Abstract
Sarcopenia, characterized by loss of muscle mass and strength, particularly affects older adults and is linked to increased morbidity and mortality. The study aimed to investigate the relationship between biomarkers, including hemoglobin (Hb), lactate dehydrogenase (LDH), and Systemic Immune-Inflammation Index (SII), and sarcopenia in the US population. Utilizing NHANES data from 2003 to 2018, the study analyzed 5,615 participants, categorizing them based on quartiles of Hb, SII, and LDH levels. It employed logistic regression models to assess the relationship between these biomarkers and sarcopenia risk, adjusting for various confounders. High levels of LDH, Hb and SII were significantly associated with sarcopenia, with higher risk in the highest quartile. The AUC for all indicators in predicting sarcopenia was 0.925 (sensitivity 0.925; specificity 0.743). The study concludes that elevated Hb, LDH, and SII levels are significant biomarkers associated with sarcopenia, emphasizing the role of inflammation in its development and the potential for these markers in early detection and intervention.
Similar content being viewed by others
Introduction
Sarcopenia is a progressive, generalized disorder of skeletal muscle, characterized by the loss of muscle mass, strength, and function, primarily affecting older adults. This condition poses a significant health risk, as it is associated with adverse outcomes, including physical disability, diminished quality of life, and increased mortality rates1,2. Risk factors associated with telework, such as prolonged sitting in a non-neutral position, are one of the causes of the occurrence and aggravation of musculoskeletal disorders in teleworkers3. Recent longitudinal studies and meta-analyses integrating multi-omics analyses have provided valuable insights into the complex interplay of genetic, epigenetic, proteomic, and metabolomic factors in the development of sarcopenia. For instance, a comprehensive study has highlighted the role of multi-omics data in identifying novel biomarkers and understanding the molecular mechanisms underlying sarcopenia. Additionally, the integration of multi-omics approaches in research has been instrumental in uncovering the intricate relationships between muscle wasting, inflammation, and other physiological processes4,5. The underlying pathophysiology of sarcopenia is complex and multifactorial, encompassing age-related muscular changes, hormonal imbalances, chronic inflammation, oxidative stress, and reduced physical activity6,7,8. Early detection of at-risk individuals is critical for the initiation of preventive and therapeutic strategies. Several predictive markers have been identified to evaluate the presence and severity of sarcopenia, with muscle mass, strength, and physical performance serving as central criteria in clinical assessments. Dual-energy X-ray absorptiometry (DEXA) and bioelectrical impedance analysis (BIA) are widely used to estimate muscle mass, while handgrip strength and gait speed are frequently employed to assess muscle strength and physical performance9.
In addition to direct measures of muscle health, several biomarkers have been explored for their potential role in predicting or diagnosing sarcopenia. One area of interest is the link between sarcopenia and systemic inflammation markers. Chronic low-grade inflammation, or “inflammaging,” has been implicated in sarcopenia development10,11. The Systemic Immune-Inflammation Index (SII), which integrates platelet, neutrophil, and lymphocyte counts, provides a comprehensive measure of systemic inflammation and immune status, and is strongly associated with chronic inflammation. Lactate dehydrogenase (LDH), an enzyme involved in glycolysis, serves as a marker of tissue damage and cell stress, with elevated levels reflecting cellular injury, particularly in metabolically active tissues like skeletal muscle, liver, and heart. Previous analyses of population-based data from China have shown that hemoglobin levels are associated with sarcopenia, muscle mass, and physical function in Chinese people aged 60 years and older12.
The National Health and Nutrition Examination Survey (NHANES), conducted by the Centers for Disease Control and Prevention (CDC), is an ongoing program designed to evaluate the health and nutritional status of the U.S. population. In this study, we utilized NHANES data to investigate the relationship between specific biomarkers and sarcopenia among American adults.
Materials and methods
Data source
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative, cross-sectional survey conducted by the National Center for Health Statistics (NCHS) since 1999. It uses multistage probability sampling to assess the health and nutrition status of the U.S. civilian, non-institutionalized population, in two-year cycles. Data collection includes health-related questionnaires, physical exams, and laboratory tests, and all information is publicly accessible through the NCHS website (https://www.cdc.gov/nchs/nhanes/index.htm). Written informed consent was obtained from all participants, and study protocols were approved by the NCHS Institutional Review Board. Clinical trial number: not applicable.
Study population
The National Health and Nutrition Examination Survey (NHANES) is a population-based, cross-sectional study led by the Centers for Disease Control and Prevention (CDC) to evaluate the health and nutritional status of adults and children in the U.S. NHANES includes both interview and examination components, though it relies on self-reported data for certain conditions. We used dual-energy X-ray absorptiometry (DEXA) and bioelectrical impedance analysis (BIA) to identify participants with sarcopenia, based on relevant body composition measurements13. In our analysis of the NHANES data, the common challenge associated with public health databases is incomplete data. Sarcopenia, unlike conditions like hypertension or diabetes that have more readily available diagnostic information, requires specific body composition measurements and diagnostic details that were not fully reported for all participants. To ensure the accuracy and stability of our models, we applied strict inclusion criteria, which led to the exclusion of a significant portion of the original cohort. This rigorous approach was essential for the propensity score matching (PSM) analysis, as it allowed us to create well-matched groups and control for potential confounders, thereby enhancing the internal validity of our study. Propensity scores were calculated using logistic regression, incorporating predictors linked to sarcopenia. Each case was matched to two controls using the nearest neighbor algorithm, ensuring similarity in observed covariates and minimizing selection bias. Balance was assessed by calculating SMDs for all covariates; SMDs below 10% indicate good balance, which was achieved for the majority of covariates post-matching.
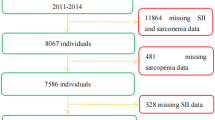
This population-based cohort study included participants aged 20 to 60 years from the 2003–2018 NHANES cycles. We analyzed data from 82,914 participants across five consecutive two-year cycles, incorporating sociodemographic data, personal history, clinical examination results, and laboratory findings. The exclusion criteria were: (1) age under 20 years, (2) missing data on complications, (3) missing data on sarcopenia, and (4) incomplete laboratory results. After applying these criteria, 5,615 participants, representing 33,172,588 U.S. individuals, were included in the analysis, of whom 592 had sarcopenia (Fig. 1). This study was conducted in accordance with the Declaration of Helsinki.
Outcome definitions and exposure definitions
We utilized NHANES 2003–2018 data, which included sarcopenia-related information, and categorized participants based on quartiles of hemoglobin (Hb), Systemic Immune-Inflammation Index (SII), and lactate dehydrogenase (LDH) levels. The Hb quartiles were: Q1 (n = 456, 8.4–13.7 g/L), Q2 (n = 440, 13.7–14.6 g/L), Q3 (n = 448, 14.6–15.5 g/L), and Q4 (n = 424, 15.5–19.5 g/L). SII quartiles were: Q1 (n = 448, 64.47–357.25 /L), Q2 (n = 440, 357.25–498.82 /L), Q3 (n = 440, 498.82–705.50 /L), and Q4 (n = 448, 705.50–2567.14 /L). LDH quartiles were: Q1 (n = 456, 63.0–111.0 U/L), Q2 (n = 448, 111.0–124.0 U/L), Q3 (n = 440, 124.0–143.0 U/L), and Q4 (n = 432, 143.0–292.0 U/L). Quartile grouping was used to examine potential nonlinear associations.
Due to the lack of established reference ranges for Hb, SII, and LDH levels, we categorized participants into quartiles. This approach allowed us to explore potential nonlinear associations between these biomarkers and sarcopenia without adding complexity to the presentation of the results.
Statistical analysis
Variables for this study were derived from four sections of the NHANES dataset: demographic, examination, laboratory, and questionnaire data. Demographic variables included sex, age, race, education level, marital status, and income-poverty ratio (PIR). Examination and laboratory data included body mass index (BMI), hemoglobin (Hb), platelet count, neutrophil (Neu) count, lymphocyte (Lym) count, and urine creatinine levels, all measured according to NHANES Laboratory/Medical Technologist Manual of Procedures. BMI was calculated as weight in kilograms divided by height in meters squared. Smoking status was categorized as never, former, or current smokers. Alcohol consumption was evaluated based on never versus ever having consumed alcohol. Hypertension was identified through self-reported history.
All patients who met the inclusion criteria were eventually subjected to propensity matching analysis in a 1:2 ratio for further statistics. After applying propensity score matching (PSM), we conducted balance diagnostics to assess the comparability of the study groups. Standardized mean differences (SMDs) were calculated for all covariates to evaluate the balance between the matched groups. An SMD of less than 10% is generally considered to indicate good balance. We assessed demographic differences across urinary creatinine quartiles using weighted Student’s t-tests and chi-square tests. In our analysis of the relationship between inflammatory markers and sarcopenia, we employed a stepwise approach in the logistic regression models to account for potential confounders systematically. This methodological strategy allows to assess the incremental effect of adding covariates on the model’s estimates. The process is detailed as follows: Model 1 (Unadjusted): This initial model does not include any covariates, providing an unadjusted estimate of the association between the inflammatory markers and sarcopenia risk. Model 2 (Adjusted for Age): Recognizing age as a fundamental demographic factor that could influence both sarcopenia and inflammatory marker levels, we adjusted for age in this model to account for age-related variations. Model 3 (Adjusted for Age and BMI): Building upon Model 2, we further adjusted for body mass index (BMI), as BMI is a key indicator of nutritional status and body composition, which are relevant to sarcopenia. Model 4 (Fully Adjusted): In this final model, we included additional adjustments for hypertension, diabetes mellitus, chronic kidney disease, cardiovascular disease, and chronic obstructive pulmonary disease. These conditions are known to be associated with sarcopenia and can confound the relationship between inflammatory markers and sarcopenia risk. A smoothed curve-fitting model was employed to explore the nonlinear associations between inflammatory markers and sarcopenia. To determine the knots for our restricted cubic splines (RCS) analysis, we utilized the ‘optimal_nKnots (fit)’ function in R. This function evaluates the statistical significance of various knot positions within the range of the predictor variable. By selecting knots based on their statistical significance, we ensure that our model captures the most relevant nonlinear patterns without overfitting. After selecting the knots, we further optimized our RCS model by assessing the model fit and the statistical significance of each knot. This iterative process allowed us to adjust the knots and achieve a model that best represents the relationship between the biomarkers and sarcopenia risk. The final selection of knots was based on both statistical criteria and the clinical interpretability of the resulting spline functions. A two-sided p-value of < 0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the NHANES sample collected from 2003 to 2018. Median values (IQRs) and percentages for age, sex, BMI, race, education, smoking status, and demographic factors such as prevalence of hypertension, chronic kidney disease, heart disease, stroke, diabetes, and COPD are presented in the table.
A total of 82,914 individuals were included in the study, weighted to represent an estimated 33,172,588 Americans. A total of 44,920 individuals were able to be followed up, excluding a total of 30,436 individuals for baseline conditions (poverty level, education level, ethnicity, and comorbidities for missing information populations). Further exclusion of those with incomplete laboratory tests resulted in 16,239 individuals being included, and combined with the need for patients to have diagnostic information on sarcopenia, a total of 5,615 individuals were included in the study. Of these, 592 (10.5%) were diagnosed with sarcopenia. A propensity matching analysis was utilized to match patients’ age, gender, race, education, household income level, alcohol consumption, smoking, and underlying comorbidities resulted in a 1:2 matched study population (Figs. 1 and 2).
The mean age of the study population was 45.30 years, while the mean age of patients with sarcopenia was higher at 46.11 years (P < 0.001). There were no significant differences in the prevalence of sarcopenia by gender or marital status and household income level. However, the diagnosis of sarcopenia varied by race, with blacks and Mexicans exhibiting lower relative rates. Individuals diagnosed with sarcopenia had a significantly higher BMI compared to the undiagnosed group (43.97 ± 9.06 kg/m² in the sarcopenia group versus 30.74 ± 6.28 kg/m² in the non-sarcopenia group, P < 0.001). Furthermore, individuals with chronic obstructive pulmonary disease and those who smoked had a significantly higher morbidity rate.
The mean leukocyte count of the study population was 8.13 (± 2.65) x 1012/L, mean lymphocyte count was 2.28 (± 0.81) x 1012/L and mean platelet count was 245.59 (± 67.50) x 109/L. The mean percentage of neutrophils was 60.35 (± 9.43) %. There were statistically significant differences between the diseased and non-diseased populations in terms of leukocyte count, percentage of neutrophils, lymphocyte count, and the combined statistical SII index. In addition, the mean LDH level was significantly higher in the diseased population, with a mean of 141.30 (± 35.31) U/L, whereas the mean LDH level in the non-diseased population was 124.73 (± 23.66) U/L (P < 0.001). Hemoglobin contained slightly lower levels in the sarcopenic population than in the non-sarcopenic population (Table 1).
Results of multifactors and stratified analyses specific relationships between Hb/LDH/SII and sarcopenia prevalence
Combined with the results of Table 1, further multifactorial Logistics regression was performed to analyze the relationship between the participants’ age, BMI index, household income, and blood test. Age, family income level and lymphocyte count and LDH were statistically different (P < 0.05, see Table 2). Further forest plots showed the effect of different factors on OR (Fig. 3).
We developed logistic regression models to assess the relationship between Hb, LDH, and SII index levels and the risk of sarcopenia. The unadjusted models did not account for any factors. Model 1 was adjusted for age, model 2 corrected for age and sex, and model 3 added adjustment for hypertension, diabetes mellitus, chronic kidney disease, cardiovascular disease, and chronic obstructive pulmonary disease to model 2. All three metrics Pfor trend in all three models showed trend differences at different levels, but only high levels of LDH were statistically different between groups. Participants with high levels of LDH had a higher risk of sarcopenia (Q4 OR: Crud Model 4.24, 95%CI 1.21–14.79 P = 0.02; Model 1 4.28, 95%CI 1.22-15.00 P = 0.02; Model 2 4.42, 95%CI 1.20-16.33 P = 0.02; Model 3 4.78, 95%CI 1.28–17.88 P = 0.01) (Table 3).
The application of restricted cubic spline analysis (RCS), a robust nonlinear statistical method, has elucidated the relationship dynamics between LDH, SII, and the risk of sarcopenia. Our analysis, as depicted in Fig. 4A and B, disclosed that the risk of sarcopenia escalates with increasing LDH and SII values, underscoring a monotonic increase without a distinct inflection point, indicative of a consistent trend rather than a complex, multi-phasic response. Notably, the RCS-estimated slopes for the SII index became more pronounced at values exceeding 1000, and for LDH, the risk of sarcopenia significantly escalated beyond 100 U/L, highlighting a critical threshold where the risk accelerates (Fig. 4A and B). Conversely, the relationship between Hb levels and the risk of sarcopenia exhibited a downward trajectory, with a pronounced slope, suggesting a protective role of higher Hb levels within the specified range of 13–15.5 g/L, despite minor fluctuations observed within this interval (Fig. 4C). The RCS analysis allows us to capture these nuanced nonlinear trends, providing a more accurate depiction of how these biomarkers modulate the risk of sarcopenia.
Predictive performance of different metrics for sarcopenia
After weighting the data, the predictive performance of the constructed models was assessed. The area under the curve (AUC) for the prediction of sarcopenia using only the SII metric was 0.56. The AUC for Hb was lower at 0.539, while the AUC for lactate dehydrogenase (LDH) was 0.648. After integrating these indicators and adjusting for age, race, and household income, the predictive performance of the model showed a substantial improvement, with an AUC of 0.925, a sensitivity of 0.926, and a specificity of 0.743 (Fig. 5). Additionally, bootstrapping yielded a 95% confidence interval of (-13.41, -9.10) for a key parameter, indicating its significant impact on sarcopenia. Cross-validation demonstrated an accuracy of 84.40% and a Kappa coefficient of 0.63, showing the model’s strong predictive performance and consistency across different data subsets.
Discussion
The analysis of NHANES data from 2003 to 2018 reveals significant associations between elevated LDH, Hb, and SII with sarcopenia in the U.S. adult population. This relationship underscores the potential role of systemic inflammation in the development of sarcopenia. Yan’s team analyzed the cell subtype distribution and function of the human myotendinous junction (MTJ) at the single-cell level and discovered clusters expressing muscle and tendon markers, MTP. Basic experiments were used to verify that the mTOR signaling pathway may be involved in the phenotypic maintenance of MTP14. Chronic low-grade inflammation, often termed ‘inflammaging,’ has been implicated in muscle degradation, especially in older adults15. Higher Hb and LDH levels, a readily available marker of systemic inflammation, align with this theory, suggesting that inflammation contributes to muscle wasting and functional decline16.
The demographic characteristics outlined in Table 1 reveal notable differences between the sarcopenic and non-sarcopenic groups, influencing our understanding of sarcopenia’s prevalence and risk factors. Age, a pivotal factor in sarcopenia development, was slightly higher in the sarcopenic group, aligning with the recognized age-related muscle loss. The similarity in gender distribution suggests equal susceptibility across men and women in our cohort. Racial disparities, with lower sarcopenia rates among blacks and Mexicans, may indicate variations in sarcopenia awareness and diagnostic practices. Socioeconomic status, reflected by the poverty index ratio (PIR), showed minor differences, potentially impacting access to healthcare and nutritional resources. The higher prevalence of hypertension, stroke, CKD, COPD, and DM in the sarcopenic group underscores the intricate relationship between chronic conditions and muscle health. Smoking habits, a modifiable risk factor, differed significantly between groups, suggesting a role in sarcopenia progression through inflammation and oxidative stress. The significantly higher BMI in the sarcopenic group corresponds with the sarcopenic obesity phenomenon, highlighting the dual challenge of muscle atrophy and excess adiposity. Hematological and biochemical markers, including elevated SII, point to systemic inflammation’s contribution to muscle degeneration. The higher mortality rate in the sarcopenic group underscores the urgency of early sarcopenia identification and intervention.
As WBC count is a routine and easily accessible measure in clinical practice, it holds potential as a cost-effective and non-invasive tool for identifying individuals at higher risk for sarcopenia17. Our findings confirm previous studies indicating that higher WBC counts are associated with reduced muscle mass and strength in older adults. This association highlights the value of WBC count as a potential biomarker for sarcopenia screening, given its accessibility in clinical practice. Logistic regression models in this study further support WBC count associated SII as a robust predictor of sarcopenia, even after adjusting for factors such as age, sex, and comorbidities18. These results suggest that WBC count could be integrated into screening protocols as a cost-effective and non-invasive tool to identify individuals at higher risk for sarcopenia19.
The mechanistic relationship between lactate dehydrogenase (LDH) and systemic inflammation, particularly in the context of ‘inflammaging,’ is an area of growing interest in sarcopenia research. The observed relationship between lactate dehydrogenase (LDH) and sarcopenia adds another layer of complexity20. Elevated LDH levels, which may indicate muscle damage or metabolic stress, suggest that LDH could reflect muscle injury and impaired energy metabolism associated with sarcopenia. Inflammaging refers to the chronic, low-grade inflammatory state that accompanies aging, characterized by elevated pro-inflammatory markers21. This condition is thought to contribute to sarcopenia through multiple pathways, including the promotion of muscle protein breakdown and the inhibition of muscle protein synthesis22,23. Some studies have suggested the association between inflammatory or common indicators such as cfDNA, CRP, IL-1β and bilirubin and sarcopenia. Studies have also shown that cfDNA and bilirubin have high clinical practicality in predicting sarcopenia24. LDH, an enzyme critical to glycolysis, is released by damaged cells and is often elevated in conditions involving tissue injury and inflammation25. In sarcopenia, elevated LDH levels may indicate increased muscle cell damage and disrupted energy metabolism, both of which are key features of muscle degradation26. Moreover, elevated LDH in sarcopenic individuals could serve as a biomarker of muscle damage and reflect impaired repair mechanisms, which are exacerbated by chronic inflammation27. Moreover, elevated LDH in sarcopenic individuals could serve as a biomarker of muscle damage and reflect impaired repair mechanisms, which are exacerbated by chronic inflammation28.
While LDH is traditionally used as an indicator of cellular damage, its significant association with sarcopenia in our study points to its potential role as a biomarker for muscle health and sarcopenia progression, particularly when paired with WBC levels29. The predictive models developed here demonstrate that combining SII which is associated with WBC and LDH significantly enhances the ability to identify individuals at risk for sarcopenia. In a study by Balcer et al., the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were used as proxies for the inflammatory response. Their findings indicated that, particularly in patients with systemic inflammation, muscle-reducing obesity was associated with a poor prognosis following pancreaticoduodenectomy (PD) for pancreatic adenocarcinoma (PDAC)17. Similarly, previous research has demonstrated a correlation between biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine (Cr), and cystatin C (CysC), and the risk of out-of-hospital death (OFH) in elderly populations (≥ 80 years) with reduced muscle mass30. The association between lactate dehydrogenase (LDH), an enzyme involved in cellular energy production, and sarcopenia further deepens our understanding of muscle health. Elevated LDH levels may reflect muscle damage or metabolic stress, suggesting a role for LDH in impaired muscle repair and energy metabolism in sarcopenia. The significant associations observed between both white blood cell (WBC) count and LDH with sarcopenia point to a complex interplay between inflammation, muscle metabolism, and muscle mass loss.
The predictive value of Hb and LDH was further illustrated by the area under the curve (AUC) values31. While Hb alone showed a moderate AUC of 0.539, LDH 0.648 and SII 0.560, combining these markers increased predictive accuracy (AUC = 0.925). This suggests that multi-marker approaches incorporating both inflammatory and metabolic markers could improve sarcopenia risk prediction32. However, while the predictive performance of these markers is promising, they may not be sufficient as standalone indicators and should be integrated with clinical assessments and other biomarkers for a comprehensive evaluation. The restricted cubic spline analysis revealed a nonlinear relationship between LDH, SII, and sarcopenia, showing that higher levels of these inflammatory markers are associated with an increased risk of sarcopenia. This monotonic increase without an inflection point suggests that any elevation in these biomarkers could signal heightened risk, which may assist in risk stratification and early intervention efforts.
Our predictive models highlight the value of SII index and LDH in identifying individuals at risk for sarcopenia, even after adjusting for potential confounders. The area under the curve (AUC) values for these markers underscore their clinical utility in screening, particularly when combined with other biomarkers or clinical assessments. The relationship between LDH and sarcopenia can be understood as a reflection of muscle damage, chronic inflammation, and metabolic dysfunction. Elevated LDH levels may indicate underlying muscle cell damage and metabolic disturbances, especially in patients with chronic inflammation or comorbid conditions33,34. Thus, LDH has the potential to serve as a biomarker for assessing the severity and prognosis of sarcopenia. However, given the nonspecific nature of LDH, it should be evaluated in conjunction with other clinical indicators.
While this study offers valuable insights, several limitations should be noted. First, this study concludes that elevated Hb, LDH, and SII are strongly associated with sarcopenia in the U.S. adult population. These findings underscore the importance of inflammation in the pathophysiology of sarcopenia and highlight the potential of Hb, LDH, and SII as practical and accessible biomarkers for identifying individuals at higher risk. However, it is important to note that the cross-sectional nature of this study limits our ability to infer causality or temporality in the observed associations. Future research should explore longitudinal designs or experimental interventions to better understand the causal relationships between these biomarkers and the development of sarcopenia. Such studies will be crucial for developing targeted interventions and advancing our understanding of sarcopenia’s etiology. Second, the assessment of sarcopenia in NHANES relies on self-reported data and certain indirect measurements, which may introduce bias or inaccuracies. More direct and standardized methods of sarcopenia diagnosis, such as muscle biopsies or objective functional performance tests, could offer a clearer understanding of the relationship between biomarkers and sarcopenia. Third, although the study adjusted for several potential confounders, other unmeasured or unknown factors may influence the relationship between SII index, LDH, Hb, and sarcopenia. Future studies incorporating more comprehensive data on lifestyle, dietary habits, and genetic factors could further elucidate these relationships.
Conclusion
This study concludes that elevated Hb, LDH, and SII are strongly associated with sarcopenia in the U.S. adult population. These findings underscore the importance of inflammation in the pathophysiology of sarcopenia and highlight the potential of Hb, LDH, and SII as practical and accessible biomarkers for identifying individuals at higher risk. Future research should investigate the underlying mechanisms linking inflammation to sarcopenia and develop multi-marker predictive models incorporating these biomarkers to improve early detection and intervention strategies. As our understanding of sarcopenia deepens, integrating genomic and proteomic data presents a promising avenue for research. Genomic analyses can uncover genetic predispositions linked to sarcopenia, while proteomic approaches can identify protein expression patterns associated with muscle wasting and inflammation35. Combining these datasets enables the exploration of interactions between genetic factors, protein expression, and environmental influences, offering insights into sarcopenia pathophysiology. These multi-omics approaches hold potential for identifying novel biomarkers and therapeutic targets, paving the way for personalized prevention and treatment strategies36,37.
Data availability
This study analyzed publicly available datasets, which can be accessed at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
References
Zou, H. B. et al. Sarcopenia is a predictive factor of poor quality of life and prognosis in patients after radical gastrectomy. Eur. J. Surg. Oncol. 47 (8), 1976–1984 (2021).
Liu, C. et al. Sarcopenic obesity and outcomes for patients with Cancer. JAMA Netw. Open. 7 (6), e2417115 (2024).
Milaković, M. et al. Telework-related risk factors for musculoskeletal disorders. Front. Public. Health 11, 1155745 (2023).
Chen, Y. et al. Exercise-Induced reduction of IGF1R sumoylation attenuates neuroinflammation in APP/PS1 Transgenic mice. J. Adv. Res. (2024).
Okamura, T. et al. A multi-omics approach to overeating and inactivity-induced muscle atrophy in Db/db mice. J. Cachexia Sarcopenia Muscle 15 (5), 2030–2045 (2024).
Yu, B. et al. Sarcopenic obesity is associated with cardiometabolic Multimorbidity in Chinese middle-aged and older adults: a cross-sectional and longitudinal study. J. Nutr. Health Aging 28 (10), 100353 (2024).
Li, Q. et al. Does high-frequency resistance exercise offer additional benefits to older adults? Learnings from a randomized controlled trial. BMC Sports Sci. Med. Rehabil. 16 (1), 186 (2024).
Reichelt, S. et al. Shining a spotlight on sarcopenia and myosteatosis in liver disease and liver transplantation: potentially modifiable risk factors with major clinical impact. Liver Int. 44 (7), 1483–1512 (2024).
Marín Baselga, R. et al. Ultrasound for Body Composition Assessment: a Narrative Review. (Intern Emerg Med, 2024).
Coperchini, F. et al. Inflamm-ageing: How Cytokines and Nutrition Shape the Trajectory of Ageing. (Cytokine Growth Factor Rev, 2024).
Inoue, D. S., Janini, M. & Gomes Integrative insights into PNI: Low-grade chronic inflammation, skeletal muscle wasting, and brain impairments. Brain Behav. Immun. Health 40, 100838 (2024).
Liu, Q. et al. Hemoglobin level is negatively associated with sarcopenia and its components in Chinese aged 60 and above. Front. Public. Health 11, 1081843 (2023).
Studenski, S. A. et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci, 69(5), 547 – 58. (2014).
Yan, R. et al. Discovery of Muscle-Tendon Progenitor Subpopulation in Human Myotendinous Junction at Single-Cell Resolution. Research (Wash D C), 2022, 9760390. (2022).
Ding, J. et al. Association of interleukin-6 with sarcopenia and its components in older adults: a systematic review and meta-analysis of cross-sectional studies. Ann. Med. 56 (1), 2384664 (2024).
Guo, A. et al. TRIM16 facilitates SIRT-1-dependent regulation of antioxidant response to alleviate age-related sarcopenia. J. Cachexia Sarcopenia Muscle, (2024).
Balcer, K. et al. Impact on survival of sarcopenia, systemic inflammatory response and anthropometric factors after pancreatectomy for resectable pancreatic adenocarcinoma. World J. Surg. Oncol. 22 (1), 232 (2024).
Tian, Q. et al. The mediation roles of intermuscular fat and inflammation in muscle mitochondrial associations with cognition and mobility. J. Cachexia Sarcopenia Muscle 15 (1), 138–148 (2024).
Nie, L. et al. Sarcopenia in peripheral arterial disease: Establishing and validating a predictive nomogram based on clinical and computed tomography angiography indicators. Heliyon 10 (7), e28732 (2024).
Deike-Hofmann, K. et al. Macroangiopathy is a positive predictive factor for response to immunotherapy. Sci. Rep. 9 (1), 9728 (2019).
Franceschi, C. et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 128 (1), 92–105 (2007).
Pin, F., Bonewald, L. F. & Bonetto, A. Role of myokines and osteokines in cancer cachexia. Exp. Biol. Med. (Maywood) 246 (19), 2118–2127 (2021).
Webster, J. M. et al. Inflammation and skeletal muscle wasting during Cachexia. Front. Physiol. 11, 597675 (2020).
Morawin, B. et al. Diagnostics of inflammaging in relation to sarcopenia. Front. Public. Health 11, 1162385 (2023).
Vujic, A. et al. Mitochondrial redox and TCA cycle metabolite signaling in the heart. Free Radic Biol. Med. 166, 287–296 (2021).
Wilkinson, D. J., Piasecki, M. & Atherton, P. J. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 47, 123–132 (2018).
Wiedmer, P. et al. Sarcopenia–Molecular Mech. Open. Questions. 65, 101200. (2021).
He, Y. et al. Exploring the impact of interleukins on sarcopenia development: A systematic review and meta-analysis. Exp. Gerontol. 193, 112480 (2024).
Veasey Rodrigues, H. et al. Body composition and survival in the early clinical trials setting. Eur. J. Cancer 49 (15), 3068–3075 (2013).
Liu, L. et al. Effectiveness of sarcopenia screening markers in predicting out-of-hospital death in the oldest (≥ 80 years) older. Geriatr. Nurs. 60, 79–84 (2024).
Li, X. Y. et al. m7G Methylation-Related Genes as Biomarkers for Predicting Overall Survival Outcomes for Hepatocellular Carcinoma. Front. Bioeng. Biotechnol. 10, 849756 (2022).
Zheng, R. et al. Chinese admission warning strategy for predicting the hospital discharge outcome in patients with traumatic brain injury. J. Clin. Med., 11(4). (2022).
Iqbal, H. et al. Anti-inflammatory, anti-oxidant and cardio-protective properties of novel fluorophenyl benzimidazole in L-NAME-induced hypertensive rats. Eur. J. Pharmacol. 929, 175132 (2022).
Ying, H. Q. et al. Cancer-elicited inflammation attenuates response and outcome in tyrosine kinase inhibitor Naive patients with advanced NSCLC. Pharmacol. Res. 170, 105734 (2021).
Jones, G. et al. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat. Commun. 12 (1), 654 (2021).
Qin, H. et al. Low-intensity pulsed ultrasound promotes skeletal muscle regeneration via modulating the inflammatory immune microenvironment. Int. J. Biol. Sci. 19 (4), 1123–1145 (2023).
Liu, J. C. et al. Multi-omics research in sarcopenia: current progress and future prospects. Ageing Res. Rev. 76, 101576 (2022).
Acknowledgements
We thank all authors for their technical and review guidance on this paper.
Funding
The research received the grant from the National Natural Science Foundation of China (NSFC) (NO. 82202366), Wu Jieping Medical Foundation Runze Fund for Critical Care Medicine(NO320.6750.2022-2-34), Beijing NaturalScience Foundation(L244067), and Beijing Key Clinical Specialty Outstanding Project.
Author information
Authors and Affiliations
Contributions
Research concept and study design: YS, ZLX and SGY. Data acquisition: SY. Data analysis/interpretation: SY and ZLX. Statistical analysis: SY and SGY. Supervision and financial support: HYZ and YZA. SY had full access to all study data and take responsibility for data integrity and data analysis accuracy. All authors contributed to interpretation of data, article drafting or revising of the intellectual content, and final approval of the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement.
The studies involving human participants were reviewed and approved by National Center for Health Statistics (NCHS) research ethics review board. The patients/participants provided their written informed consent to participate in this study. Clinical trial number: not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, Y., Yang, S., Xiao, Z. et al. Risk factors and predictive modeling in a US population with sarcopenia: a propensity score cohort study. Sci Rep 15, 6953 (2025). https://doi.org/10.1038/s41598-025-91437-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91437-7