention. Forty-one patients with a total of 49 lesions were included (tumor progression n = 40, treatment-related changes n = 9). Above the rCBV threshold of 2.4, MRI was 100% accurate (21/21 patients) in diagnosing tumor progression. Below the threshold, MRI identified 9 true negatives but produced 19 false negatives. 18F-FET PET reclassified 18/19 (95%) false negatives resulting in an overall accuracy of 48/49 (98%) for the workflow. Our MRI DSC perfusion rCBV-based threshold workflow for triaging patients for additional 18F-FET PET imaging in post-treatment high grade glioma has the potential to optimize 18F-FET PET resource allocation.
Similar content being viewed by others
Introduction
High grade gliomas are the most common malignant brain tumors in adults, with an incidence of 3–4 per 100,000 people. The current standard of care includes maximal safe resection followed by radiotherapy with or without concomitant chemotherapy, followed by maintenance temozolomide-based chemotherapy1,2,3,4. Due to high and often inevitable recurrence rates, short-term follow-up imaging is routinely performed. Magnetic resonance imaging (MRI), being non-invasive and widely available, is the preferred modality for follow-up imaging despite presenting a diagnostic dilemma in the early post-treatment setting. Treatment-related changes can mimic tumor progression on MRI through new, size-increasing contrast enhancing lesions but are histologically usually transient, inflammatory processes and do not represent tumor progression. The term “treatment-related changes” includes pseudoprogression, typically occurring 3 to 6 months after chemoradiation, and radiation necrosis, which appears after 6 months and possibly years later. Treatment-related changes are common, occurring in 9–36% of patients1,3,5,6,7,8,9, and must be differentiated from tumor progression to guide appropriate patient management.
Dynamic Susceptibility Contrast (DSC) MRI perfusion-weighted imaging (henceforth denoted PWI) is the most validated and widely employed advanced imaging technique for differentiation of treatment-related changes from tumor progression in glioma4,8. Relative cerebral blood volume (rCBV), a parameter derived from PWI, characterizes angiogenesis and serves as a surrogate marker for malignancy, being elevated in cases of tumor progression and not elevated with treatment-related changes5,8.
Positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine) (18F-FET PET), a nuclear medicine study, characterizes amino acid metabolism in tumor cells through uptake of amino acid analogs, thus proving unique functional information complementary to MRI. Furthermore, uptake may be independent of BBB disruption10,11. True tumor progression will demonstrate high amino acid uptake and wash-out, as opposed to healthy brain tissue that shows no significant uptake1,7,8. Unfortunately, the availability of 18F-FET PET is limited due to reimbursement issues and its lack of approval as a medical drug for gliomas in many countries and healthcare systems12,13. As a result, MRI is still considered more suitable than 18F-FET PET as a follow-up tool, despite the superior diagnostic accuracy of 18F-FET PET14,15. The purpose of this study was to propose a sequential, threshold-based workflow to triage patients for additional 18F-FET PET scans based on MRI DSC-Perfusion derived rCBV values, to optimize 18F-FET PET resource allocation.
Materials and methods
Prior to commencing the study, ethical approval was obtained through the Institutional Review Board (Kantonale Ethikkommission Zuerich, BASEC Nr. 2021–02297). Informed consent was obtained for all patients included. All methods were performed in accordance with the relevant guidelines and regulations. A full-text Radiological Information System (RIS)-based search was performed for MRI studies with DSC Perfusion for the terms “progression vs pseudoprogression,” “pseudoprogression,” and “radiation necrosis” in patients with post-treatment high-grade gliomas from 1/2016 to 12/2021. Patients were included according to the following eligibility criteria: (1) high-grade (WHO III/3 or IV/4) gliomas confirmed by histology based on the WHO classification at the time of diagnosis16,17; (2) completed standard-of-care treatment including radiotherapy; 4) new or enlarged contrast-enhancing lesion on post-treatment follow-up imaging as defined per Response Assessment in Neuro-Oncology (RANO) Criteria for Gliomas18; 5) had MRI including PWI; 6) had 18F-FET PET performed within 4 months of the PWI without interval treatment change or significant differences in the lesion between the two modalities. Patients with motion degraded MRI studies and SWI hypointensity overlapping the contrast-enhancing lesions (limiting DSC perfusion) were excluded.
Outcome
The gold standard for differentiating tumor progression from treatment-related changes is histopathological verification, however this was only available in a subset of patients. In all other patients, outcome was determined through MRI follow-up data, where continued contrast-enhancing lesion progression was considered indicative of progression while a spontaneously regressing lesion (without administration of bevacizumab) was determined to represent treatment-related changes.
Image analysis
MRI
DSC perfusion (Echo planar 2D; EP2D) and contrast-enhanced T1-weighted (T1-CE) images were acquired on Siemens Skyra 3.0 T (Siemens Healthineers, Erlangen, Germany) systems with the following parameters: Single-dose Dotarem (Guerbet, Villepint, France) without pre-loading, TR/TE 1750/29 ms; slice thickness 4 mm; matrix: 128 × 128; flip angle = 90°.
DSC perfusion data was evaluated by one reader (NH) with 8 years of neuroradiology experience including dedicated fellowship training using Olea Sphere 3.0 – SP28 (Olea Medical, Cambridge, Massachusetts, USA) with built-in leakage correction19. Regions of interest (ROI) were drawn on axial T1-CE using the slice with the largest lesion diameter, encompassing as much of the contrast-enhancing component as possible. Obvious areas of necrosis were avoided. A reference ROI with approximately the same size was drawn on the same axial slice on normal-appearing white matter in the contralateral brain hemisphere. The normalized rCBV was calculated as rCBVtumor/rCBVcontralateral using mean rCBV values derived from the ROIs.
18F-FET PET
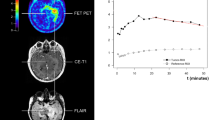
18F-FET PET images were acquired on a PET/MR (3.0 T) scanner (Signa PET/MR; GE HealthCare). The patients were injected with a standardized dose of 130 MBq 20 min immediately before the dynamic PET acquisition. All PET images were reconstructed using the ordered-subsets expectation maximization algorithm in conjunction with point-spread-function modeling. The reconstructed dynamic datasets consisted of 8 time frames (8 × 5 min).
18F-FET PET data was evaluated by one reader (MH) with 14 years of experience in neuroradiology and nuclear medicine. Mean lesional 18F-FET uptake was determined by a 3-dimensional auto-contouring process. Max TBR (TBR) was calculated by dividing the SUVmean of the hottest five pixels within the ROI by the SUVmean of a larger reference ROI placed in the normal appearing contralateral hemisphere including white and gray matter. A TBR threshold of 2.1 was used to differentiate treatment-related changes from tumor progression. The following additional MRI criteria were used for 18F-FET PET/MRI acquisitions to establish the final 18F-FET PET diagnosis: Contrast uptake, presence of susceptibility on T2*-weighted images, signal characteristics of lesion on FLAIR-weighted and T2-weighted images.
Numerical variables are presented as median (range), categorical variables are presented as number (percentage). Sensitivity, specificity and area under the receiver operating characteristics curve (AUC) were calculated. All statistical analyses were performed using R statistical software (version 4.4.1, R Foundation for Statistical Computing, Vienna, Austria). Number Needed to Image (NNI)20 was calculated using 18F-FET PET sensitivity as the control and the sequential rCBV threshold-based workflow sensitivity as the experimental arm.
Results
Patient cohort
Upon full-text search, 73 patients were identified. Thirty-two patients were excluded due to the following reasons: full-text search terms only found in the clinical history or query of the radiological report (n = 28), quality degraded images (n = 2), SWI hypointensity limiting DSC-Perfusion assessment (n = 2) (Fig. 1). The final cohort consisted of 41 patients (males = 23, females = 18, median age = 59 years (range 21 to 77 years)). Forty-nine lesions fulfilling the inclusion criteria were identified (8 patients harboured 2 lesions). Diagnosis based on histology; glioblastoma, n = 36; anaplastic astrocytoma, WHO grade 3, n = 12; astrocytoma, IDH-mutant, WHO 4 = 1. Radiation was performed in all patients (n = 41) for all lesions (n = 49), and dose was specified for 38 patients: mean 57 Gy (range 40.05 Gy to 60 Gy). 37 patients had surgical tumor resection (with an additional re-resection in seven patients), biopsy only was performed in three patients, and biopsy followed by laser ablation in one patient. Thirty-three patients received first line chemotherapy (temozolomide) and three patients were included into trials (concomitant temozolomide and pembrolizumab (n = 1), nivolumab (n = 1), not further specified (n = 1)). Twelve patients received second line chemotherapy (temozolomide (n = 3), lomustine (n = 4), rindopepimut (n = 2), not further specified (n = 3)). Bevacizumab was not administered in any patient immediately before or at the time of image acquisition. Median time from completion of treatment to MRI was 5 months (range 1 month to 153 months). A study on MRI perfusion post-processing was previously performed using the same cohort4.
Standards for the reporting of diagnostic accuracy studies flow diagram. 18F-FET PET = 18F fluoroethyl-L-tyrosine positron emission tomography; DSC = dynamic susceptibility contrast; rCBV = relative cerebral blood volume; SWI = susceptibility weighted imaging; TBR = tumor to background ratio (Asterisk (*) indicates number of lesions misclassified based on TBR alone, re-classified based on the morphological MRI component of 18F-FET PET/MRI scans.
Outcome
Gold standard diagnosis revealed tumor progression in 40/49 lesions (twelve determined histologically, 28 via longitudinal MRI follow-up), and treatment-related changes in 9/49 lesions (all determined through longitudinal MRI follow-up) (Table 1). Mean MRI follow-up time for diagnosis of lesions not determined histologically was 25 months (range 5 to 162 months).
Image analysis
This study builds on a previous publication using the same patient cohort, which identified an optimal rCBV threshold of 2.4 for distinguishing tumor progression from treatment-related changes4. This threshold dichotomized the cohort into 21 cases of tumor progression above 2.4 (range rCBV 2.5–10.5), all confirmed to be tumor progression by gold standard, and 28 cases of treatment-related change at or below 2.4 (range rCBV 0.3–2.4). Of the 28 below-threshold cases, gold standard identified 9 cases to be treatment-related change while the remaining 19 cases were identified as tumor progression (i.e. 19 false negatives). Overall, MRI demonstrated a sensitivity of 53%, a specificity of 100%, and an AUC of 0.76 (95% Confidence Interval (CI); 0.68 to 0.84).
All patients underwent 18F-FET PET scanning. Median time difference between MRI and 18F-FET PET was 27 days (range 2 to 112 days), with MRI being acquired first in 67% (33/49) of cases. Above the MRI threshold of rCBV 2.4, 18F-FET PET classified 20/21 cases correctly as tumor progression and classified one as treatment-related changes (false-negative i.e. true tumor progression determined by gold standard). At or below the threshold of rCBV 2.4, 18F-FET PET reclassified 95% (18/19) MRI rCBV lesions from treatment-related changes to tumor progression (Fig. 2). The remaining 1/19 case was incorrectly classified by both MRI and 18F-FET PET as treatment-related change. All 9 cases of treatment-related change were correctly identified by both MRI and 18F-FET PET. Based on a TBR threshold of 2.1, 43/49 lesions diagnosed by 18F-FET PET were on the appropriate side of the threshold, while for the remaining 6 lesions, the MRI component of the 18F-FET PET/MRI study was given greater weighting. Over the entire cohort, 18F-FET PET demonstrated a sensitivity of 95%, a specificity of 100%, and an AUC of 0.98 (95% CI; 0.94 to 1.00). The sequential, rCBV threshold-based workflow demonstrated a sensitivity of 97%, a specificity of 100%, and an AUC of 0.99 (95% CI; 0.96 to 1.00). Overall, our workflow resulted in a 42.8% (21/49) reduction in the number of 18F-FET PET scans with an NNI of 50.
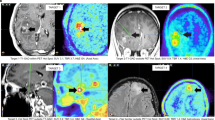
Three different high-grade glioma post-treatment patients with routine follow-up MRI studies demonstrating no contrast enhancement initially (1a, 2a, 3a; all contrast-enhanced T1-weighted images). Upon further MRI follow-up, a new contrast-enhancing lesion was discovered in the right temporal lobe (1b; arrow), right frontal lobe (2b; arrow) and left temporal lobe (3b; arrow). rCBV (relative cerebral blood volume) values derived from Dynamic susceptibility weighted perfusion MRI performed at the time of lesion discovery revealed below-threshold rCBV values for all three patients (1c, 2c, 3c; arrows), suggesting the diagnosis of treatment-related changes. 18F-FET PET performed at the time of MRI = T1 demonstrated high tracer uptake (TBR; tumor to background ratio) and correctly overturned the diagnosis to tumor progression in all cases (1d, 2d, 3d; arrows).
Discussion
In this study, we propose a threshold-based sequential workflow utilizing PWI to triage post-treatment high grade glioma patients for additional 18F-FET PET imaging. Below the rCBV threshold, 18F-FET PET reclassified 95% of PWI-based diagnoses while above the cutoff, PWI alone was 100% accurate compared to the gold standard. Overall, this workflow correctly classified 98% (48/49) of cases. The greatest benefit of 18F-FET PET was observed when PWI did not clearly indicate tumor progression.
A recent meta-analysis of 28 articles reported pooled sensitivity and specificity of DSC MRI perfusion for the differentiation of tumor progression from treatment-related changes at 90 and 89%, respectively21. This is comparable to FET PET, which has reported sensitivity ranges of 78 to 99% and specificity ranges of 67 to 100%12. Despite 18F-FET PET’s slightly higher diagnostic accuracy than MRI, it is typically used as a second-line imaging modality in equivocal cases14,15. The advent of hybrid 18F-FET PET/MRI has improved patient logistics, but the technology is not widely available22 and has not demonstrated a significant difference in clinical outcomes when compared to sequential scanning in of patients with gliomas after treatment23.
Only one other study has previously assessed an rCBV threshold-based workflow to guide additional 18F-FET PET imaging for distinguishing treatment-related change from tumor progression. In their study, Steidl et al. reported an rCBVmax cutoff of 2.85, above which MRI correctly classified 100% of patients, whereas in the remaining 60 below-threshold patients, 18F-FET PET correctly diagnosed 78% of cases (using either stand-alone 18F-FET PET or 18F-FET PET/MRI)24. Overall, the accuracy of their sequential workflow achieved 87%. Notably, the authors included low-grade gliomas (WHO II), representing a major difference to our study, and their reporting of rCBVmax compared to our rCBVmean. Furthermore, their study included more heterogeneity in terms of MRI vendor, field strength, DSC perfusion methodology and pulse sequence parameters, with both studies being single-center. Finally, our 18F-FET PET diagnosis was based on a different TBR-threshold value (2.1) as opposed to 1.95 in their study. Given the known limitations of using TBR alone, with accuracies of around 70% in this setting25, the potential for false positive and false negatives associated with 18F-FET uptake26,27, and the wide range of TBR values for differentiating these two entities27,28, we integrated MRI findings from 18F-FET PET/MRI acquisitions to arrive at the final 18F-FET PET diagnosis, representing a further difference to Steidl et al.
Despite Response Assessment in Neuro-Oncology (RANO) Working Group’s recommendation for the integration of amino acid PET at all stages of brain tumor management29, 18F-FET PET remains limited in availability. Approval for 18F-FET as a medical drug for gliomas is currently restricted to Switzerland, Germany, and France, as the lack of prospective multi-center trials has hampered approval in other European countries and North America12,13. Notably, 18F-FET has recently been granted FDA fast track designation and is on course to become the first FDA approved radiolabeled amino acid for use in diagnostic imaging of brain tumors in the United States30. Moreover, re-imbursement varies even within countries where 18F-FET PET is approved, further complicating access to the modality12. Cost-effectiveness is another significant factor contributing to the limited availability of 18F-FET PET31,32,33, yet numerous recent publications from Asia reflect growing global interest and acceptance34,35,36,37,38. This corroborates the rising interest among primary care physicians13,39,40, emphasizing the importance of optimizing 18F-FET PET allocation given the constraints on resources.
Our sequential, rCBV threshold-based workflow resulted in an NNI of 50 when compared to 18F-FET PET, with a simultaneous 42.8% reduction in the number of 18F-FET PET scans required. These metrics reiterate how the workflow could be used to triage patients from centers without access to amino acid PET (but with PWI MRI) to more comprehensive centers as part of a regional cancer care network, optimizing resource allocation and ensuring appropriate imaging when required.
The retrospective nature of our study represents a limitation, with 18F-FET PET and MRI with DSC Perfusion not being acquired on the same day. Our inclusion criteria allowed for a time interval of up to four months between MRI and 18F-FET PET, which exceeds the recommended three-month follow up period. While this represents a further limitation of our study, it reflects the realities of clinical practice, including waitlists and scheduling constraints that patients experience. A shorter time interval cutoff in our inclusion criteria would have significantly reduced the size of our cohort. MRI DSC perfusion parameters were not aligned to current recommendations41, again a consequence of the retrospective nature of our study. ROIs were not co-registered between the two modalities, though this ultimately represents the clinical reality. The imbalance of tumor progression and treatment-related changes in our cohort also represents what is seen in clinical routine and provides an incidence-corrected rCBV threshold. The interpretation of PET findings were based on Max TBR using SUVmean of the hottest five pixels and did not include further parameters such as maximum and mean TBR or PET-positive volume, which are recommended for amino acid PET in glioma42. Finally, histopathological correlation was only obtained in a subset of patients.
To conclude, we have demonstrated the feasibility of an rCBV-based threshold workflow to triage patients for additional 18F-FET PET imaging in post-treatment high grade glioma. Our study further validates the findings from Steidl et al., despite differences in glioma grades included and PWI methodology24. This workflow could improve 18F-FET PET resource allocation as its use expands globally.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- 18F:
-
FET PET O-(2-[18F]fluoroethyl)-L-tyrosine) positron emission tomography
- AUC:
-
Area under the curve
- BBB:
-
Blood-brain barrier
- DSC:
-
Dynamic susceptibility contrast
- FDA:
-
U.S. food and drug administration
- MRI:
-
Magnetic resonance imaging
- NNI:
-
Number needed to image
- RIS:
-
Radiological information system
- RANO:
-
Response assessment in neuro-oncology
- rCBV:
-
Relative cerebral blood volume
- SUV:
-
Standardized uptake value
- SWI:
-
Susceptibility weighted imaging
- TBR:
-
Tumor to background ratio
- T1-CE:
-
T1-weighted, contrast enhanced
- ROI:
-
Region of interest
- WHO:
-
World Health Organization
References
Soni, N., Ora, M., Mohindra, N., Menda, Y. & Bathla, G. Diagnostic performance of PET and perfusion-weighted imaging in differentiating tumor recurrence or progression from radiation necrosis in posttreatment gliomas: A review of literature. Am. J. Neuroradiol. https://doi.org/10.3174/ajnr.A6685 (2020). ajnr;ajnr.A6685v1.
Weller, M. et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 18, e315–e329. https://doi.org/10.1016/S1470-2045(17)30194-8 (2017).
Sidibe, I., Tensaouti, F., Roques, M., Cohen-Jonathan-Moyal, E. & Laprie, A. Pseudoprogression in glioblastoma: Role of metabolic and functional MRI-systematic review. Biomedicines 10, 285. https://doi.org/10.3390/biomedicines10020285 (2022).
Nierobisch, N. et al. Comparison of clinically available dynamic susceptibility contrast post processing software to differentiate progression from pseudoprogression in post-treatment high grade glioma. Eur. J. Radiol. 167, 111076. https://doi.org/10.1016/j.ejrad.2023.111076 (2023).
Fink, J., Born, D. & Chamberlain, M. C. Pseudoprogression: Relevance with respect to treatment of high-grade gliomas. Curr. Treat. Options Oncol. 12, 240–252. https://doi.org/10.1007/s11864-011-0157-1 (2011).
Wang, W. et al. Glioblastoma pseudoprogression and true progression reveal spatially variable transcriptional differences. Acta Neuropathol. Commun. 11, 192. https://doi.org/10.1186/s40478-023-01587-w (2023).
Young, J. S., Al-Adli, N., Scotford, K., Cha, S. & Berger, M. S. Pseudoprogression versus true progression in glioblastoma: What neurosurgeons need to know. J. Neurosurg. 139, 748–759. https://doi.org/10.3171/2022.12.JNS222173 (2023).
Strauss, S. B., Meng, A., Ebani, E. J. & Chiang, G. C. Imaging glioblastoma posttreatment. Neuroimaging Clin. 31, 103–120. https://doi.org/10.1016/j.nic.2020.09.010 (2021).
Chinot, O. L. et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 709–722. https://doi.org/10.1056/NEJMoa1308345 (2014).
Koh, E-S. et al. [18 F]-fluoroethyl-L-tyrosine (FET) in glioblastoma (FIG) TROG 18.06 study: Protocol for a prospective, multicentre PET/CT trial. BMJ Open 13, e071327. https://doi.org/10.1136/bmjopen-2022-071327 (2023).
Smith, N. J. et al. Hybrid 18 F-fluoroethyltyrosine PET and MRI with perfusion to Distinguish disease progression from treatment-related change in malignant brain tumors: The quest to beat the toughest cases. J. Nucl. Med. 64, 1087–1092. https://doi.org/10.2967/jnumed.122.265149 (2023).
Galldiks, N. et al. Challenges, limitations, and pitfalls of PET and advanced MRI in patients with brain tumors: A report of the PET/RANO group. Neuro Oncol. 26, 1181–1194. https://doi.org/10.1093/neuonc/noae049 (2024).
Heinzel, A. et al. Two decades of brain tumour imaging with O-(2-[18F]fluoroethyl)-L-tyrosine PET: The Forschungszentrum Jülich experience. Cancers 14, 3336. https://doi.org/10.3390/cancers14143336 (2022).
Galldiks, N. et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 42, 685–695. https://doi.org/10.1007/s00259-014-2959-4 (2015).
Galldiks, N. et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro-Oncol 17, 1293–1300. https://doi.org/10.1093/neuonc/nov088 (2015).
Louis, D. N. et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 131, 803–820. https://doi.org/10.1007/s00401-016-1545-1 (2016).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 23, 1231–1251. https://doi.org/10.1093/neuonc/noab106 (2021).
Leao, D. J., Craig, P. G., Godoy, L. F., Leite, C. C. & Policeni, B. Response assessment in neuro-oncology criteria for gliomas: Practical approach using conventional and advanced techniques. AJNR Am. J. Neuroradiol. 41, 10–20. https://doi.org/10.3174/ajnr.A6358 (2020).
Boxerman, J. L., Schmainda, K. M. & Weisskoff, R. M. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am. J. Neuroradiol. 27, 859–867 (2006).
Haller, S. The Concept of Number needed to image. AJNR Am. J. Neuroradiol. 38, E79–80. https://doi.org/10.3174/ajnr.A5276 (2017).
Patel, P. et al. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: A systematic review and meta-analysis. Neuro Oncol. 19, 118–127. https://doi.org/10.1093/neuonc/now148 (2017).
Langen, K-J. et al. Hybrid PET/MRI in cerebral glioma: Current status and perspectives. Cancers 15, 3577. https://doi.org/10.3390/cancers15143577 (2023).
Ziegenfeuter, J. et al. Sequential and hybrid PET/MRI acquisition in follow-up examination of glioblastoma show similar diagnostic performance. Cancers 15, 83. https://doi.org/10.3390/cancers15010083 (2023).
Steidl, E. et al. Sequential implementation of DSC-MR perfusion and dynamic [18F]FET PET allows efficient differentiation of glioma progression from treatment-related changes. Eur. J. Nucl. Med. Mol. Imaging 48, 1956–1965. https://doi.org/10.1007/s00259-020-05114-0 (2021).
Maurer, G. D. et al. 18F-FET PET imaging in differentiating glioma progression from treatment-related changes: A single-center experience. J. Nucl. Med. 61, 505–511. https://doi.org/10.2967/jnumed.119.234757 (2020).
Lohaus, N., Mader, C., Jelcic, I., Reimann, R. & Huellner, M. W. Acute disseminated encephalomyelitis in FET PET/MR. Clin. Nucl. Med. 47, e137–e139. https://doi.org/10.1097/RLU.0000000000003879 (2022).
Galldiks, N. et al. Treatment monitoring of Immunotherapy and targeted therapy using 18F-FET PET in patients with melanoma and lung cancer brain metastases: Initial experiences. J. Nucl. Med. https://doi.org/10.2967/jnumed.120.248278 (2020).
Rapp, M. et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J. Nucl. Med. 54, 229–235. https://doi.org/10.2967/jnumed.112.109603 (2013).
Albert, N. L. et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 18, 1199–1208. https://doi.org/10.1093/neuonc/now058 (2016).
Bheda, K. TLX101-CDx (Pixclara™) Granted FDA Fast Track Designation. Telix Pharm. https://telixpharma.com/news-views/tlx101-cdx-pixclara-granted-fda-fast-track-designation/. Accessed 21 Oct 2024 (2024).
Heinzel, A., Stock, S., Langen, K-J. & Müller, D. Cost-effectiveness analysis of FET PET-guided target selection for the diagnosis of gliomas. Eur. J. Nucl. Med. Mol. Imaging 39, 1089–1096. https://doi.org/10.1007/s00259-012-2093-0 (2012).
Heinzel, A. et al. The use of O-(2-18F-fluoroethyl)-l-tyrosine PET for treatment management of Bevacizumab and Irinotecan in patients with recurrent high-grade glioma: A cost-effectiveness analysis. J. Nucl. Med. 54, 1217–1222. https://doi.org/10.2967/jnumed.113.120089 (2013).
Moreau, A., Febvey, O., Mognetti, T., Frappaz, D. & Kryza, D. Contribution of different positron emission tomography tracers in glioma management: Focus on glioblastoma. Front. Oncol. 9, 1134. https://doi.org/10.3389/fonc.2019.01134 (2019).
Puranik, A. D. et al. Brain FET PET tumor-to-white mater ratio to differentiate recurrence from post-treatment changes in high-grade gliomas. J. Neuroimaging 31, 1211–1218. https://doi.org/10.1111/jon.12914 (2021).
Chan, D. L. et al. FET PET in the evaluation of indeterminate brain lesions on MRI: Differentiating glioma from other non-neoplastic causes—a pilot study J. Clin. Neurosci. 58, 130–135. https://doi.org/10.1016/j.jocn.2018.09.009 (2018).
Cheng, Y. et al. Glioma imaging by O-(2-18F-fluoroethyl)-L-tyrosine PET and diffusion-weighted MRI and correlation with molecular phenotypes, validated by PET/MR-guided biopsies. Front. Oncol. 11, 743655. https://doi.org/10.3389/fonc.2021.743655 (2021).
Song, S. et al. Simultaneous FET-PET and contrast-enhanced MRI based on hybrid PET/MR improves delineation of tumor spatial biodistribution in gliomas: A biopsy validation study. Eur. J. Nucl. Med. Mol. Imaging 47, 1458–1467. https://doi.org/10.1007/s00259-019-04656-2 (2020).
Kang, S. Y., Moon, B. S., Yoo, M. Y., Yoon, H-J. & Kim, B. S. Clinical usefulness of 18 F-FET PET in a pediatric patient with suspected demyelinating disease. Clin. Nucl. Med. 47, e562–e564. https://doi.org/10.1097/RLU.0000000000004201 (2022).
Stegmayr, C., Willuweit, A., Lohmann, P. & Langen, K-J. O-(2-[18F]-fluoroethyl)-L-tyrosine (FET) in neurooncology: A review of experimental results. Curr. Radiopharm. 12, 201–210. https://doi.org/10.2174/1874471012666190111111046 (2019).
Langen, K-J. et al. Imaging of amino acid transport in brain tumours: Positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine (FET). Methods 130, 124–134. https://doi.org/10.1016/j.ymeth.2017.05.019 (2017).
Boxerman, J. L. et al. Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas. Neuro Oncol. 22, 1262–1275. https://doi.org/10.1093/neuonc/noaa141 (2020).
Albert, N. L. et al. PET-based response assessment criteria for diffuse gliomas (PET RANO 1.0): A report of the RANO group. Lancet Oncol. 25, e29–e41. https://doi.org/10.1016/S1470-2045(23)00525-9 (2024).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: P.A.-L., N.H. Data curation: K.K., N.N., F.M., N.H. Formal analysis: K.K., N.N., F.M., P.H., P.A.-L., M.H., M.W., Z.K., N.H. Resources: M.H., M.W., Z.K. Software: K.K., N.N., F.M., P.H., M.H., N.H. Validation: M.H., M.W., Z.K., N.H. Writing, original draft: K.K., N.H. Writing, review and editing: K.K., N.N., F.M., P.H., P.A.-L., M.H., M.W., Z.K., N.H. Approval of manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: MH is a recipient of grants by GE Healthcare, the Alfred and Annemarie von Sick legacy and the Clinical Research Priority Program (CRPP) Artificial Intelligence in Oncological Imaging Network (University of Zurich). None of the other authors has any conflict of interest to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kadali, K.R., Nierobisch, N., Maibach, F. et al. An effective MRI perfusion threshold based workflow to triage additional 18F-FET PET in posttreatment high grade glioma. Sci Rep 15, 7749 (2025). https://doi.org/10.1038/s41598-025-90472-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90472-8