Abstract
Recycled polyethylene terephthalate (RPET) was doped with Neodymium Oxide (Nd2O3: 0, 1, 2, 4, and 8 wt.%) to investigate its structural, optical, dielectric, and mechanical properties. X-ray diffraction (XRD) analysis revealed that pure RPET exhibited an amorphous structure, while the incorporation ofNd2O3 induced the formation of crystalline phases, with crystallinity increasing as the Nd2O3 concentration increased. Fourier-transform infrared (FTIR) spectroscopy identified chemical interactions between RPET and Nd2O3, evidenced by a new band around 535 cm−1. Optical analysis using diffuse reflectance UV–Vis spectroscopy showed a reduction in the band gap from 3.75 eV for pure RPET to 2.25 eV in 8wt.% doped samples, indicating enhanced optical properties. Dielectric studies revealed that Nd2O3 doping significantly decreased the dielectric constant of RPET, contributing to the thermal stability of the dielectric constant. Furthermore, the dielectric loss and conductivity improved, with enhanced stability observed across varying temperatures. Dynamic mechanical analysis (DMA) revealed that adding 8 wt.% Nd2O3 reduced the storage modulus of RPET from 1.62 GPa to approximately 0.26 GPa at 35 °C, attributed to structural softening. These improvements suggest that Nd2O2-doped RPET is suitable for applications requiring conductive REPT, low storage modulus, thermal stability, and enhanced energy dissipation capabilities.
Similar content being viewed by others
Introduction
Plastic is one of the most important consumer materials due to its low production cost and ease of formation1. However, the continuous and increasing use of plastic, coupled with its immense production, has resulted in its widespread entry into the environment, making it a significant concern2. The accumulation and degradation of plastic waste in the environment cause serious harm to humans and other organisms3. Moreover, plastic plays a major role in climate change, contributing to global warming through carbon dioxide emissions during its production and manufacturing processes.
Addressing the impacts of plastic waste on human health and the environment has become a critical priority. Communities must adopt safe disposal methods to mitigate its detrimental effects4,5. Notably, plastic takes several years to decompose in soil due to its resistance to degradation, even when buried in landfills6. To tackle this challenge, recycling has emerged as a practical solution, helping to preserve the environment while conserving raw plastic materials for future generations7,8. Reports about the application of polymers with nanoparticles have been studied for storage energy as well as electronic devices applications including, electrospinning of polyaniline/poly acrylonitrile/reduced graphene oxide doped with aluminum oxide nanoparticles for supercapacitors application9, Several reports about the nanoparticles doped in polymers have been studied including; PMMA/PEG polymer nanocomposites doped with different oxides nanoparticles10, PEVA composite membrane embedded with conductive copper fluoroborate glass powder11, and effect of V2O5 nanoparticles into PMMA composite membranes to study the physical properties for conductive polymeric membrane applications11. Synthesis, characterization of polyelectrolyte membranes based on phosphorylated-PVA/cellulose acetate performance testing direct methanol fuel cell applications12. Moreover, the effect of bismuth sodium potassium titanate (Bi0.5Na0.5−xKxTiO2: BNKT) nanoceramics on the organic–inorganic conductive membranes based on oxidized cellulose as an intelligent and innovative antibacterial nano-system for bio-applications13.
In this study, we focused on one specific type of plastic: Recycled polyethylene terephthalate (PET). RPET is among the most widely used plastics, commonly found in soda bottles, mineral water containers, and various other applications. Consequently, RPET waste constitutes a significant proportion of plastic waste14,15. Recycling has recently gained prominence as an effective, environmentally friendly, and cost-efficient approach to managing plastic waste.
Several research studies have combined plastic recycling methods with nanotechnology techniques, not only to address plastic waste abundance but also to tackle other environmental challenges16,17,18,19. Nanotechnology is considered one of the three core technologies of the twenty-first century, given its diverse applications and transformative potential in fields such as physics, biomedical science, chemistry, and environmental protection20,21,22,23,24. Rare earth (RE) oxide micro- and nanomaterials, with their distinct morphologies and structures, have drawn significant attention due to their unique properties and wide-ranging applications25,26,27,28,29. Several research studies have used plastics recycling methods together with nanotechnology techniques, not only to solve the issues of plastic waste abundance but also to solve another environmental problem30,31,32,33,34,35. Recently, the designs of rare earth (RE) oxide micro- and nanomaterials with different morphologies and structures have attracted considerable interest because of their unique properties because they possess a large band gap (Eg = 4 − 6 eV), high-resistivity (ρ = 1012 − 1015 Ω.cm), high-crystallization temperature, high-relative permittivity, thermodynamic stability and most significantly have a high-k value (ε = 7–20) as compared to different type of materials36,37,38,39,40. Among rare-earth oxides, neodymium oxide (Nd2O3) is one of potential candidates because it has high-k dielectric constant (10–15), a large band gap (5.8 eV), perfect electrical and thermal stability, and excellent optical transmission41. Furthermore, it exhibits excellent magnetic, catalytic, and mechanical properties, making it suitable for a wide range of applications42. Studies have demonstrated that Nd2O3 is an appropriate material for high-k gate dielectrics, paving the way for the realization of future ultra-large-scale integration devices43,44,45. Despite this interest, limited research has explored the integration of RPET with rare earth oxides. In this study, focusing on Recycled Polyethylene Terephthalate (RPET), a sustainable alternative to virgin PET commonly used in packaging and textiles. Enhancing the properties of RPET through doping with rare earth oxides, such as Nd2O3, holds promise for advanced applications. This research investigates the impact of rare earth oxide doping on the optical and electrical properties of RPET, aiming to expand its potential for innovative applications.
Owing to the above advantage of Nd2O3, this motivated us to study the additive of Nd2O3 into RPET plastics for the interaction to enhance the electrical properties of RPET. The structure and morphology of the prepared samples were characterized by X-ray diffraction (XRD) and Fourier-transformed infrared (FTIR) spectroscopy. Optical properties of the samples were studied using Ultraviolet–Visible spectroscopy (UV/Vis). In addition, the dielectric properties as functions of frequency (1 kHz to 200 kHz) and temperature (30 to 100 °C) were studied. Dynamic mechanical analysis (DMA) studies was investigated to show the impact of Nd2O3 into RPET plastics. This article examines the impact of rare earth oxides doping on the optical, electrical, and mechanical properties of RPET.
Experimental work
Materials and synthesis process
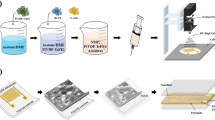
Figure 1 illustrates the experimental procedure followed for sample preparation, outlining each step in detail. Recycled PET was chopped up, and ground mechanically, converting the RPET waste into powder. RPET powder was then mixed with Nd2O3 in different concentrations (0, 1, 2, 4, 8 wt.%) as shown in Table 1.
Sample preparation
Plastic waste collection and cleaning
The process begins with collecting plastic waste. After collection, the plastic is sorted to select the RPET (Recycled Polyethylene Terephthalate) material. The cleaning process is conducted in two stages; firstly, the plastic waste is placed in a water washing tank for rinsing. A plastic mixing machine is then used to remove loose dirt, such as sand and mud. Secondly, strongly adhered contaminants, such as printing inks and paper labels with adhesives, are removed by soaking the plastic in a hot alkaline water solution, typically containing sodium hydroxide (NaOH; 99.9%) or potassium hydroxide (KOH; 99.9%), at a temperature of 70–90 °C for 30–60 min. The plastic is agitated in the solution, causing the materials to rub against each other to dislodge the dirt. Finally, the cleaned plastic is dried with hot air to remove any remaining moisture.
Cutting and grinding RPET into small pieces
The RPET is cut into small parts using a homemade machine designed specifically for this purpose. The grinding process involves cutting and crushing the RPET material into powder through centrifugation and friction. The small RPET pieces are placed in a device that rotates at a speed of 730 rpm. The rough surface of the cylinder facilitates the crushing process through friction. The resulting plastic powder is then sieved through a fine mesh to ensure a uniform particle size.
Mixing RPET with rare earth oxides (Nd2O3)
The RPET powder was mixed with varying proportions of rare earth oxide (Nd2O3) with a purity of 99.99%, procured from Sigma-Aldrich, at concentrations of 1, 2, 4, and 8 wt.% (as shown in Table 1). The mixture is compressed using a hydraulic press and formed into small discs. Finally, the surfaces of the samples are coated with silver paste to ensure proper Ohmic contact electrodes for the LCR analyzer.
Samples characterization techniques
The XRD patterns of the prepared samples of RPET and RPET doped with Nd2O3 were obtained using a PANalytical Empyrean 3rd generation diffractometer at the Nanotechnology Research Center, British University in Egypt. The measurements were performed using Cu-Kα radiation (λ = 1.540Å) with an operating voltage of 30 kV. The scans were conducted with a step size of 0.02 s−1 over an angular range of 2θ from 10° to 80°. The interaction of infrared radiation with the samples, through absorption, was studied using FT-IR spectroscopy to identify chemical substances or functional groups. The measurements were carried out using an ALPHA II—FT-IR spectrometer at the National Research Center, Cairo, Egypt. The optical properties of the samples were analyzed using UV/Vis spectroscopy with a Jasco V-630 spectrophotometer at the National Research Center, Cairo, Egypt. The dielectric properties of the samples were measured as a function of frequency, ranging from 1 to 200 kHz, under varying temperature conditions from 30 to 100 °C. The measurements were conducted using a Hioki IM-3533 LCR Analyzer equipped with active Kelvin electrodes (Cairo University, Egypt). A Dynamic Mechanical Analyzer (Metravib-DMA 25) was used to analyze the prepared composites to evaluate their viscoelastic properties as a function of temperature (35–100℃) at a fixed frequency of 50 Hz.
Results and discussion
X-ray diffraction (XRD)
Figure 2 shows the structural of pure RPET and doped-RPET with varying concentrations of neodymium oxide (Nd2O3) (1, 2, 4, and 8 wt.%). The XRD pattern of pure RPET (labelled at the bottom) shows broad, diffuse peaks, characteristic of its amorphous nature. This indicates a lack of long-range order in the polymer structure, which is typical of thermoplastic polymers like RPET. With the addition of Nd2O3, distinct crystalline peaks appear, and their intensity increases progressively with higher Nd2O3 concentrations (1 to 8 wt.%). This suggests that Nd2O3 acts as a nucleating agent, enhancing the crystallinity of the RPET matrix. At 1 wt.% Nd2O3, new peaks begin to emerge, signifying the formation of crystalline phases associated with Nd2O3. However, the crystallinity improvement is minimal at this concentration. At 2 wt.% and 4 wt.% Nd2O3, the crystalline peaks become more pronounced, indicating a significant increase in structural order. The peaks align with the characteristic planes of Nd2O3, which are indexed in the figure using the JCPDS card No. 74-2139 (e.g., (001), (101), and (110)). At 8 wt.% Nd2O3, the crystalline peaks reach their highest intensity, reflecting maximum crystallinity in the composite. This concentration introduces a substantial amount of Nd2O3 particles, which act as centers for crystallite formation. The indexed peaks in the XRD pattern correspond to the planes of Nd2O3. This confirms the incorporation of Nd2O3 into the RPET matrix, contributing to the observed crystallinity. The intensity of these peaks suggests that Nd2O3 remains crystalline and does not undergo significant structural changes during the blending process. In the amorphous and crystalline regions, the broad hump observed in the pure RPET sample decreased in intensity, and the peaks with indices (010) and (100) disappeared in the doped samples. This suggests that a portion of the RPET matrix remains amorphous, as indicated by the oriented poly(ethylene terephthalate) (PET) pattern (PDF 00–061-1413). Meanwhile, the addition of Nd2O3 primarily contributes to the formation of crystalline regions. The increase in crystallinity with higher Nd2O3 concentrations can enhance the mechanical and thermal stability of RPET. Crystalline regions typically improve the material’s rigidity and resistance to thermal deformation. However, excessive crystallinity may reduce the material’s flexibility. This XRD analysis demonstrates the ability of Nd2O3 to enhance the structural order of RPET by increasing its crystallinity. The findings suggest that Nd2O3-doped RPET composites could be tailored for applications requiring improved mechanical and thermal properties. The variations in crystallinity and peak intensities across different Nd2O3 concentrations provide insight into optimizing the doping level for specific applications46,47.
FT-IR spectra analysis of RPET with Nd2O3
Fourier Transform Infrared (FT-IR) spectroscopy is a valuable technique for identifying functional groups and molecular interactions. It provides insights into the chemical and structural properties of RPET plastic and the effects of doping with Nd2O3. In this study, FT-IR analysis was performed on RPET plastic and RPET samples doped with Nd2O3. As shown in Fig. 3, the ester functional group in RPET contains a carbonyl carbon (C = O), an alpha carbon to its left, and an ester oxygen to its right. If the alpha carbon is saturated, the ester is classified as a saturated ester, whereas it is classified as an aromatic ester if the alpha carbon is part of an aromatic ring. Esters exhibit a distinct infrared (IR) spectral pattern due to their molecular structure, which includes a carbonyl bond and two C–O bonds. These bonds produce characteristic absorption peaks: Firstly, Carbonyl Stretching (C = O); Strong peaks typically observed between 1800 and 1600 cm−1. Secondly, C–O Stretching; Intense peaks generally seen between 1300 and 1000 cm−1. Because one of the C–O bonds in the ester group is attached to the carbonyl carbon and the other is not, they are chemically distinct and have different force constants. Consequently, these C–O bonds give rise to two separate peaks within the 1300–1000 cm−1 range48. The experimental results confirm these predictions. Esters consistently display a memorable pattern of three intense peaks: A peak at ~ 1700 cm−1 corresponding to the C = O stretching and peaks at ~ 1200 cm−1 and ~ 1100 cm−1 corresponding to the two distinct C–O stretches. This pattern is often referred to as the "Rule of Three" for esters. In RPET, the ester group is part of the polymer backbone and is identified as an aromatic ester due to the aromatic alpha carbon. These findings underscore the utility of FT-IR spectroscopy in confirming the presence of ester groups and understanding their behavior, particularly in the context of RPET and its interaction with Nd2O349,50.
Figure 3a, b illustrates the FT-IR spectra of RPET and the effects of Nd2O3 doping on the characteristic peaks. The addition of rare earth oxides influences the vibrational modes, suggesting molecular-level interactions. The ester functional group, formed through esterification when alcohol and carboxylic acid react, plays a key role in this context. The FT-IR spectra of RPET and Nd2O3 with varying concentrations (1, 2, 4, and 8 wt.%) were recorded over the range of 400–4000 cm−1. Here there are four points should be noted; Firstly, O–H Stretching Vibrations; A broad band is observed at approximately 3593, 3680, 3686, 3675, and 3689 cm−1, as shown in the inset of Fig. 3b. The data is presented in Table 2, corresponding to the O–H stretching vibrations of water molecules absorbed from the environment. Secondly; C–O and Organic Network Vibrations, a small peak at ~ 870 cm−1 and 871 cm−1 may be attributed to the C–O group or an organic network as in Fig. 3a. Thirdly; Nd-OH Vibrations, an absorption band at 535 cm−1 is associated with Nd–OH vibrations, observed prominently at higher Nd2O3 concentrations. Fourthly, Metal Oxide Vibrations (Nd–O), a small peak at 491 cm−1 corresponds to the characteristic stretching vibrations of the Nd–O bond as shows in Fig. 3c. Notably, the intensity of these peaks increases with theNd2O3 content, indicating a stronger presence of the metal oxide. Fifthly; New Peak Formation and Shifts; a new peak at 535 cm−1 emerges prominently at 8 wt.% Nd2O3 concentration, and a noticeable peak shift occurs from 443 to 427 cm−1. These shifts suggest significant interactions between RPET andNd2O3, which likely contribute to enhanced material properties51. The observed changes in peak intensity, formation, and shifts with increasing Nd2O3 content underscore the molecular-level interactions and modifications introduced by the rare earth oxide. These interactions are pivotal in improving the structural and functional properties of RPET.
UV–Vis spectroscopy and optical band gap analysis
Figure 4a showing the absorbance spectra plots for recycled polyethylene terephthalate (RPET) and Nd2O3-doped RPET samples. The absorbance spectra display how the material absorbs light across the UV–Vis spectrum. The x-axis represents the wavelength of light (in nm), while the y-axis shows the absorbance. The pure RPET sample shows a characteristic peak in the UV region, which corresponds to electronic transitions within the material. The addition of Nd2O3 at different weight percentages (1 wt.%, 2 wt.%, 4 wt.%, and 8 wt.%) alters the absorbance profile. As the doping level increases, there might be a slight shift in the absorption edge, indicating changes in the electronic structure and interaction of Nd2O3 with RPET. The intensity of absorbance in some regions increases with doping, which could be due to the presence of Nd2O3 nanoparticles introducing localized states or enhancing light absorption.
The UV–Vis absorbance spectra of the samples (Fig. 4a) were used to calculate the optical energy band gap (E) of RPET. The absorption spectrum model was applied to the UV absorption data, enabling the determination of the optical gap energy based solely on the samples’ absorbance measurements52,53,54.
The optical band gap depends on the wavelength (λ) of the incident photon and is expressed by the relation:
Figure 4b–f illustrates the variation of photon energy (hν) versus (αhν)2 for pure RPET and Nd2O3-doped RPET. The band gap values as in Table 3 are derived from the Tauc plots, highlighting the influence of Nd2O3 doping on the electronic transitions within RPET. The direct band gap of pure RPET is measured as 3.75 eV. Upon doping with Nd2O3 at various weight percentages (1, 2, 4, and 8 wt.%), the band gap decreases slightly to 3.69, 3.65, 2.5, and 2.25 eV, respectively. This reduction is attributed to the displacement of the absorption edge, indicating a decrease in the gap energy with increasing Nd2O3 content. The reduction in the optical band gap confirms the presence of significant interactions between Nd2O3 and the RPET polymer matrix. These interactions lead to modifications in the optical properties of RPET. Shifts observed in the XRD patterns and FT-IR spectra further support changes in the crystalline structure and molecular interactions due to doping. The narrowing of the band gap with Nd2O3 incorporation enhances the material’s electronic conductivity, suggesting potential applications in optoelectronic devices. The combined effects of optical, structural, and molecular changes make Nd2O3-doped RPET a promising material for advanced technological applications.
Table 3 shows that, the gradual reduction in band gap energy with increasing Nd2O3 doping levels highlights the significant impact of dopant incorporation on the electronic structure of RPET. The introduction of defect states by Nd2O3 enables lower-energy electronic transitions, enhancing the optical properties of the material. This modification makes Nd2O3-doped RPET a promising candidate for advanced applications requiring improved visible light absorption, such as in photovoltaic devices and photocatalytic processes.
Electrical properties
Dielectric properties provide crucial insights into materials for electronic and insulating applications. The dielectric constant (∈′) reflects a material’s ability to store electrical energy under an applied electric field, while the dielectric loss (∈′′) indicates energy dissipation in an alternating electric field. The dielectric loss tangent (tan δ), the ratio of ∈′′ to ∈′, highlights the material’s energy efficiency and stability, particularly at temperatures like 30 °C and 100 °C. AC conductivity (σac) reveals the response of Recycled PET (RPET) doped with Nd2O3 to alternating current, offering insights into charge transport and dielectric behavior55.
Figure 5a illustrates the variation of the dielectric constant (∈′) with frequency at 30 °C for RPET samples doped with different concentrations of Nd2O3. Pure RPET; The dielectric constant (∈′≈150) remains stable across all frequencies, indicating a high and consistent polarization ability with minimal frequency dependence. RPET with 1 wt.% Nd2O3; ∈′ decreases significantly compared to pure RPET, especially at higher frequencies (f > 10 kHz), suggesting that the Nd2O3ions at this concentration do not significantly enhance dielectric properties. RPET with 2 wt.% Nd2O3; ∈′ increases (∈′≈130) compared to 1 wt.% Nd2O3, showing slight frequency dependence and some dielectric relaxation effects. RPET with 4 wt.% Nd2O3; the dielectric constant decreases further, but a more pronounced frequency dependence emerges, with ∈′ decreasing at higher frequencies. RPET with 8 wt.% Nd2O3; This sample exhibits the lower ∈′ among the doped samples, but a significant drop in ∈′ is observed at higher frequencies (f > 100 kHz), indicating strong dielectric relaxation effects. For all doped samples, ∈′ decreases with increasing frequency, reflecting typical dielectric relaxation behavior56. This behavior is typical of dielectric materials, where dipole orientation cannot keep pace with rapidly changing electric fields at higher frequencies, leading to a decrease in the dielectric constant. At lower frequencies, dipoles align with the field, enhancing polarization and increasing ∈′. Adding Nd2O3 to RPET improves ∈′ due to its high polarizability, but at very high frequencies, even samples with the highest Nd2O3 content show significant reductions (∈′ < 20), reflecting the limitations of polarization mechanisms46.
Figure 5b shows the dielectric loss (∈′′) vs. frequency for RPET doped with varying Nd2O3 concentrations at 30 °C. Pure RPET exhibits low, stable ∈′′, indicating minimal energy dissipation. At 1 wt.% Nd2O3, ∈′′ fluctuates, especially at lower frequencies (f < 10 kHz), suggesting increased dissipation. For 2 wt.% NNd2O3, ∈′′ remains higher than pure RPET but stabilizes at higher frequencies. At 4 wt.%Nd2O3, ∈′′ becomes more frequency-dependent, indicating greater dissipation. The 8 wt.% Nd2O3 sample shows the highest ∈′′, decreasing with frequency but remaining higher overall. For all samples, ∈′′ decreases as frequency increases due to limited dipole reorientation at high frequencies. Higher Nd2O3 content increases ∈′′ due to added dipoles and defects, enhancing energy dissipation, particularly at low frequencies where dipoles align more easily with the field.
Figure 5c shows the dielectric loss tangent (tan δ) vs. frequency for RPET and RPET-doped with varying Nd2O3 concentrations at 30 °C. Pure RPET exhibits low, stable tan δ, indicating minimal energy dissipation relative to stored energy. At 1 wt.% Nd2O3, tan δ increases slightly, particularly at lower frequencies (f < 10 kHz). With 2 wt.% Nd2O3, tan δ remains higher than pure RPET but stabilizes at higher frequencies (f > 10 kHz). At 4 wt.% Nd2O3, tan δ becomes more frequency-dependent, indicating greater relative energy dissipation. The highest doping (8 wt.% Nd2O3) shows the largest tan δ, decreasing with frequency but remaining above other samples. Tan δ generally decreases with increasing frequency as dipole reorientation lags behind the rapidly changing electric field. Higher Nd2O3 concentrations increase tan δ due to added dipoles and defects, raising energy dissipation relative to stored energy57.
Figure 5d presents AC conductivity (σac) vs. frequency for RPET with different Nd2O3 concentrations. At low frequencies (f < 10 kHz), pure RPET shows low σac due to limited charge carrier mobility. At higher frequencies (f > 10 kHz), σac rises due to enhanced dipole relaxation and charge hopping. Adding 1 wt.% Nd2O3 slightly increases σac, indicating improved charge carrier density or mobility. For 2 wt.% and 4 wt.% Nd2O3, σac rises further, reflecting better charge transport mechanisms, especially at high frequencies. At 8 wt.% Nd2O3, σac is highest, with significant contributions from free charge carriers and improved mobility. σac increases with frequency for all samples, driven by enhanced dipole relaxation and charge hopping. Higher Nd2O3 concentrations boost σac by increasing charge carriers and altering the polymer matrix, facilitating conduction. These trends suggest Nd2O3-doped RPET’s potential for applications requiring tailored electrical properties, particularly at high frequencies58.
Figure 6a illustrates the dielectric constant for RPET and Nd2O3-doped RPET at 100 °C. At low frequencies (f < 10 kHz), pure RPET shows a higher dielectric constant due to active polarization mechanisms. Adding Nd2O3 (1 wt.%) increases the dielectric constant, while further increases in Nd2O3 (up to 8 wt.%) progressively enhance polarization and energy storage. At higher frequencies (f > 10 kHz), the dielectric constant decreases as dipoles fail to align with the alternating field. Doping with Nd2O3mitigates this decline, with higher concentrations maintaining better frequency stability. Higher temperatures enhance dipole mobility, increasing the dielectric constant at low frequencies, but the lag in reorientation at high frequencies causes a more noticeable reduction. Overall, Nd2O3 improves electrical properties, enabling high energy storage and stability across frequencies and temperatures59.
Figure 6b highlights the dielectric loss (∈′′) behavior. At low frequencies, pure RPET exhibits high losses due to dipole and ionic conduction. Increasing Nd2O3 content amplifies energy dissipation, with 8 wt.% showing the highest losses. At high frequencies, losses decrease as dipoles and ions fail to keep up with the field, though higher Nd2O3 levels retain greater dissipation.
Dielectric loss tangent and AC conductivity
Figure 6c presents the dielectric loss tangent (tan δ), with pure RPET showing significant energy dissipation relative to storage at low frequencies. Adding Nd2O3 increases tan δ, with higher concentrations maintaining energy dissipation mechanisms at both low and high frequencies. This indicates improved ionic conduction and dipole activity60.
Figure 6d shows AC conductivity (σac) behavior. Pure RPET has low σac at low frequencies due to limited charge carrier mobility. Nd2O3 doping enhances σac by introducing or mobilizing charge carriers. Higher Nd2O3 levels lead to improved charge transport across all frequencies, with the highest doping (8 wt.%) showing the most pronounced increases. Temperature (100 °C), frequency, and doping level significantly influence dielectric properties. Higher temperatures enhance dipole and charge carrier mobility, while increasing Nd2O3 concentrations improve polarization, energy dissipation, and charge transport. These findings highlight Nd2O3-doped RPET as a promising material for applications requiring stable dielectric and electrical performance across a wide frequency and temperature range46.
Dielectric properties with temperature
Figure 7a shows the dielectric constant (∈′) of pure RPET increases slightly with temperature (T < 50 °C) due to enhanced dipolar polarization. Adding Nd2O3 increases ∈′, with higher doping levels (1–8 wt.%) showing progressively greater and more stable values over a wide temperature range. At high temperatures (T > 50 °C), pure RPET shows a decline in ∈′ due to molecular relaxation and degradation. Doped samples maintain better thermal stability, with 8 wt.% Nd2O3 exhibiting the highest ∈′ and best thermal stability due to a high density of polarizable entities61.
Figure 7b presented the dielectric loss (∈′′) increases with temperature for all samples. Pure RPET shows low ∈′′ at low temperatures, increasing due to molecular motion and dipolar relaxation. Doped samples exhibit higher ∈′′ due to energy dissipation from Nd2O3. Higher doping levels (4–8 wt.%) show greater ∈′′ across the temperature range, with 8 wt.% Nd2O3 displaying the most pronounced energy dissipation29.
Figure 7c depicted the dielectric loss tangent (tan δ) follows trends similar to ∈′′. Pure RPET shows low values that increase with temperature due to dipolar relaxation. Doping with Nd2O3 enhances tan δ, with higher concentrations (4–8 wt.%) leading to greater energy dissipation. The 8 wt.% Nd2O3 sample shows the sharpest increase in tan δ, indicating significant energy loss mechanisms.
Figure 7d shows the AC conductivity (σac) of pure RPET is low but increases with temperature due to charge carrier mobility. Doping with Nd2O3 significantly enhances σac, with higher doping levels (4–8 wt.%) leading to a pronounced nonlinear increase. The improved conductivity is attributed to the introduction of charge carriers by Nd2O3 and enhanced interaction with the polymer matrix. In summary, Nd2O3 doping improves the dielectric and conductivity properties of RPET, with higher doping levels providing greater stability and performance across a broad temperature range62.
Dynamic mechanical analysis (DMA)
Dynamic mechanical analysis (DMA) provides valuable insight into the impact of adding Nd2O3 to recycled PET (RPET) nanocomposite. The storage modulus (E') is a measure of a material’s ability to store and release elastic energy when subjected to dynamic deformation. It represents the stiffness of the material under oscillatory stress and is a key parameter in DMA. In polymers like RPET, a higher storage modulus indicates greater rigidity or elasticity, while a lower value suggests more flexibility or reduced stiffness. The storage modulus is temperature-dependent and reflects changes in the material’s structural properties under varying conditions62.
DMA was used to characterize the Nd2O3-doped RPET nanocomposites polymer at different temperatures (35–100 °C) under a strain of 2 × 10−5, with an angular frequency of 5.25 rad/s, a heating rate of 1 °C/min, and a stabilization time of 3 min. The temperature dependence of the storage modulus (Eʹ) for the RPET and Nd2O3-doped RPET composites was studied, as shown in Fig. 8a. For the pristine recycled RPET polymer, the storage modulus (E') of the pure RPET sample has a relatively high value (∼1.62 GPa) at room temperature (35 °C). As the temperature increases up to 100 °C, Eʹ decreases drastically to (1.18 GPa) about 22% of its value at room temperature. Other samples of Nd2O3-doped RPET nanocomposite, the storage modulus (Eʹ) at room temperature decreased to 0.5 MPa for Nd2O3-RPET sample. The impact of Nd2O3 on the mechanical properties of the RPET is very clear for all samples, it was improved the storage modulus for application of the RPET at room temperature. This decrease can be attributed to structural softening caused by the increased volume available for the motion of the molecular main chains. The same behavior is observed for all the Nd2O3-doped RPET composites63,64.
(a) The temperature dependence of the storage modulus at an angular frequency of 5.25 rad/s of pure RPET and RPET with additives of Nd2O3 (1, 2, 4 and 8 wt.%). (b) The dependence of the storage modulus at an angular frequency of 5.25 rad/s on the wt.% content of pure RPET and RPET with additives of Nd2O3 (1, 2, 4 and 8 wt.%) at 35℃.
However, the relation between the storage modulus (Eʹ) and Nd2O3-contents was illustrated in Fig. 8b. The storage modulus (Eʹ) decreases with increasing Nd2O3 content linearly. At room temperature (∼35 °C), the storage modulus decreases from 1.6 GPa for the pure RPET sample to approximately 0.26 GPa for the sample doped with 8 wt.% Nd2O3. This reduction may be attributed to structural softening that likely accompanies the addition of Nd2O3. this finding confirmed thermal stability of the Nd2O3-PET for application in infrastructure and housing as supporting walls64.
Dynamic mechanical analysis (DMA) studies on various nanocomposites have provided valuable comparative insights into the impact of fillers like Nd2O3, carbon nanotubes (CNTs), and others on the storage modulus and overall mechanical properties of polymers like RPET65. This study indicates that the addition of Nd2O3 decreases the storage modulus of RPET from 1.62 GPa to approximately 0.26 GPa with 8 wt.% Nd2O3 at 35 °C, attributed to structural softening. Similarly, for PET/CNT nanocomposites, it was observed that increasing CNT content up to 5 wt.% enhanced the storage modulus by up to 1.2 times at room temperature, showing a reinforcing effect66. In another case, storage modulus changes in PMMA/PS blends highlighted how interfacial properties between incompatible components significantly alter stiffness67.
The comparative study highlights the advancements achieved in this work on RPET/Nd2O3composites compared to PBMA/Nd-TiO2 systems as in Table 4. XRD analysis reveals that Nd2O3 significantly enhances the crystallinity of RPET, contributing to improved mechanical and thermal stability, similar to the effect of Nd-TiO2 in PBMA. FTIR spectra confirm molecular-level interactions in both systems, with new peaks at 535 cm−1 for RPET/Nd2O3 and 665 cm−1 for PBMA/Nd-TiO2, indicating structural modifications that enhance material properties. UV–Vis studies show a reduction in the optical band gap for RPET/Nd2O3 (minimum 2.25 eV at 8 wt.%), suggesting potential optoelectronic applications, while PBMA/Nd-TiO2 exhibits a concentration-dependent absorption edge shift. Dielectric studies demonstrate that RPET/Nd2O3 achieves its highest dielectric constant at 8 wt.% but exhibits frequency-dependent relaxation, whereas PBMA/Nd-TiO2 shows optimal performance at 7 wt.% before agglomeration impacts properties. This work underscores the potential of Nd2O3 to enhance RPET’s functional and structural properties for advanced applications.
Conclusion
Doping RPET with Nd2O3 significantly enhances its optical, dielectric, mechanical, and electrical properties. Higher Nd2O3 concentrations decrease the dielectric constant and improve thermal stability. AC conductivity and dielectric loss tangent (tan δ) also improved with doping. Higher Nd2O3 concentrations enhance charge carrier mobility and energy dissipation, particularly at elevated temperatures (e.g., 80–100 °C). At 200 kHz, doping decreases the dielectric constant due to enhanced charge mobility, with higher concentrations delaying degradation at extreme temperatures. Optical band gap energies changed from 3.75 eV for pure-RPET to 3.69, 3.65, 2.5, and 2.25 eV for 1, 2, 4, and 8wt.% of Nd2O3 doped-samples, respectively. These findings demonstrate the potential of Nd2O3-doped RPET in applications requiring optimized dielectric and conductive properties over wide temperature and frequency ranges. Dynamic mechanical analysis (DMA) studies confirmed the addition of Nd2O3 decreases the storage modulus of RPET from 1.62 GPa to approximately 0.26 GPa with 8 wt.% Nd2O3 at 35 °C. These improvements suggest that Nd2O3-doped RPET is suitable for applications requiring low storage modulus and thermal stability. This study aligns with the 3R (Reduce-Recycle-Reuse) principles, highlighting the potential of recycled materials in high-performance applications while supporting sustainability efforts.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Fellner, J. & Brunner, P. H. Plastic waste management: is circular economy really the best solution?. J. Mater. Cycles Waste Manag. 24(1), 1–3 (2022).
Rahman, M. H. & Bhoi, P. R. An overview of non-biodegradable bioplastics. J. Clean. Prod. 294, 126218 (2021).
Dey, S. et al. Degradation of plastics waste and its effects on biological ecosystems: A scientific analysis and comprehensive review. Biomed. Mater. Dev. 2(1), 70–112 (2024).
Kehinde, O. et al. Plastic wastes: environmental hazard and instrument for wealth creation in Nigeria. Heliyon 6(10), e05131 (2020).
Gopinath, K. P. et al. A critical review on the influence of energy, environmental and economic factors on various processes used to handle and recycle plastic wastes: Development of a comprehensive index. J. Clean. Prod. 274, 123031 (2020).
Monkul, M. M. & Özhan, H. O. Microplastic contamination in soils: A review from geotechnical engineering view. Polymers 13(23), 4129 (2021).
Thompson, R. C. et al. Plastics, the environment and human health: current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 364(1526), 2153–2166 (2009).
Ayeleru, O. O. et al. Challenges of plastic waste generation and management in sub-Saharan Africa: A review. Waste Manag. 110, 24–42 (2020).
Kenawy, E.-R. et al. Electrospinning of polyaniline/poly acrylonitrile/reduced graphene oxide doped with aluminium oxide nanoparticles for supercapacitors application. J. Inorg. Organomet. Polym. Mater. https://doi.org/10.1007/s10904-024-03402-y (2024).
Salam, O. A. et al. A comparative study of PMMA/PEG polymer nanocomposites doped with different oxides nanoparticles for potential optoelectronic applications. Sci. Rep. 14(1), 19295 (2024).
Kamoun, E. A. et al. Effect of gamma irradiation on the electrical and optical properties of PEVA composite membrane embedded with conductive copper fluoroborate glass powder. Mater. Adv. 5(13), 5658–5670 (2024).
Khalaf, M. et al. Polyelectrolyte membranes based on phosphorylated-PVA/cellulose acetate for direct methanol fuel cell applications: synthesis, instrumental characterization, and performance testing. Sci. Rep. 13(1), 13011 (2023).
Ali, A. I. et al. Dielectric and dynamic antibacterial investigations of organic–inorganic conductive membranes based on oxidized cellulose with BNKT nanoceramics. Cellulose 30(14), 9027–9046 (2023).
Marathe, K., Chavan, K. R. & Nakhate, P. Life cycle assessment (LCA) of PET bottles. In Recycling of Polyethylene Terephthalate Bottles 149–168 (Elsevier, 2019).
Ferrara, C., De Feo, G. & Picone, V. LCA of glass versus pet mineral water bottles: An Italian case study. Recycling 6(3), 50 (2021).
Evode, N. et al. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 4, 100142 (2021).
Narancic, T. & O’Connor, K. E. Plastic waste as a global challenge: are biodegradable plastics the answer to the plastic waste problem?. Microbiology 165(2), 129–137 (2019).
Kibria, M. G. et al. Plastic waste: challenges and opportunities to mitigate pollution and effective management. Int. J. Environ. Res. 17(1), 20 (2023).
Mihai, F.-C. et al. Plastic pollution, waste management issues, and circular economy opportunities in rural communities. Sustainability 14(1), 20 (2021).
Singh, N. et al. Nano revolution: exploring the frontiers of nanomaterials in science, technology, and society. Nano-Struct. Nano-Objects 39, 101299 (2024).
Mohan, S. et al. Biopolymers–application in nanoscience and nanotechnology. Recent Adv. Biopolym. 1(1), 47–66 (2016).
Babatunde, D. E. et al. Environmental and societal impact of nanotechnology. IEEE Access 8, 4640–4667 (2019).
Sharma, A. et al. Cutting edge technology for wastewater treatment using smart nanomaterials: recent trends and futuristic advancements. Environ. Sci. Pollut. Res. 31, 58263–58293 (2024).
Suhailath, K., Thomas, M. & Ramesan, M. T. Effect of temperature on AC conductivity of poly(butyl methacrylate)/cerium dioxide nanocomposites and applicability of different conductivity modeling studies. Res. Chem. Intermed. 46(5), 2579–2594 (2020).
Suhailath, K., Bahuleyan, B. K. & Ramesan, M. T. Synthesis, characterization, thermal properties and temperature-dependent AC conductivity studies of poly (butyl methacrylate)/neodymium oxide nanocomposites. J. Inorg. Organomet. Polym. Mater. 31(1), 365–374 (2021). https://doi.org/10.1007/s10904-020-01665-9.
Nandi, S. S. et al. Rare earth based nanocomposite materials for prominent performance supercapacitor: a review. Appl. Mech. Mater. 908, 3–18 (2022).
Li, B. et al. Exploring the influence of polymeric and non-polymeric materials in synthesis and functionalization of luminescent lanthanide nanomaterials. Coord. Chem. Rev. 514, 215922 (2024).
Suhailath, K. & Ramesan, M. T. Effect of ceria nanoparticles on mechanical properties, thermal and dielectric properties of poly (butyl methacrylate) nanocomposites. Polym. Compos. 41(6), 2344–2354 (2020).
Ramesan, M. T. & Sampreeth, T. Synthesis, characterization, material properties and sensor application study of polyaniline/niobium doped titanium dioxide nanocomposites. J. Mater. Sci. Mater. Electron. 28(21), 16181–16191 (2017).
Rai, P. K. et al. Micro-and nano-plastic pollution: Behavior, microbial ecology, and remediation technologies. J. Clean. Prod. 291, 125240 (2021).
Carroccio, S. C. et al. Impact of nanoparticles on the environmental sustainability of polymer nanocomposites based on bioplastics or recycled plastics–A review of life-cycle assessment studies. J. Clean. Prod. 335, 130322 (2022).
Ahire, S. A. et al. The augmentation of nanotechnology era: A concise review on fundamental concepts of nanotechnology and applications in material science and technology. Results Chem. 4, 100633 (2022).
Tegart, G. Nanotechnology: the technology for the twenty-first century. Foresight 6(6), 364–370 (2004).
Suhailath, K. & Ramesan, M. T. Effect of neodymium-doped titanium dioxide nanoparticles on the structural, mechanical, and electrical properties of poly(butyl methacrylate) nanocomposites. J. Vinyl Addit. Technol. 25(1), 9–18 (2019).
Suhailath, K. et al. Synthesis by in situ-free radical polymerization, characterization, and properties of poly (n-butyl methacrylate)/samarium-doped titanium dioxide nanoparticles composites. Adv. Polym. Technol. 37, 1114–1123 (2018).
Pathan, H. & Lokhande, C. Deposition of metal chalcogenide thin films by successive ionic layer adsorption and reaction (SILAR) method. Bull. Mater. Sci. 27, 85–111 (2004).
Korotcenkov, G. Handbook of II-VI Semiconductor-Based Sensors and Radiation Detectors: Volume 1, Materials and Technology (Springer Nature, 2023).
Okasha, N. et al. Comparative study on the influence of rare earth ions doping in Bi0. 6Sr0. 4FeO3 nanomultiferroics. J. Alloys Compd. 689, 1051–1058 (2016).
AboZied, A.E.-R.T. et al. Structure, magnetic and magnetocaloric properties of nano crystalline perovskite La0. 8Ag0. 2MnO3. J. Magn. Magn. Mater. 479, 260–267 (2019).
Ali, A. I. et al. Ferroelectric enhancement of La-doped BaTiO3 thin films using SrTiO3 buffer layer. Thin Solid Films 551, 127–130 (2014).
Karuppiah, H. Neodymium Oxide Thin Film Gate Oxide on Silicon Substrate (University of Malaya, 2018).
El-Tantawy, A. et al. Low cost paints reinforced with an Al2O3/Y2O3/graphene nanocomposite for fire-resistant wood coating applications. Mater. Adv. 5(18), 7377–7386 (2024).
Stefanov Y. The Application of Atomic Force Microscopy in Semiconductor Technology-Towards High-K Gate Dielectric Integration. (2012). https://tuprints.ulb.tu-darmstadt.de/2931/1/PhD_Thesis.pdf.
Reddy, D. S. et al. Modification of interface properties of Au/n-GaN Schottky junction by rare-earth oxide Nd2O3 as an interlayer and its microstructural characterization. Vacuum 215, 112300 (2023).
Yang, J. -J. et al. Effective Modulation of Quadratic Voltage Coefficient of Capacitance in MIM Capacitors Using Sm2O3/SiO2 Dielectric Stack. IEEE Electron Device Lett. 30, 460–462 (2009). https://doi.org/10.1109/LED.2009.2015970.
Sima, W. et al. Investigation of dielectric properties of polyethylene terephthalate under different aging temperatures. IEEE Trans. Dielectr. Electric. Insul. 24(5), 3015–3023 (2017).
Park, C. et al. Effects of mechanicl stresses on the dielectric breakdown strengths of PET and FRP. IEEE Trans. Electr. Insul. 3, 234–240 (1982).
Weyer, L. & Lo, S. Spectra-structure correlations in the near-infrared. Handb. Vib. Spectrosc. 3, 1817–1837 (2002).
Chatjigakis, A. et al. FT-IR spectroscopic determination of the degree of esterification of cell wall pectins from stored peaches and correlation to textural changes. Carbohydr. Polym. 37(4), 395–408 (1998).
Yang, C. Q. Characterizing ester crosslinkages in cotton cellulose with FT-IR photoacoustic spectroscopy 1. Text. Res. J. 61(5), 298–305 (1991).
Wang, K. et al. High value-added conversion and functional recycling of waste polyethylene terephthalate (PET) plastics: a comprehensive review. J. Environ. Chem. Eng. 12, 113539 (2024).
Sammon, C., Yarwood, J. & Everall, N. An FT–IR study of the effect of hydrolytic degradation on the structure of thin PET films. Polym. Degrad. Stab. 67(1), 149–158 (2000).
Awaja, F. & Pavel, D. Recycling of PET. Eur. Polym. J. 41(7), 1453–1477 (2005).
Wang, Y. & Lehmann, S. Interpretation of the structure of poly (ethylene terephthalate) by dynamic FT-IR spectra. Appl. Spectrosc. 53(8), 914–918 (1999).
Neagu, E. et al. Dielectric relaxation spectroscopy of polyethylene terephthalate (PET) films. J. Phys. D Appl. Phys. 30(11), 1551 (1997).
Singh, K. & Gupta, P. Study of dielectric relaxation in polymer electrolytes. Eur. Polym. J. 34(7), 1023–1029 (1998).
Kopřiva J et al. Comparison of Dielectric Properties of Pure and Modified PET-G Materials for 3D Prototyping. In 2024 IEEE International Conference on High Voltage Engineering and Applications (ICHVE). IEEE. (2024).
Park, E. S. Morphology, mechanical, and dielectric breakdown properties of PBT/PET/TPE, PBT/PET/PA66, PBT/PET/LMPE, and PBT/PET/TiO2 blends. Polym. Compos. 29(10), 1111–1118 (2008).
Pop, T., Iordache, D. & Jonas, A. Dielectric properties of PET below its glass transition temperature. Microelectron. Eng. 33(1–4), 377–384 (1997).
Kosloski-Oh, S. C. et al. Catalytic methods for chemical recycling or upcycling of commercial polymers. Mater. Horiz. 8(4), 1084–1129 (2021).
Hafiz, M. et al. Synthesis, Structure, and Gamma-Ray Shielding Properties of Phosphate Glass Doped with Naturally Extracted Neodymium. J. Rad. Nucl. Appl. 3, 255–264 (2024). https://doi.org/10.18576/jrna/090309.
Anis, A. et al. Mouldable conductive plastic with optimised mechanical properties. Polymers 16(3), 311 (2024).
Chan, K. & Zinchenko, A. Design and synthesis of functional materials by chemical recycling of waste polyethylene terephthalate (PET): Opportunities and challenges. J. Clean. Prod. 433, 139828 (2023).
Enache, A.-C., Grecu, I. & Samoila, P. Polyethylene terephthalate (PET) recycled by catalytic glycolysis: A bridge toward circular economy principles. Materials 17(12), 2991 (2024).
Li, X. et al. High-toughness and antistatic PET/EMAG/CNTs nanocomposites from recycled sources by reactive compatibilization. Compos. Commun. 47, 101855 (2024).
Dorigato, A. & Fredi, G. Effect of nanofillers addition on the compatibilization of polymer blends. Adv. Ind. Eng. Polym. Res. 7(4), 405–427 (2024).
Huang, C.-H. et al. Design of super-toughened, heat-resistant and antistatic polyethylene terephthalate-based blend composites by constructing a tenacious interface. Polymer 277, 125966 (2023).
Acknowledgements
This work was not supported by any organizations. Software and Equipment utilization support from the Physics Research Institute, National Research Centre (NRC), Cairo, Egypt, is gratefully appreciated.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Amr Antar, Galal H. Ramzy, Medhat A. Ibrahim, Nasser Ayoub, and M. M. Maghawry: investigation, methodology, characterization. Ahmed I. Ali and Jong Yeog Son write the original draft, Ahmed I. Ali, Amr Antar, Medhat A. Ibrahim, and Jong Yeog Son reviewed & edited the final version of the manuscript. Dongwhi Choi and Ahmed I. Ali revised experimental results, reviewed & edited the revised version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Antar, A., Ibrahim, M.A., Maghawry, M.M. et al. Functionalized recycled polyethylene terephthalate plastic by rare earth oxide for electronic device and housing infrastructure applications. Sci Rep 15, 10156 (2025). https://doi.org/10.1038/s41598-025-91895-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91895-z
Keywords
This article is cited by
-
Tailoring bandgap and dielectric properties of RPET using lanthanum oxide nanofillers for UV-shielding and optoelectronic applications
Journal of Materials Science: Materials in Electronics (2025)