Abstract
The purpose of this study is to evaluate left atrial function using cine-magnetic resonance imaging (cine-MRI) in patients with pulmonary vein stump thrombus (PVST) after left upper lobectomy (LUL). The study population comprised 91 patients (30 with PVST and 61 without PVST) who underwent LUL for pulmonary lesions and evaluation by cine-MRI. Left atrial functional parameters were evaluated and compared between patients with and without the development of PVST after LUL using the Mann–Whitney U test. The diagnostic capabilities of these parameters for predicting PVST development were assessed using receiver-operating characteristic (ROC) curve analysis. Clinical and left atrial functional parameters were analyzed by multivariate logistic regression models to determine predictors of PVST. Left atrial end-systolic volume (LAESV), left atrial end-diastolic volume (LAEDV), LAESV index (LAESVI), and LAEDV index (LAEDVI) were significantly greater in patients who developed PVST than in those without PVST (p = 0.009, < 0.001, 0.004, and < 0.001, respectively). Left atrial ejection fraction (LAEF) was significantly lower in patients who developed PVST than in those without PVST (p < 0.001). The area under the ROC curve for predicting PVST was 0.668, 0.769, 0.688, 0.792, and 0.803 for LAESV, LAEDV, LAESVI, LAEDVI, and LAEF, respectively. In the multivariate logistic regression analysis, only LAEF was identified as an independent predictor of PVST (OR 0.896; 95% CI 0.846–0.950). In conclusion, left atrial enlargement and left atrial dysfunction were associated with the development of PVST after LUL.
Similar content being viewed by others
Introduction
Pulmonary vein stump thrombus (PVST) has been increasingly recognized as a frequent complication following surgical treatment for lung cancer, particularly after left upper lobectomy (LUL)1–5. The occurrence rate of PVST following LUL surgery ranges from 13.5 to 34.0% 1,2,6. This complication can lead to embolism in various organs, including the brain4,7, spleen8, and kidney9. Therefore, it is vitally important to understand the mechanism that underlies PVST after LUL. Although the exact pathogenesis of PVST is unclear, several factors potentially contribute to its development. Some recent studies have suggested that blood stasis and turbulent blood flow near the pulmonary vein (PV) stump may contribute to the development of thrombus10,11,12. However, it is clinically challenging to assess the state of blood flow in the left atrium (LA) and the PV stump after LUL.
The LA appendage, the most common site of LA thrombus formation—particularly in patients with atrial fibrillation—shares structural similarities with the post-LUL PV stump, suggesting that its pathogenesis may similarly apply to PVST. LA enlargement has been linked to appendage dysfunction and thrombus formation13,14, thereby elevating the risk of stroke and systemic embolization15,16. Although LA size and function are routinely assessed by transthoracic echocardiography (TTE), cardiac MR offers more reliable evaluations17,18. We hypothesized that LA size and function would be associated with PVST after LUL. The purpose of this study was to clarify the association between LA function as evaluated by cine-magnetic resonance imaging (cine-MRI) and the development of PVST after LUL. The novelty of this study lies in using cine-MRI to comprehensively evaluate LA function and clarify its relationship with PVST formation after LUL. By leveraging cine MRI’s superior structural and functional assessment capabilities over conventional echocardiography, our approach offers unique insights into the hemodynamic mechanisms underlying PVST and may contribute to more effective risk stratification and patient management.
Results
Interobserver agreement was excellent for all quantitative measurements (ICC = 0.99 for LAESV, ICC = 0.96 for LAEDV, ICC = 0.99 for LAESVI, ICC = 0.96 for LAEDVI, ICC = 0.85 for LASV, ICC = 0.90 for LAEF, and ICC = 0.98 for length of PV stump). Measured values were significantly greater in patients who developed PVST than in those without PVST in terms of LAESV (74.2 ± 34.3 vs. 58.7 ± 18.6mL, p = 0.009), LAEDV (49.5 ± 30.0 vs. 33.1 ± 16.3 mL, p < 0.001), LAESVI (45.8 ± 19.8 vs. 36.5 ± 10.2 mL/m2, p = 0.004), and LAEDVI (30.5 ± 17.6 vs. 20.4 ± 9.6 mL/m2, p < 0.001) (Table 1). LAEF was significantly lower in patients with PVST (35.0 ± 8.5%) than in those without PVST (45.0 ± 10.1%, p < 0.001). There was no significant difference in LASV between patients with and without development of PVST (24.6 ± 8.2 vs. 25.7 ± 8.3 mL/m2, p = 0.627). PV stump length was significantly longer in patients with PVST than in those without PVST (22.9 ± 4.5 vs. 20.6 ± 5.0 mm, p = 0.033).
The AUC value for predicting the development of PVST was 0.668 for LAESV (95% confidence interval (CI), 0.562–0.764), 0.769 for LAEDV (95%CI, 0.669–0.851), 0.688 for LAESVI (95%CI, 0.582–0.781), 0.792 for LAEDVI (95%CI, 0.694–0.870), and 0.803 for LAEF (95%CI, 0.706–0.879) (Fig. 1). AUC values for LAEDV and LAEDVI, and LAEF were significantly greater than those for LAESV (p = 0.001, 0.002, and 0.016, respectively) and LAESVI (p = 0.016, 0.002, and 0.031, respectively). ROC analysis yielded a cut-off value of 66.4 mL for LAESV, 35.3 mL for LAEDV, 35.9 mL/m2 for LAESVI, 23.3 mL/m2 for LAEDVI, and 40.2% for LAEF.
In the univariate logistic regression analysis, PV stump length (OR: 1.103; 95%CI: 1.003–1.213), LAESV (OR: 1.028; 95%CI: 1.005–1.051), LAEDV (OR: 1.044; 95%CI: 1.013–1.075), LAESVI (OR: 1.054; 95%CI: 1.011–1.099), LAEDVI (OR: 1.082; 95%CI: 1.025–1.141), and LAEF (OR: 0.896; 95%CI: 0.846–0.950) were significantly associated with PVST (Table 2). In the multivariate analysis, variables such as LAESV, LAEDV, LAESVI, LAEDVI, and SV exhibited high VIF values (VIF = 1591.596, 1418.090, 342.474, 821.579, and 166.538, respectively) and were therefore excluded from the analysis. In the multivariate analysis with stepwise selection, only LAEF remained significantly associated with PVST (OR: 0.896; 95%CI: 0.846–0.950).
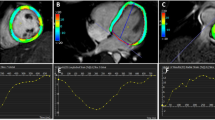
Representative cases are shown in Fig. 2.
A 76-year-old man with lung cancer who underwent left upper lobectomy. Postoperative cine-MRI (a) and contrast-enhanced cardiac CT (b) show thrombus in the left superior pulmonary vein stump (arrows). Volumetric analysis of the cine-MR images reveals a large left atrial end-systolic volume (102.7 mL) (LAESVI, 62.5 mL/m2), large left atrial end-diastolic volume (75.8 mL) (LAEDVI, 46.1 mL/m2), and low ejection fraction (26.2%). LAESVI = left atrial end-systolic volume index; LAEDVI = left atrial end-diastolic volume index.
Discussion
This is the first report to reveal an association between LA function and development of PVST after LUL. The major finding of the present study is that LA enlargement and dysfunction, as evaluated by cine-MRI, were significantly associated with the development of PVST after LUL. These results suggest that assessing LA function using cine-MRI may be a critical step in identifying patients at an increased risk for development of PVST. Understanding the relationship between LA function and PVST will potentially enable clinicians and surgeons to improve patient outcomes through early intervention and more targeted treatment approaches.
Virchow described three critically important factors in the development of venous thrombosis: blood stasis, activation of blood coagulation, and venous intimal damage19. It is important to note that activation of blood coagulation and venous intimal damage occur to some degree in all lung surgeries and are not exclusive to LUL. In other words, it is possible that blood stasis might play a significant role in PVST formation after LUL. Certain distinct regional blood flow patterns (blood stasis or turbulent blood flow) around the PV stump have been observed more frequently after LUL than after other lung lobectomies, which could be related to thrombus formation10,11,12. Recent studies using 4D flow MRI have suggested that LUL probably induces blood turbulence around the PV stump by complicated blood streams in the LA, and have also suggested that PVST is prone to develop under certain hemodynamic conditions11,12. Ohtaka et al. found that slow blood flow and the presence of spontaneous echo contrast (SEC) in the left superior PV stump on ultrasonography were associated with PVST10. A long PV stump after LUL has been proposed as a significant risk factor for PVST as it can create a procoagulant environment characterized by turbulent flow or blood stasis20. The left superior PV stump is reported to be significantly longer than other PV stumps, primarily because it has the longest intrapericardial segment1. In our results, PV stump length was significantly longer in patients with thrombus formation than in those without PVST formation, which is consistent with their findings. However, in the multivariate analysis conducted in this study, it did not remain as an independent predictor. As shown in several reports, attempts to prevent PVST after lobectomy by shortening the PV stump have not always been effective, and PVST has still occurred in patients who underwent proximal PV ligation after LUL21,22. Accordingly, factors other than long PV stump length might cause blood stasis near the PV stump and development of PVST.
LA blood stasis is associated with LA enlargement and dysfunction, which have been reported to be associated with an increased incidence of cardiovascular events such as LA appendage thrombus formation, atrial fibrillation, ischemic heart disease, heart failure, stroke, and cardiovascular death15,23,24. Low LAEF has been reported to have a significant association with SEC in LA and with LA appendage thrombus development14,25, in agreement with the present finding of an association between LAEF and PVST development. SEC in the LA has also been observed in patients with a large LA25,26. LAESVI and LAEDVI have been reported to be associated with LA appendage thrombus13. LAESVI, as an indicator of the largest LA volume, is a known predictor of cardiovascular outcomes and is the recommended measure of LA size27. LA enlargement can promote blood stasis, which facilitates thrombus formation in the LA appendage14. Similarly, PVST formation after LUL may be affected by blood stasis associated with LA dilation. A previous study has reported that LA volume as measured by preoperative non-ECG gated CT was significantly greater in patients who developed PVST than in those without thrombus6. In the present study, LA volume and function were evaluated using ECG-gated cine-MRI, which provides more accurate assessment than conventional non-ECG gated CT and does not require injection of contrast material. LAESVI > 32 mL/m2 and LA volume index (LAVI) ≥ 34 mL/m2 are widely recognized risk factors for cardiovascular events28,29. In the present study, LAESVI > 35.9 mL/m2 was significantly associated with PVST development, which is similar to those reported values. According to a recent report, LAEDVI is also a strong predictor of cardiovascular events30. LAEDVI has been reported to be significantly higher in patients with LA appendage thrombus than in those without thrombus13.
To address potential biases and confounding factors, we conducted a multivariate analysis that included age, gender, and underlying conditions such as hypertension, diabetes, atrial fibrillation, and medication of anticoagulant or antiplatelet drugs. These factors were selected based on their known influence on LA function and thrombus formation. The analysis demonstrated that LAEF remained the only independent predictor significantly associated with PVST. This finding suggests that the observed relationship between LAEF and PVST is robust and not confounded by these variables.
There are some limitations in the present study. First, there were fewer patients with PVST than those without. Second, the present evaluation focused solely on the presence of PVST development on the seventh postoperative day, and long-term assessment was not performed. Third, this study evaluated the LA function using only LAEF. However, this approach does not account for other important aspects of left atrial function, such as conduit function (left atrial passive ejection fraction, LAPEF) and pump function (left atrial active ejection fraction, LAAEF), nor does it include advanced techniques such as strain analysis. Future research should incorporate a more comprehensive assessment of left atrial function using these additional parameters and advanced imaging techniques to provide deeper insights into the relationship between left atrial function and PVST. Finally, we recognize that our study only evaluated the relationship between postoperative LA function and PVST development. From a clinical perspective, it would be highly beneficial to determine patients’ risk of thrombus formation preoperatively. Nevertheless, this is the first study to focus on the relationship between LA function and PVST after LUL, and we believe that our findings offer an important starting point and will encourage future large-scale multicenter, prospective studies that include preoperative LA function assessments, ultimately leading to improved risk stratification and patient management.
In conclusion, this study demonstrated a significant association of LA enlargement and LA dysfunction with PVST after LUL, suggesting that LA dysfunction may contribute to the development of PVST. Therefore, assessment of LA function by cine-MRI could be useful for predicting PVST development after LUL.
Materials and methods
This study was approved and the requirement for informed consent from the study subjects was waived by the institutional review board (Ethics Committee on Epidemiological and its related Studies, Sakuragaoka Campus, Kagoshima University; approval number, revised edition 1 of 220009) due to the retrospective study design. This study was conducted in accordance with the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan.
Patients
Our institutional ethics review board approved this retrospective study and the requirement for patients’ informed consent was waived. We reviewed the imaging database of our radiology department and patients’ electronic medical records to identify those who had undergone surgical treatment for lung neoplasm as well as a postoperative MR examination between January 2018 and April 2024. Among the 294 consecutive patients identified, 91 (36 women and 55 men; mean age, 69.3 ± 9.2 years; age range, 41 to 85 years) met the following inclusion criteria and were enrolled in the study: (1) treated with LUL, and (2) evaluated by cine-MRI for the presence or absence of PVST at 7 days after surgery. One case was excluded due to poor image quality. PVST was defined as a structure situated in the PV stump, possessing boundaries that are discernible from the PV wall and can be distinguished from artifacts. For all cases of PVST suspected on cine-MRI, contrast-enhanced ECG-gated CT was performed to confirm the existence of PVST. PVST was confirmed in 30 of the 91 patients. Table 3 summarizes the patients’ clinical characteristics according to the presence or absence of PVST after LUL.
Cine-MRI protocol and evaluation of left atrial function
MRI examinations were performed on the seventh postoperative day, using a 3T system (Prisma, Siemens Medical Systems, Erlangen, Germany) with a 30-channel body array coil. At our institute, cardiac cine-MRI is included in the routine clinical protocol for assessing potential PVST formation following LUL because it involves no radiation exposure or contrast material injection, and has been recognized as a reliable diagnostic tool for assessing thrombi in the LA or LA appendage31–33. Cine-MR images were obtained in the coronal plane using a balanced steady-state free precession (bSSFP) sequence with short periods of breath-holding. The imaging parameters were as follows: field-of-view = 360 × 360 mm, in-plane spatial resolution = 1.9 × 1.9 mm, slice thickness = 5 mm, number of slices = 27, repetition time = 40–80 ms, echo time = 1.1 ms, flip angle = 48°, number of cardiac phases = 10–15, and number of signals averaged = 1. All MR images were transferred and analyzed using a 3D image-analysis system (SYNAPSE VINCENT; Fujifilm Medical Co., Tokyo, Japan). Two radiologists with 21 and 2 years of chest radiology experience performed manual LA segmentation. They were blinded to the final results concerning the presence or absence of PVST. LA functional parameters (LA end-systolic volume [LAESV], LA end-diastolic volume [LAEDV], LAESV index [LAESVI], LAEDV index [LAEDVI], LA stroke volume [LASV], and LA ejection fraction [LAEF]) were calculated. LAEF was calculated using the formula: (the maximum LA volume – the minimum LA volume) / the maximum LA volume×100. LAESVI and LAEDVI were calculated according to the body surface area (BSA) of each patient, as LAESV/BSA and LAEDV/BSA, respectively. The radiologists also measured the length of the left superior PV stump.
Statistical analysis
Intraclass correlation coefficient (ICC) was calculated to assess inter-observer agreement of all LA functional parameters (κ = 0.00–0.20, poor correlation; κ = 0.21–0.40, fair correlation; κ = 0.41–0.60, moderate correlation; κ = 0.61–0.80, good correlation; κ = 0.81–1.00, excellent correlation). Comparisons of all LA functional parameters and PV stump length between patients with and without development of PVST were conducted using the Mann–Whitney U test. Receiver-operating characteristic (ROC) curve analysis was performed to evaluate the predictive accuracy of LA functional parameters for PVST. Optimal cutoff values were selected based on the maximum Youden index for predicting PVST. Areas under the ROC curve (AUC) were compared using DeLong’s test. Clinical and LA functional parameters were analyzed by univariate and multivariate logistic regression models to determine predictors of PVST. For the multivariate analysis, the variance inflation factor (VIF) was calculated to assess multicollinearity among the predictor variables. Variables with VIF values exceeding 10 were excluded. Subsequently, a stepwise regression analysis was performed to identify the best predictor variables for PVST. Continuous variables are displayed as the mean ± standard deviation (SD). A P-value < 0.05 was considered to indicate statistical significance in all analyses. Statistical analyses were performed using MedCalc version 20.211 (MedCalc Software, Mariakerke, Belgium) and SPSS version 28.0 (SPSS, Chicago, IL).
Data availability
The datasets generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- PVST:
-
Pulmonary vein stump thrombus
- LUL:
-
Left upper lobectomy
- PV:
-
Pulmonary vein
- LA:
-
Left atrium
- TTE:
-
Transthoracic echocardiography
- cine-MRI:
-
cine-magnetic resonance imaging
- LAESV:
-
Left atrial end-systolic volume
- LAEDV:
-
Left atrial end-diastolic volume
- LAESVI:
-
Left atrial end-systolic volume index
- LAEDVI:
-
Left atrial end-diastolic index
- LAEF:
-
Left atrial ejection fraction
- BSA:
-
Body surface area
- ICC:
-
Intraclass correlation coefficient
- ROC:
-
Receiver-operating characteristic
- AUC:
-
Area under the ROC curve
- SD:
-
Standard deviation
- SEC:
-
Spontaneous echo contrast
References
Ohtaka, K. et al. Left upper lobectomy can be a risk factor for thrombosis in the pulmonary vein stump. J. Cardiothorac. Surg. 9 https://doi.org/10.1186/1749-8090-9-5 (2014).
Ohtaka, K. et al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann. Thorac. Surg. 95, 1924–1928. https://doi.org/10.1016/j.athoracsur.2013.03.005 (2013).
Ichimura, H., Ozawa, Y., Nishina, H. & Shiotani, S. Thrombus formation in the pulmonary vein stump after left upper lobectomy: a report of four cases. Ann. Thorac. Cardiovasc. Surg. 20 Suppl, 613–616. https://doi.org/10.5761/atcs.cr.13-00079 (2014).
Hattori, A. et al. Risk factor analysis of cerebral infarction and clinicopathological characteristics of left upper pulmonary vein stump thrombus after lobectomy. Gen. Thorac. Cardiovasc. Surg. 67, 247–253 (2019).
Ohtaka, K. et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J. Thorac. Cardiovasc. Surg. 143, e3–5. https://doi.org/10.1016/j.jtcvs.2011.09.025 (2012).
Takumi, K. et al. Left atrial CT volume and CHA(2)DS(2)-VASc score predict early pulmonary vein stump thrombus after left upper lobectomy. Sci. Rep. 13, 4965. https://doi.org/10.1038/s41598-023-32240-0 (2023).
Yamamoto, T. et al. Is left upper lobectomy for lung cancer a risk factor for cerebral infarction? Surg. Today. 46, 780–784 (2016).
Harada, R. et al. Left superior pulmonary venous thrombosis complicated with Splenic infarction after video-assisted thoracoscopic left upper lobectomy. J. Cardiol. Cases. 16, 1–4. https://doi.org/10.1016/j.jccase.2017.03.003 (2017).
Song, C. Y. et al. Left superior pulmonary vein stump thrombosis and right renal infarction after left upper lobectomy: case report and literature review. Gen. Thorac. Cardiovasc. Surg. 68, 1047–1050. https://doi.org/10.1007/s11748-019-01200-9 (2020).
Ohtaka, K. et al. Blood stasis May cause thrombosis in the left superior pulmonary vein stump after left upper lobectomy. J. Cardiothorac. Surg. 9 https://doi.org/10.1186/s13019-014-0159-8 (2014).
Umehara, T. et al. Four-dimensional flow magnetic resonance imaging study to explain high prevalence of pulmonary vein stump thrombus after left upper lobectomy. J. Thorac. Disease. 12, 5542–5551. https://doi.org/10.21037/jtd-20-1606 (2020).
Umehara, T. et al. Hemodynamic features underlying pulmonary vein stump thrombus formation after left upper lobectomy: Four-dimensional flow magnetic resonance imaging study. Quant. Imaging Med. Surg. 12, 992–1003. https://doi.org/10.21037/qims-21-472 (2022).
Osawa, K. et al. Predicting left atrial appendage Thrombus from left atrial volume and confirmation by computed tomography with delayed enhancement. Tex. Heart Inst. J. 47, 78–85. https://doi.org/10.14503/thij-17-6290 (2020).
Hautmann, M. et al. Left atrial appendage thrombus formation, potential of resolution and association with prognosis in a large real-world cohort. Sci. Rep. 13, 889. https://doi.org/10.1038/s41598-023-27622-3 (2023).
Xu, Y. et al. Left atrial enlargement and the risk of stroke: A meta-analysis of prospective cohort studies. Front. Neurol. 11, 26. https://doi.org/10.3389/fneur.2020.00026 (2020).
Hamatani, Y. et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci. Rep. 6, 31042. https://doi.org/10.1038/srep31042 (2016).
Agner, B. F. et al. Assessment of left atrial volume and function in patients with permanent atrial fibrillation: Comparison of cardiac magnetic resonance imaging, 320-slice multi-detector computed tomography, and transthoracic echocardiography. Eur. Heart. J. Cardiovasc. Imaging. 15, 532–540. https://doi.org/10.1093/ehjci/jet239 (2014).
Peters, D. C., Lamy, J., Sinusas, A. J. & Baldassarre, L. A. Left atrial evaluation by cardiovascular magnetic resonance: Sensitive and unique biomarkers. Eur. Heart J. Cardiovasc. Imaging. 23, 14–30. https://doi.org/10.1093/ehjci/jeab221 (2021).
Brotman, D. J., Deitcher, S. R., Lip, G. Y. & Matzdorff, A. C. Virchow’s triad revisited. South. Med. J. 97, 213–214. https://doi.org/10.1097/01.Smj.0000105663.01648.25 (2004).
Chaaya, G. & Vishnubhotla, P. Pulmonary vein thrombosis: A recent systematic review. Cureus 9, e993, (2017). https://doi.org/10.7759/cureus.993 (2017).
Nakano, T., Kaneda, H., Kawaura, T., Kitawaki, T. & Murakawa, T. Ligating the pulmonary vein at the pericardial reflection is useful for preventing thrombus formation in the pulmonary vein stump after left upper lobectomy. Gen. Thorac. Cardiovasc. Surg. 67, 450–456. https://doi.org/10.1007/s11748-018-1032-9 (2019).
Miyoshi, R. et al. Pulmonary vein thrombosis after lobectomy with vein stump closure by ligation. Asian Cardiovasc. Thorac. Ann. 26, 546–551. https://doi.org/10.1177/0218492318802141 (2018).
Faustino, A. et al. Which method of left atrium size quantification is the most accurate to recognize thromboembolic risk in patients with non-valvular atrial fibrillation? Cardiovasc. Ultrasound. 12, 28. https://doi.org/10.1186/1476-7120-12-28 (2014).
Ayirala, S., Kumar, S., O’Sullivan, D. M. & Silverman, D. I. Echocardiographic predictors of left atrial appendage thrombus formation. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 24, 499–505. https://doi.org/10.1016/j.echo.2011.02.010 (2011).
Kim, H. D. et al. Left atrial dysfunction, fibrosis and the risk of thromboembolism in patients with paroxysmal and persistent atrial fibrillation. Int. J. Heart Fail. 4, 42–53. https://doi.org/10.36628/ijhf.2021.0043 (2022).
Kim, M. N. et al. Improvement of predictive value for thromboembolic risk by incorporating left atrial functional parameters in the CHADS2 and CHA2DS2-VASc scores. Int. Heart J. 56, 286–292. https://doi.org/10.1536/ihj.14-380 (2015).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 28, 1–39e14. https://doi.org/10.1016/j.echo.2014.10.003 (2015).
Osranek, M. et al. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: Three-decade follow-up. Eur. Heart J. 26, 2556–2561. https://doi.org/10.1093/eurheartj/ehi483 (2005).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 29, 277–314. https://doi.org/10.1016/j.echo.2016.01.011 (2016).
Thadani, S. R., Shaw, R. E., Fang, Q., Whooley, M. A. & Schiller, N. B. Left atrial End-Diastolic volume index as a predictor of cardiovascular outcomes: The heart and soul study. Circ. Cardiovasc. Imaging. 13, e009746. https://doi.org/10.1161/circimaging.119.009746 (2020).
Vira, T., Pechlivanoglou, P., Connelly, K., Wijeysundera, H. C. & Roifman, I. Cardiac computed tomography and magnetic resonance imaging vs. transoesophageal echocardiography for diagnosing left atrial appendage thrombi. Europace 21, e1–e10. https://doi.org/10.1093/europace/euy142 (2019).
Chen, J. et al. Cardiac MRI for detecting left atrial/left atrial appendage thrombus in patients with atrial fibrillation: Meta-analysis and systematic review. Herz 44, 390–397. https://doi.org/10.1007/s00059-017-4676-9 (2019).
Rathi, V. K. et al. Contrast-enhanced CMR is equally effective as TEE in the evaluation of left atrial appendage thrombus in patients with atrial fibrillation undergoing pulmonary vein isolation procedure. Heart Rhythm. 10, 1021–1027. https://doi.org/10.1016/j.hrthm.2013.02.029 (2013).
Acknowledgements
This work was supported by JSPS KAKENHI (Grant number JP21K15811).
Author information
Authors and Affiliations
Contributions
K. T.: Conceptualization, Methodology, Writing - Original Draft; H. N.: Investigation, Data Curation; Y. K.: Data Curation, Investigation; K. U.: Investigation; T. U.: Resources; G. K.: Resources; R. N.: Investigation; M. N.: Formal analysis; K. K.: Data Curation; F. K.: Resources; T. Y.: Writing- Reviewing and Editing, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Takumi, K., Nagano, H., Kamimura, Y. et al. Association between left atrial function and pulmonary vein stump thrombus after left upper lobectomy: insights from cine-MRI. Sci Rep 15, 6629 (2025). https://doi.org/10.1038/s41598-025-91030-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91030-y