Abstract
Shift workers have an increased risk to develop type 2 diabetes. We aimed to investigate the underlying mechanisms and the role of the timing of food intake by subjecting rats to an acute phase inversion of the light/dark (L/D) cycle. In the first experiment, with food available ad libitum, male Wistar rats were implanted with jugular vein catheters and intravenous glucose tolerance tests were performed at either ZT2 or ZT14. Three days after the 12 h phase shift, these glucose tolerance tests were repeated. In the second experiment, rats were housed in metabolic cages for the continuous measurement of multiple behavioral and metabolic parameters after the 12 h phase shift, food was available ad libitum or restricted to the light or dark period. The daily rhythm of glucose tolerance, and the peak and trough corticosterone levels, adapted within three days after exposure to the inverted L/D cycle. However, phase inversion caused insulin resistance at the onset of the active phase. Under ad libitum feeding conditions, the daily rhythms of locomotor activity and energy expenditure adapted faster to the inverted L/D cycle compared to the other behavioral rhythms measured. Food restriction to the dark period facilitated behavioral adaptation to the new circadian phase.
Similar content being viewed by others
Introduction
Acute inversions of the L/D cycle often occur in the setting of shift work and jet lag. Both shift work and jetlag are associated with metabolic disease1,2,3.
Shift work refers to a schedule that deviates from daytime working hours. Shift work is highly prevalent in our society, with approximately 20% of the work force doing some form of shift work4,5,6,7. Shift work is crucial to keep our society going, for example in health care, hospitality, and transportation. Unfortunately, recent systematic reviews have shown that shift work is a risk factor for a number of health problems, including type 2 diabetes mellitus and cardiovascular disease8,9.
Jet lag results from crossing multiple time zones in a short period, which causes a temporary misalignment between the body’s internal clocks and local time at the destination. This disruption often leads to well-known jet lag symptoms such as fatigue, insomnia, and gastrointestinal discomfort10,11. In addition, people who perform night shift work for many years’ experience chronic jet lag, which may increase the risk of obesity, diabetes, and cognitive deficits12,13.
The negative health effects of shift work and jet lag are probably due to a disruption of the internal circadian timing system, which is responsible for the coordination of many endocrine and physiological rhythms with the behavioral 24-hour sleep-wake cycle. The daily rhythm of glucose tolerance, for example, refers to the higher glucose utilization at the beginning of the activity period14,15. The circadian timing system consists of a central brain clock in the hypothalamic suprachiasmatic nucleus (SCN) and various peripheral clocks in other tissues including muscle, liver, and adipose tissue. The SCN synchronizes its own endogenous rhythm of approximately 24 h with the exact 24 h rhythm of the environment by receiving light information via a direct projection from the retina. The entrained timing signal is forwarded from the SCN to other brain areas, as well as to the peripheral clocks via neural and hormonal signals, body temperature and behavioral activities15. In addition to the entraining signals from the SCN, also behavioral activities such as food intake and exercise can serve as Zeitgebers for the peripheral clocks, including those in metabolic tissues such as the liver, pancreas, adipose tissue and muscle. The timing and composition of meals can affect the expression of clock genes and the rhythmic activity of metabolic pathways in these organs16,17. The same is true for exercise18. Disturbances in the alignment of these different endogenous clock systems can have profound effects on health, performance and well-being.
Some studies in rodents have shown that restricting food intake to the dark period, i.e. the normal wake period of these nocturnal species, facilitates the adjustment of the circadian clock system after a phase inversion19 and improves insulin sensitivity and glucose tolerance20. By contrast, feeding during the light phase (i.e. the wrong time-of-day) attenuated re-entrainment of the melatonin rhythm (a marker of the endogenous circadian rhythm)21.
In 2018, a human study showed that 3 days after an acute 12 h phase shift, muscle insulin sensitivity at the onset of the active period was reduced22. In rodent studies, it was observed that after reversal of the light/dark cycle, clock gene rhythms in liver, heart, kidney, pancreas and duodenum remain desynchronized for at least 7 days23,24,25. However, still little is known about the adaptation speed of the daily rhythm in glucose tolerance after a 12 h phase shift. Therefore, in the present study we measured glucose tolerance at the beginning of the light period and dark period, both before and after rats had been subjected to an acute phase inversion of the L/D cycle. In order to better understand the pathophysiological mechanisms underlying adaptation to L/D phase inversions, we also investigated the adaptation speed of behavioral and metabolic parameters, including locomotor activity, food and water intake, energy expenditure (EE), and the respiratory exchange ratio (RER), after the same 12 h phase shift. Finally, we investigated the effects of time-restricted food intake after the phase inversion as a potential strategy to mitigate the negative effects of such phase-inversions.
Materials and methods
Animals and ethics
Adult male Wistar WU rats (Charles River, Harland, Germany) were used for all our experiments. Animals were group-housed (4–5 animals per cage) during one week of acclimatization before the experiments, under a standardized 12 h/12 h light/dark (L/D) cycle (lights on at 07:00 h/ Zeitgeber Time 0 (ZT0) and lights off at 19:00 h (ZT12)), with ad libitum access to water and chow diet (3.1 kcal/gram, Irradiated Global diet 2918, Horst, the Netherlands). All experimental procedures were performed in accordance with Dutch law, the Council Directive 2010/63EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes. The study was approved by the Animal Ethics Committee of the Royal Dutch Academy of Arts and Sciences (KNAW, Amsterdam, the Netherlands) and in accordance with the guidelines on animal experimentation of the Netherlands Institute for Neuroscience and the ARRIVE guidelines.
Intravenous glucose tolerance test
In the first experiment, jugular vein cannulation surgery was performed in 25 rats (body weight 323 ± 2 g) as described previously26,27. Rats were injected subcutaneously (s.c.) with meloxicam (1 mg/kg; Metacam, Hyperdrug C.O., United Kingdom) ~ 30 min prior to surgery and then were anesthetized with the mixture of ketamine (64 mg/kg BW; Anesketin, Dechra C.O., United Kingdom) and metedomidine (0.26 mg/kg BW; Sedastart, AST Farma, Utrecht, the Netherlands). Subsequently, four holes were drilled in the skull in which stainless steel screws were placed. A silicone catheter was implanted in the internal jugular vein so that the tip reached the right atrium. The other side of the catheter was connected with a bended blunt 20G needle and filled with 0.05 ml PVP solution (0.1 ml heparin (1000 IU/ml), LEO Pharma B.V.; 0.9 ml amoxicillin, Centrafarm B.V.; and 0.9 g polyvinylpyrrolidone, Sigma Aldrich, Germany), and fixed to the screws on the skull with dental cement. Rimadyl (Carprofen, 0.6–1.5 mg/rat/day; Zoetis, the Netherlands) drinking water was provided for 3–4 days after procedure and rats were housed individually till the end of this experiment.
Seven to ten days later, an intravenous glucose tolerance test (ivGTT) was performed at ZT2 or ZT14. After another 7–10 days, rats underwent a complete 12 h L/D phase reversal with a transitional 4 h light period/8 h dark period and 3 days later the ivGTTs were repeated (Fig. 1). Food was withdrawn 2 h prior to the glucose injection. All rats were tested twice, under the regular L/D cycle (Baseline/BL) and after the 12 h phase shift (Desynchronization/DS), respectively. They were randomly assigned to either of two groups: in group 1, ivGTTs were done at the same time points, but different clock times, i.e. ZT2 (9:00) in the BL condition and ZT2 (21:00) in the DS condition (n = 8), or ZT14 (21:00) in the BL condition and ZT14 (9:00) in the DS condition (n = 8); in group 2, ivGTTs were done at the same clock time but different ZT time points, i.e. ZT2 in the BL condition and ZT14 in the DS condition (9:00; n = 4), or at ZT14 in the BL condition and at ZT2 in the DS condition (21:00, n = 5) (Fig. 3). All animals with catheters that stopped working were excluded. The primary outcomes were the effect of a 12 h phase shift on ivGTT glucose and insulin excursions (assessed as area under the curve/AUC), comparing identical ZTs as well as the daily rhythm between before and after desynchronization.
Timeline of the intravenous glucose tolerance test. All rats underwent jugular vein cannulation surgery. Seven to ten days later, an intravenous glucose tolerance test (ivGTT) was performed at Zeitgeber Time 2 (ZT2) or ZT14. After another 7–10 days rats underwent a complete 12 h phase light-dark reversal, and 3 days later the ivGTTs were repeated. Created with BioRender.
During the ivGTT, a baseline blood sample (0.2 ml) was drawn at t = 0 min before the glucose injection (1 g/ml/kg BW, Sigma Aldrich, USA). Subsequently, another five blood samples (0.2 ml) were taken at t = 5, 10, 20, 30, and 60 min. Glucose was measured immediately using a glucometer with blood-glucose test strips (FreeStyle Freedom Lite, Abbott Diabetes Care Ltd., United Kingdom). Plasma was separated from blood cells after centrifugation (11,000 rpm, 10 min, 4 °C) and stored at -20 °C. After completion of all experiments plasma was thawed and insulin and corticosterone levels were measured in duplicates using an enzyme-linked immunosorbent assay (ELISA, Merck, USA) for insulin and a high-sensitivity ELISA (Immunodiagnostic systems, Boldon Business Park, UK) for corticosterone. A coefficient of variation (CV) less than 15% between duplicates was considered acceptable28,29.
Metabolic cages
In the second experiment, 22 animals (body weight 263 ± 4 g) were housed individually in metabolic cages (PhenoMaster, TSE system, Bad Hombourg, Germany). The metabolic cages were placed in a climate chamber with constant temperature (22–23 °C), humidity (40–60%), and a 12/12 light (60–80 lx)/ dark (0 lx) cycle. All animals were habituated to the metabolic cages for 3–4 days in a normal L/D cycle with standard chow available ad libitum, the data from the last two days were used as the baseline measurement. Next, a complete L/D reversal was done in all animals, with a transitional 4 h light period/8 h dark period (Fig. 2)22. After the 12 h phase shift rats were randomly divided into the food ad libitum group (ad libitum feeding/ALF; n = 8 at the start of the experiment, but after day 11 some drop outs occurred due to technical issues), food-restricted to the dark period group (dark feeding/DF; active phase; n = 7 at the start of the experiment, but after day 11 some drop outs occurred due to technical issues) and the food-restricted to the light period group (light feeding/LF; inactive phase; n = 7 at the start of the experiment, but after day 11 some drop outs occurred due to technical issues). The food modules of the metabolic cages opened or closed exactly at the light/dark transitions for the two food-restricted groups. Throughout the whole experiment, multiple behavioral and metabolic parameters as mentioned above were measured continuously for each animal with the indirect calorimetry system (TSE system, Bad Hombourg, Germany). The RER was calculated by the ratio of between the amount of CO2 produced in the citric acid cycle and the amount of O2 consumed during the oxidative phosphorylation. For EE, we took body weight 100% into account. Secondary outcomes were the adaptation speed of metabolic parameters to the new circadian phase after desynchronization, as well as the effect of time restricted eating on these parameters.
Timeline of the metabolic cages experiment. Animals were habituated in the metabolic cages for 3–4 days in a normal L/D cycle with standard chow available ad libitum. Next, a complete 12 h light-dark cycle reversal was done in all animals. Then all rats were divided into the food ad libitum group (AL), food-restricted to the dark period group (DF) and food-restricted to the light period group (LF) till the end of the experiment (11–14 days in total). After the L/D shift, the food modules of the metabolic cages opened or closed exactly at the light/dark transitions for the two food-restricted groups. Created with BioRender.
Statistical analysis
All data are expressed as the mean ± standard error of mean (SEM). Using R studio version 4.0.3 (2020) a cosinor analysis30 with package cosinor and cosinor2 was used to calculate the acrophase (the peak of the fitted curve within 24 h) and amplitude (peak-to-trough difference divided by 2)31. First, significance of curve fitting was estimated for the group mean, followed by calculations of acrophase and amplitude for individual animals. With GraphPad Prism 9.5.1 software, the individual acrophase and amplitude of all parameters, were analysed with a repeated measures two-way analysis of variance (ANOVA) with the factors Acrophase or Amplitude, Days, and their Interaction, followed by Dunnett’s multiple comparisons post hoc test. Differences between groups in percentage of all parameters, the absolute RER values and the positive area under curve (AUC, from t = 0 min to t = 60 min) of blood glucose were analyzed with two-tailed student’s t-test for normally distributed values. Actograms were measured by Image J software with Actogram J package. The results were considered statistically significant when P < 0.05.
Results
Desynchronization causes insulin resistance at activity onset
Previously we and others have shown a clear daily rhythm in glucose tolerance in rats, with glucose tolerance being highest at the onset of the dark period (the active period for nocturnal rodents) and lowest at the onset of the light period14,32. In the current study, we again confirmed this daily rhythm in glucose tolerance, since in the baseline condition glucose tolerance was higher at ZT14 than at ZT2. Besides, we found a similar day/night difference 3 days after the phase-inversion. This rapid adaptation of the daily rhythm in glucose tolerance to the inversed L/D cycle was apparent in group 1 (tested at the same Zeitgeber Time before and after the inversion) (Fig. 3A-D), as well as in the group 2 animals that were tested at different Zeitgeber Times before and after the phase inversion, i.e. ZT2 → ZT14 or ZT14 → ZT2 (Fig. 3E-H). In addition, when we combined the results from groups 1 and 2, a normal rhythm in glucose tolerance was found before the phase inversion (P < 0.05; Fig. 3I, J) and after the phase inversion (P < 0.001; Fig. 3K, L), with the improved glucose tolerance at the beginning of the dark period compared to the beginning of the light period14.
The daily rhythm in glucose tolerance appears to normalize within 3 days after a 12 h phase shift. (A–H) Show the comparison between the BL and DS condition for all groups. (A,C,E,G) show the absolute blood glucose values and (B,D,F,H) show the positive area under curve (AUC) of the blood glucose response (A,B): group 1/ ZT2 BL condition and ZT2 DS condition; (C,D): group 1/ ZT14 BL condition and ZT14 DS condition; (E,F): group 2/ ZT2 BL condition and ZT14 DS condition; (G,H): group 2/ZT14 BL condition and ZT2 DS condition. (I–L) Shows the combined results of groups 1 and 2 for the BL condition (I,J) and the DS condition (K,L). The data are presented as the mean ± SEM, n = 8 (for group 1), n = 4–5 (for group 2); * P < 0.05, ** P < 0.01.
On the other hand, despite the apparent normalization of the daily rhythm in glucose tolerance three days after the 12 h L/D inversion, the higher insulin response at ZT14 after desynchronization compared to baseline (Fig. 4C, D) indicated insulin resistance since the glucose responses did not differ (Fig. 3C, D). On the other hand, at ZT2 the lower insulin response after desynchronization compared to baseline (Fig. 4A, B) (with similar glucose responses (Fig. 3A, B)) indicated increased insulin sensitivity after the L/D inversion. No statistically significant difference was found between ZT2 and ZT14 before and after inversion in group 2 (Fig. 4E–H). The combined results from groups 1 and 2 showed the normal daily fluctuation of insulin responses before the inversion, with the highest response at ZT2 (P < 0.001; Fig. 4I, J). However, after the inversion still a non-significant trend towards higher responses at ZT14 was observed (P = 0.09; Fig. 4K, L).
Desynchronization causes insulin resistance at activity onset 3 days after a 12 h phase shift. (A–H) Show the comparison between the BL and DS condition for all groups. (A,C,E,G) show the absolute insulin values and (B,D,F,H) show the positive area under the curve (AUC) of the insulin response. (A,B): group 1/ ZT2 BL condition and ZT2 DS condition; (C,D): group 1/ ZT14 BL condition and ZT14 DS condition; (E,F): group 2/ ZT2 BL condition and ZT14 DS condition; (G,H): group 2/ ZT14 BL condition and ZT2 DS condition. (I–L) Shows combined results of groups 1 and 2 for the BL condition (I,J) and the DS condition (K,L). The data are presented as the mean ± SEM, n = 8 (for group 1), n = 4–5 (for group 2); * P < 0.05, ** P < 0.01.
Peak and trough corticosterone normalize within 3 days
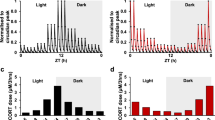
We also measured basal corticosterone values before the glucose bolus injection, i.e. at t = 0 min. In group 1, animals tested at the same ZT before and after the shift, no significant changes in mean corticosterone values were found (Fig. 5A, B). On the other hand, in the group 2, animals tested at the same clock time before and after the shift, a significant increase was observed in the ZT2 → ZT14 animals (Fig. 5C), but a significant decrease in the ZT14 → ZT2 animals (Fig. 5D). The combined results of groups 1 and 2 show a normal daily corticosterone rhythm with lowest levels at ZT2 and highest levels at ZT14, both before and after the shift (Fig. 5E, F). These results suggest that also the daily corticosterone rhythm has adapted to the new L/D cycle within three days after the inversion.
Peak and trough corticosterone appear to normalize within 3 days after a 12 h phase shift. (A–D) show the absolute corticosterone values and the comparison between the BL condition and DS condition for all four groups (A: group 1/ ZT2 BL condition and ZT2 DS condition; B: group 1/ ZT14 BL condition and ZT14 DS condition; C: group 2/ ZT2 BL condition and ZT14 DS condition; D: group 2/ ZT14 BL condition and ZT2 DS condition. E and F show the combined results of group 1 and 2 for the BL condition (E) and the DS condition (F). The data are presented as the mean ± SEM, n = 8 (for group 1), n = 4–5 (for group 2); * P < 0.05, *** P < 0.001.
The daily rhythms of locomotor activity and energy expenditure adapt faster than other behavioral rhythms
We used metabolic cages to assess the time-course of the behavioral and metabolic adaptation of rats to the 12 h phase shift. Rats gradually adapted their behavior to the inverted L/D cycle. The daily rhythms of locomotor activity and energy expenditure adapted faster to the inverted L/D cycle as compared to the rhythms of food intake, water intake and RER (Fig. 6A–J). The acrophase of the daily rhythm in locomotor activity returned to baseline values on day 9 after the inversion (Fig. 6A), the percentage of activity in the 12 h dark period returned to baseline levels on day 5 after the comparison (Fig. 6B). Similar days were found for EE, acrophase back to baseline on day 9 (Fig. 6G) and dark period percentage back on day 5 (Fig. 6H), respectively. In contrast, for food intake the acrophase and dark phase percentage returned to baseline values only on day 12 (Fig. 6C) and day 9 (Fig. 6D). For water intake these numbers were day 6 (Fig. 6E) and day 12 (Fig. 6F), and for RER day 8 (Fig. 6I) and day 6 (Fig. 6J), respectively.
Locomotor activity and EE adapt faster to an L/D shift than other rhythms. The individual acrophase of locomotor activity (A), food intake (C), water intake (E), EE (G) and RER (I); each line represents one animal, same color means same animal. The percentage of 24-h locomotor activity (B), food intake (D), water intake (F) and EE (H) during the light (white bars) and dark (black bars) phase. The absolute RER values during the light (white bars) and dark (black bars) phase (J). A return to baseline was defined as follows: For acrophase, as the day after the last day with a significant acrophase difference compared to baseline. For light phase % or average, as the day after the last day with a light phase value significantly above baseline (if the baseline light phase value is lower than baseline dark phase value) or below baseline (if baseline light phase value is higher than baseline dark phase value). The data are presented as the mean ± SEM, n = 8 at the start of the experiment, after day 11 some drop outs occurred due to technical issues; * P < 0.05, ** P < 0.01 compared to baseline. Open symbols represent data points with no significant Cosine fit in (G).
Dark feeding facilitates adaptation to phase inversion
Food restriction to the dark period facilitated adaptation of the locomotor activity rhythm, whereas food restriction to the light period reduced the adaptation speed (Fig. 7; Supplementary Figure S3A, S3B, S1B). Specifically, food restriction to the dark period caused the locomotor activity rhythm to entrain in terms of acrophase on day 5 (Fig. 7D) and dark phase percentage on day 5 (Supplementary Figure S3A), contrary to day 9 for acrophase (Figs. 6A and 7D) and day 5 for percentage (Fig. 6F) in the ad libitum feeding group. The first return of the rhythm amplitude to baseline values was quite similar in the three groups, but showed a subsequent overshoot in the DF group (i.e. stronger rhythmicity), whereas in the LF group it decreased again and remained below baseline values for the remainder of the experiment (Fig. 7E). Besides, the corresponding actograms confirmed a slower adaptation of the ALF group as compared to the DF group (Fig. 7A, B; Supplementary Figure S1A, S1B). In contrast, light feeding caused the locomotor activity acrophase to return to baseline only on day 13 (Fig. 7D), and the dark phase percentage and amplitude of the rhythm did not return to baseline before the end of the experiment, i.e. >14 days (Fig. 7E and Supplementary Figure S3B).
DF facilitates the rhythm adaptation of locomotor activity. (A) shows a representative actogram of rat 1 of the ALF group, (B) shows a representative actogram of rat 1 of the DF group, (C) shows a representative actogram of rat 1 of the LF group. Rats were first exposed to L/D for 2 days as baseline, and subsequently to D/L for 13–14 days. Grey stars indicate missing part of the data at that day because of cage cleaning. The blue double-arrowed line on the right side of A, B and C indicates the number of days required for the corresponding animal to return to baseline (which is defined as no significant acrophase difference anymore compared to baseline). The acrophase (D) and amplitude (E) as calculated from the Cosine fits of locomotor activity in the ALF group (Black), DF group (Green) and LF group (Red). The horizontal lines indicate that there still is a difference compared to baseline (black represents the ALF group, green represents the DF group, and red represents the LF group). Note that the longer green line in (E) is mostly due to a stronger, not smaller, amplitude as compared to baseline. Data are presented as the mean ± SEM, n = 8 for the ALF groups at the start of the experiment, after day 11 some drop outs occurred due to technical issues, n = 7 for the DF group at the start of the experiment, after day 11 some drop outs occurred due to technical issues, and n = 7 for the LF group at the start of the experiment, after day 11 some drop outs occurred due to technical issues; * P < 0.05 compared to baseline (ALF group), # P < 0.05 compared to baseline (DF group), & P < 0.05 compared to baseline (LF group). Open symbols represent data points with no significant Cosine fit in (D,E). P-values for the Cosine fits can be found in Supplementary Table 1 (Table S1).
Also, the adaptation of the daily rhythms of water intake, energy expenditure and RER were facilitated by food restriction to the dark phase, whereas food restriction to the light period delayed the adaptation of all these parameters (Supplementary Figure S2A, S2C, S2E, S2G; Supplementary Figure S3C, S3D, S3E, S3F, S3G, S3H, S3I, S3J). In addition, DF animals showed higher amplitudes of their water intake and RER rhythms compared to the ALF group (Supplementary Figure S2B, S2D, S2H). On the other hand, the LF animals showed a lower amplitude of their water intake and EE rhythms (Supplementary Figure S2D, S2F), but a higher amplitude of the RER rhythm (Supplementary Figure S2H) compared to the ALF group.
Discussion
In the present rat model of shift work and jet lag, we found an apparent normalization of the daily rhythm of glucose tolerance already three days after onset of the phase inversion. However, these normalized glucose responses were only possible at the expense of insulin resistance at activity onset after the phase inversion, and increased insulin sensitivity at the onset of the inactive phase. The increased insulin resistance at the beginning of the activity period is in line with a comparable experimental study in humans22, underscoring the relevance of the rat model for translation to the human situation. Also the peak and trough corticosterone levels normalized within three days after phase inversion. On the behavioral level, we found that the acute phase inversion results in the expected gradual adaptation of the daily rhythms of locomotor activity, food intake, water intake, energy expenditure and RER to the new circadian phase, with the daily rhythms of locomotor activity and energy expenditure adapting faster than the other rhythms. Restricting food access to the dark period considerably facilitated the adaptation of the behavioral and metabolic parameters. Food restricted to the light period, however, had the opposite effect, delaying the speed of adaptation.
Our glucose tolerance experiments showed a rapid resynchronization of the plasma glucose excursions after the L/D phase inversion. Glucose tolerance was always higher at ZT14 as compared to ZT2 in the different comparisons, in line with the previously reported normal daily rhythm of glucose tolerance14. However, when analyzing the insulin responses it became clear that the normal glucose tolerance was probably due to the lack of adaptation in the insulin response. When comparing ZT14 at baseline to ZT 14 after desynchronization, glucose responses are identical, but the insulin response is higher after desynchronization, indicating insulin resistance at the onset of the active period. Since insulin resistance lays at the heart of the pathophysiology of type 2 diabetes33, this may be related to the increased risk of type 2 diabetes in chronic shift workers.
The corticosterone results showed that the changes in insulin sensitivity were not due to the effect of aberrant levels of corticosterone, since peak and trough plasma corticosterone normalized within 3 days, similar to glucose tolerance. A discordance of the adaption of insulin and corticosterone responses, resulting in a change of their phase relation, was also found in a previous study in which rats were confronted with light phase feeding34. Previously, many human studies have shown that the cortisol rhythm is robust against acute changes in the photic environment35,36. For example, a study in police officers showed that the diurnal rhythm of cortisol phase delayed with increasing number of night shifts by 33 min per day over the course of 7 days37. This is consistent with another human study which showed that salivary cortisol levels were only partially reset following 1 week of night shifts38. Thus, it seems that the daily corticosterone rhythm in rats is more affected by masking than the cortisol rhythm in humans. Important difference however is that contrary to our rat experiment these human studies are often performed in dim light conditions.
In circadian biology, daily rhythms observed in environments with a rhythmic timing signal from the light-dark cycle, can generally be explained as endogenous (regulated by the circadian timing system), masking (regulated by the exogenous light/dark cycle), or a combination of the two. Endogenous rhythms are truly circadian rhythms that are regulated by the endogenous circadian timing system, which synchronizes these rhythms with the light-dark cycle, whereas masking involves a direct stimulatory or inhibitory effect of light on physiology or behavior39,40. For example, light pulses are a strong suppressor of locomotor activity in nocturnal organisms, while they stimulate activity in diurnal organisms41,42. Similarly, even though core body temperature, melatonin, plasma cortisol and locomotor activity are well established markers of internal time in continuous darkness condition43,44, under L/D conditions the control of the endogenous clock on these rhythms may be masked by the inhibitory effect of light. Therefore, the pure endogenous component can only be studied under constant dark conditions. In our study, we observed that the rhythms of food intake and RER took longer to adapt to the new schedule compared to locomotor activity and energy expenditure, indicating that locomotor activity is probably more strongly affected by the masking effect of light than food intake and RER. This difference in “masking sensitivity” is important as it may be one of the reasons for the long-lasting disturbing effects of a phase inversion on the re-synchronization of daily rhythms. At the same time, energy expenditure is following locomotor activity. Therefore, energy expenditure also showed a faster adaptation speed. Thus, the observed reaction of metabolic and behavioral rhythms to a circadian phase inversion in our study can be due to (a combination of) a response of the endogenous clocks and masking. Prolonged desynchrony of appetite/eating behavior leads to eating at the wrong time-of-day (that is eating close to the sleep period), which may predispose to weight gain and further metabolic dysregulation45,46.
A phase inversion of the L/D cycle also affects the daily rhythms in clock gene expression in the central and peripheral clocks. One study using advanced or delayed shifts of the L/D cycle by 6–9 h in the Per1-luciferase transgenic rat model showed that Per1 in the SCN shifted almost completely after the first day, but rhythmicity in the peripheral tissues shifted differently and in all cases more slowly than the SCN47. A study in adult mice showed that mPer1, mPer2 and mCry1 in the SCN react rapidly, completing the shift together by the second day after a 6 h phase delay48. Another study showed that the resetting process of clock genes (including Bmal1, Cry1, Per1, Dec1) in the rat pineal gland was almost completed within 3–5 days after an abrupt 12 h phase shift via a 24 h dark period transition49. After the combined reversal of the feeding schedule and L/D cycle the examined clock genes finished their re-entrainment within 3–5 days in the liver, while in the heart their resetting course took 5–7 days50. Thus, also when looking at daily rhythms in clock gene expression it is clear that after an L/D inversion the central and peripheral clocks show large variability in adaptation speed, although in general they seem to adapt more quickly than behavioral rhythms.
The rhythmic output of the SCN is further fine-tuned by the various peripheral clocks. However, by now it is well known that also the metabolic signals resulting from food intake are strongly involved in the synchronization of peripheral clocks15,51. Therefore, we performed time-restricted feeding experiments in our shifted rats. These experiments showed that food restricted to the dark period (the habitual active period of nocturnal rats) facilitated adaptation of all parameters to the new L/D cycle, confirming the profound effects of feeding time. Moreover, our results align with many previous studies implicating that eating at the right time-of-day has beneficial health effects, especially to prevent health problems caused by circadian disruption19,20,21,22.
Although the comparable effects on insulin resistance in our current study and the previous study in humans22 are encouraging, translation of the current data to the human situation is complicated, also in view of the many different shift work schedules that are being used. However, based on the current experiment and all the previous knowledge that is available, it is clear that an optimal timing of both meals and physical activity is crucial for metabolic health. Specifically, restricting food intake to the new active period, may help to synchronize to a new circadian phase in jet lag and in shift work. On the other hand, in case of fast rotating shift schedules, restricting food intake to the regular active period (i.e. the light period in humans) may help to reduce the desynchronizing effects of continuous shifts.
Conclusion
In summary, our results demonstrate that the normal daily rhythm in glucose tolerance after a circadian phase shift can only be maintained at the expense of changes in insulin release and insulin sensitivity. Locomotor activity and energy expenditure are adapting more quickly to a new light-dark cycle compared to other behavioral rhythms. The slow adaptation of the food intake rhythm as well as the insulin resistance at the onset of the active period may be causally related to the increased incidence of metabolic disease in shift workers. Restricting food intake to the new active period may help to adapt to a new circadian phase, which may be beneficial in reducing the negative health effects of jet lag and shift work.
Data availability
The original contributions presented in the study are included in the article/supplementary material, and further inquiries can be directed to the corresponding author.
References
Koopman, A. D. M. et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: the new Hoorn study. J. Biol. Rhythms. 32, 359–368. https://doi.org/10.1177/0748730417713572 (2017).
Boivin, D. B., Boudreau, P. & Kosmadopoulos, A. Disturbance of the circadian system in shift work and its health impact. J. Biol. Rhythms. 37, 3–28. https://doi.org/10.1177/07487304211064218 (2022).
Reid, K. J. & Abbott, S. M. Jet lag and shift work disorder. Sleep. Med. Clin. 10, 523–535. https://doi.org/10.1016/j.jsmc.2015.08.006 (2015).
Agnès Parent-Thirion et al. Sixth european working conditions survey - overview report (2017). update. (2017).
McMenamin, T. M. A time to work: recent trends in shift work and flexible schedules. Mon. Labor. Rev. 130, 3-15. (2007).
Wickwire, E. M., Geiger-Brown, J., Scharf, S. M. & Drake, C. L. Shift work and shift work sleep disorder: clinical and organizational perspectives. Chest. 151, 1156–1172. https://doi.org/10.1016/j.chest.2016.12.007 (2017).
Sweileh, W. M. Analysis and mapping of global research publications on shift work (2012–2021). J. Occup. Med. Toxicol. 17, 22. https://doi.org/10.1186/s12995-022-00364-0 (2022).
Kecklund, G. & Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ. 355, i5210. https://doi.org/10.1136/bmj.i5210 (2016).
Barf, R. P., Meerlo, P. & Scheurink, A. J. Chronic sleep disturbance impairs glucose homeostasis in rats. Int. J. Endocrinol. 2010, 819414. https://doi.org/10.1155/2010/819414 (2010).
Herxheimer, A. Jet lag. BMJ. Clin. Evid. 2014, 2303. (2014).
Kolla, B. P. & Auger, R. R. Jet lag and shift work sleep disorders: how to help reset the internal clock. Cleve. Clin. J. Med. 78, 675–684. https://doi.org/10.3949/ccjm.78a.10083 (2011).
Cho, K., Ennaceur, A., Cole, J. C. & Suh, C. K. Chronic jet lag produces cognitive deficits. J. Neurosci. 20, RC66. https://doi.org/10.1523/jneurosci.20-06-j0005.2000 (2000).
Zheng, R. et al. Chronic jet lag alters gut microbiome and mycobiome and promotes the progression of MAFLD in HFHFD-fed mice. Front. Microbiol. 14, 1295869. https://doi.org/10.3389/fmicb.2023.1295869 (2023).
la Fleur, S. E., Kalsbeek, A., Wortel, J., Fekkes, M. L. & Buijs, R. M. A daily rhythm in glucose tolerance a role for the suprachiasmatic nucleus. Diabetes. 50, 1237-1243. https://doi.org/10.2337/diabetes.50.6.1237 (2001).
Stenvers, D. J., Scheer, F., Schrauwen, P., la Fleur, S. E. & Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15, 75–89. https://doi.org/10.1038/s41574-018-0122-1 (2019).
Oike, H., Oishi, K. & Kobori, M. Nutrients, clock genes, and chrononutrition. Curr. Nutr. Rep. 3, 204–212. https://doi.org/10.1007/s13668-014-0082-6 (2014).
Hatori, M. & Panda, S. Response of peripheral rhythms to the timing of food intake. Methods Enzymol. 552, 145–161. https://doi.org/10.1016/bs.mie.2014.10.027 (2015).
Martin, R. A. & Esser, K. A. Time for exercise?? exercise? and its influence on the skeletal muscle clock. J. Biol. Rhythms. 37, 579–592. https://doi.org/10.1177/07487304221122662 (2022).
Ren, B. et al. Impact of time-restricted feeding on adaptation to a 6-hour delay phase shift or a 12-hour phase shift in mice. Nutrients. 14, 3025. https://doi.org/10.3390/nu14153025 (2022).
de Goede, P. et al. Time-restricted feeding improves glucose tolerance in rats, but only when in line with the circadian timing system. Front. Endocrinol. (Lausanne). 10, 554. https://doi.org/10.3389/fendo.2019.00554 (2019).
Kalsbeek, A., Barassin, S., van Heerikhuize, J. J., van der Vliet, J. & Buijs, R. M. Restricted daytime feeding attenuates reentrainment of the circadian melatonin rhythm after an 8-h phase advance of the light-dark cycle. J. Biol. Rhythms. 15, 57–66. http://doi.org/10.1177/074873040001500107 (2000).
Wefers, J. et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. U S A. 115, 7789–7794. https://doi.org/10.1073/pnas.1722295115 (2018).
Wu, T. et al. Effects of light cues on re-entrainment of the food-dominated peripheral clocks in mammals. Gene. 419, 27–34. https://doi.org/10.1016/j.gene.2008.04.010 (2008).
Wu, T. et al. Regulation of circadian gene expression in the kidney by light and food cues in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R635–641. https://doi.org/10.1152/ajpregu.00578.2009 (2010).
Xu, L., Wu, T., Li, H., Ni, Y. & Fu, Z. An individual 12-h shift of the light-dark cycle alters the pancreatic and duodenal circadian rhythm and digestive function. Acta Biochim. Biophys. Sin (Shanghai). 49, 954–961. https://doi.org/10.1093/abbs/gmx084 (2017).
Foppen, E., Tan, A. A., Ackermans, M. T., Fliers, E. & Kalsbeek, A. Suprachiasmatic nucleus neuropeptides and their control of endogenous glucose production. J. Neuroendocrinol. 28. https://doi.org/10.1111/jne.12365 (2016).
Steffens, A. B. A method for frequent sampling of blood and continuous infusion of fluids in the rat without disturbing the animal. Physiol. Behav. 4, 833–836. http://doi.org/10.1016/0031-9384(69)90126-7 (1969).
Shechtman, O. The coefficient of variation as an index of measurement reliability. In: doi, S., William, G. (eds) Methods of clinical epidemiology. Springer series on epidemiology and public health. Springer, Berlin, Heidelberg. Chapter 4, 39–49. https://doi.org/10.1007/978-3-642-37131-8_4 (2013).
Hanneman, S. K., Cox, C. D., Green, K. E. & Kang, D. H. Estimating intra- and inter-assay variability in salivary cortisol. Biol. Res. Nurs. 13, 243–250. https://doi.org/10.1177/1099800411404061 (2011).
Minors, D. S. & Waterhouse, J. M. Mathematical and statistical analysis of circadian rhythms. Psychoneuroendocrinology. 13, 443–464. http://doi.org/10.1016/0306-4530(88)90030-3 (1988).
Rumanova, V. S., Okuliarova, M., Foppen, E., Kalsbeek, A. & Zeman, M. Exposure to dim light at night alters daily rhythms of glucose and lipid metabolism in rats. Front. Physiol. 13, 973461. https://doi.org/10.3389/fphys.2022.973461 (2022).
Jolin, T. & Montes, A. Daily rhythm of plasma glucose and insulin levels in rats. Horm. Res. 4, 153–156. https://doi.org/10.1159/000178303 (1973).
DeFronzo, R. A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 58, 773–795. https://doi.org/10.2337/db09-9028 (2009).
Honzlová, P., Novosadová, Z., Houdek, P., Sládek, M. & Sumová, A. Misaligned feeding schedule elicits divergent circadian reorganizations in endo- and exocrine pancreas clocks. Cell. Mol. Life Sci. 79, 318. https://doi.org/10.1007/s00018-022-04354-7 (2022).
Archer, S. N., Moller-Levet, C. S., Laing, E. E. & Dijk, D. J. Mistimed sleep and waking activity in humans disrupts glucocorticoid signalling transcripts and SP1, but not plasma cortisol rhythms. Front. Physiol. 13, 946444. https://doi.org/10.3389/fphys.2022.946444 (2022).
McHill, A. W., Hull, J. T., McMullan, C. J. & Klerman, E. B. Chronic insufficient sleep has a limited impact on circadian rhythmicity of subjective hunger and awakening fasted metabolic hormones. Front. Endocrinol. (Lausanne). 9, 319. https://doi.org/10.3389/fendo.2018.00319 (2018).
Jensen, M. A., Hansen, Å., Kristiansen, M., Nabe-Nielsen, J., Garde, A. H. & K. & Changes in the diurnal rhythms of cortisol, melatonin, and testosterone after 2, 4, and 7 consecutive night shifts in male Police officers. Chronobiol. Int. 33, 1280–1292. https://doi.org/10.1080/07420528.2016.1212869 (2016).
Koshy, A., Cuesta, M., Boudreau, P., Cermakian, N. & Boivin, D. B. Disruption of central and peripheral circadian clocks in Police officers working at night. Faseb. J. 33, 6789–6800. https://doi.org/10.1096/fj.201801889R (2019).
Mrosovsky, N. Masking: history, definitions, and measurement. Chronobiol. Int. 16, 415–429. http://doi.org/10.3109/07420529908998717 (1999).
Redlin, U. Neural basis and biological function of masking by light in mammals: suppression of melatonin and locomotor activity. Chronobiol. Int. 18, 737–758. http://doi.org/10.1081/cbi-100107511 (2001).
Gall, A. J. & Shuboni-Mulligan, D. D. Keep your mask on: the benefits of masking for behavior and the contributions of aging and disease on dysfunctional masking pathways. Front. Neurosci. 16, 911153. https://doi.org/10.3389/fnins.2022.911153 (2022).
Liu, J. M., Yang, Y. M., Xu, W. & Li, X. D. Regulation of circadian physiology and behavior by light in mammals: entrainment and masking. Life. Res. 7, 7. https://doi.org/10.53388/lr20240007 (2024).
Brown, E. N., Choe, Y., Luithardt, H. & Czeisler, C. A. A statistical model of the human core-temperature circadian rhythm. Am. J. Physiol. Endocrinol. Metab. 279, E669–683. https://doi.org/10.1152/ajpendo.2000.279.3.E669 (2000).
Zuo, L. et al. Effects of constant light and dark conditions on the locomotor activity, body mass, and body temperature rhythms of Eurasian tree sparrows (Passer montanus). Avian. Res. 14, 100126. https://doi.org/10.1016/j.avrs.2023.100126 (2023).
Kessler, K. & Pivovarova-Ramich, O. Meal timing, aging, and metabolic health. Int. J. Mol. Sci. 20, 1911. https://doi.org/10.3390/ijms20081911 (2019).
Peters, B., Vahlhaus, J. & Pivovarova-Ramich, O. Meal timing and its role in obesity and associated diseases. Front. Endocrinol. 15, 1359772. https://doi.org/10.3389/fendo.2024.1359772 (2024).
Yamazaki, S. et al. Resetting central and peripheral circadian oscillators in Transgenic rats. Science. 288, 682–685. https://doi.org/10.1126/science.288.5466.682 (2000).
Reddy, A. B., Field, M. D., Maywood, E. S. & Hastings, M. H. Differential resynchronisation of circadian clock gene expression within the Suprachiasmatic nuclei of mice subjected to experimental jet lag. J. Neurosci. 22, 7326–7330. https://doi.org/10.1523/jneurosci.22-17-07326.2002 (2002).
Wu, T. et al. Differential resetting process of circadian gene expression in rat pineal glands after the reversal of the light/dark cycle via a 24 h light or dark period transition. Chronobiol. Int. 26, 793–807. https://doi.org/10.1080/07420520903044208 (2009).
Wu, T., Ni, Y., Zhuge, F. & Fu, Z. Resetting process of peripheral circadian gene expression after the combined reversal of feeding schedule and light/dark cycle via a 24-h light period transition in rats. Physiol. Res. 59, 581–590. https://doi.org/10.33549/physiolres.931818 (2010).
Chellappa, S. L. et al. Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci. Adv. 7, eabg9910. https://doi.org/10.1126/sciadv.abg9910 (2021).
Acknowledgements
The work described here was primarily funded by a China Scholarship Council(CSC) grant of AW. No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Contributions
A.W. contributed to the conception and design, the acquisition, analysis and interpretation of data for the work, drafting the article and reviewing it. E.F. provided substantial contributions to the conception of the design, acquisition of the data, interpreted data and provided critical review for important intellectual content. V.S.R. provided substantial contributions to the analysis of the data and provided critical review for important intellectual content. T.K. contributed to the acquisition of the data and provided critical review for important intellectual content. A.K. was involved in the conception of the study design, interpreted data, revised the manuscript critically for important intellectual content. D.J.S. contributed to the conception and design, interpreted data, revised the manuscript critically for important intellectual content. All authors critically revised and approved the final version to be submitted. D.J.S is the guarantor of this work.
Corresponding author
Ethics declarations
Ethics statement
All experimental procedures were performed in accordance with Dutch law, the Council Directive 2010/63EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes. The study was approved by the Animal Ethics Committee of the Royal Dutch Academy of Arts and Sciences (KNAW, Amsterdam, the Netherlands) and in accordance with the guidelines on animal experimentation of the Netherlands Institute for Neuroscience and the ARRIVE guidelines.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, A., Foppen, E., Rumanova, V.S. et al. Circadian phase inversion causes insulin resistance in a rat model of night work and jet lag. Sci Rep 15, 9687 (2025). https://doi.org/10.1038/s41598-025-91485-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91485-z