Abstract
To evaluate the clinical performance and safety of the ONIRY system for obstetric anal sphincter injuries (OASI) detection versus three-dimensional endoanal ultrasound (EAUS). A prospective, comparative, multicentre, international study. Poland, Czechia, Slovakia, and Spain. 152 women between the first moments up to 8 weeks after vaginal delivery. Participants underwent EAUS and were allocated to groups based on OASIS classification: A (no perineal tear), B (1st or 2nd degree tear), or C (3rd or 4th degree, anal sphincters affected). Electric impedance was measured in the anal canal using the ONIRY system. The primary endpoint was the diagnostic outcome of impedance spectroscopy versus EAUS. Adverse events were collected. Part II involved in silico modelling and 10-time 10-fold cross-validation for automated analysis. Accuracy, sensitivity, and specificity. 30 women were allocated to group A, 61 to group B, and 61 to group C. The diagnostic outcome was determined for 147 participants. The accuracy, sensitivity, and specificity of the ML-assisted impedance spectroscopy were 87.0 ± 0.5%, 90.6 ± 2.0%, and 84.6 ± 1.9%, respectively, compared with EAUS. After data cleaning, the performance metrics of the proposed final ML model for ONIRY were: 90.0 ± 0.4%, 90.0 ± 1.2%, and 90.0 ± 0.7%, respectively. No adverse device effects or deficiencies were observed. By enabling early identification of sphincter injuries, ML-assisted impedance spectroscopy facilitates timely diagnosis and intervention, potentially reducing long-term complications such as faecal incontinence. Its rapid, bedside application in obstetric settings supports immediate postpartum care, complementing digital rectal examination and optimizing clinical decision-making.
Similar content being viewed by others
Introduction
Obstetric anal sphincter injuries (OASIs) are a serious yet often undiagnosed complication of vaginal delivery, directly implicated in various degrees of continence issues, including frank faecal incontinence (FI). It is estimated that one in four women who undergo vaginal delivery experience some form of OASI1,2,3,4, placing them at a 25–50% risk of developing FI, either shortly after delivery5,6 or later in life7,8,9,10. FI has a profoundly negative impact on quality of life, affecting women’s social interactions, professional activities, family dynamics, and intimate relationships11,12,13.
Despite this significant risk, early and reliable postpartum detection of OASI remains a critical gap in maternal care. There is no widely accessible, objective diagnostic tool that enables timely and accurate identification of OASI before complications arise. Currently, obstetricians rely on digital rectal examination (DRE), the only available assessment method in maternity settings, which is highly dependent on the examiner’s experience and skills. As a subjective method, DRE lacks reliability, with primary detection failure rates for OASI reported as high as 80%10.
The lack of an effective early detection tool contributes to delayed diagnosis, missed treatment opportunities, and a higher burden of FI among postpartum women9,10. While endoanal ultrasound (EAUS) is the gold standard for diagnosing anal sphincter injuries14,15, with near-perfect sensitivity in identifying structural abnormalities, it is nor routinely used in obstetric care. Its limited accessibility is primarily due to a shortage of trained specialists and the technical challenges of interpreting perianal imaging in the immediate postpartum period, when swelling, blood, and tissue changes obscure visibility16.
Nonetheless, as highlighted in a Cochrane review17, EAUS demonstrates clinical value when used immediately after childbirth, before perineal repair, as it can reduce the rate of severe FI at 6-month follow-up. Over the past decade, transperineal ultrasound (TPUS) has been explored as a more accessible alternative to EAUS for identifying OASI, demonstrating good correlation with EAUS results18,19. Studies indicate that when TPUS is performed before sphincter repair, it detects more OASIs than DRE alone20. However, both EAUS and TPUS require specialized training and interpretation, which limits their widespread adoption in real-world obstetric care.
Addressing this unmet need requires a rapid, objective, and easy-to-use bedside diagnostic tool that can be applied in standard maternity settings, reducing reliance on subjective examination and ensuring timely intervention. To this end, a novel approach using electric impedance spectroscopy has been developed, as this technique is an established method for assessing tissue condition21,22, and has been successfully applied in various medical fields23,24,25,26. This technique applies a sinusoidal electrical current below the sensation threshold to the body at various frequencies, measuring the impedance response to infer tissue characteristics. Despite its broad medical applications, impedance spectroscopy has never been explored for perianal diagnostics. A proof-of-concept study involving 22 patients, followed by two pilot clinical studies using prototype devices on a total of 69 postpartum women, demonstrated the validity of this method27,28,29,30. However, distinguishing between normal and injured tissue required advanced signal processing techniques. Due to subtle differences in impedance values high sensitivity and specificity were achieved only through the application of nonlinear machine learning (ML) algorithms for test interpretation.
Prototypes of this new diagnostic tool, called the ONIRY system, were designed and developed, with the ML module progressively trained on clinical data to refine its accuracy27,28. The overall concept for this system is to serve as a rapid, accessible, and objective bedside method for early OASI detection, addressing the longstanding gap in postpartum care.
This study aims to evaluate the clinical performance and safety of the ONIRY system—a rapid OASI detection device incorporating impedance spectroscopy and ML—against EAUS as the reference diagnostic method, following re-training of the ML model on a larger, balanced postpartum population.
Materials and methods
A prospective, comparative, multicenter, international clinical study was designed, composed of two parts: the clinical conduct (Part I) and modelling and ML (Part II). Part I of the study was conducted from 2021 to 2022 at five European centers in Czech Republic, Slovakia, Poland, and Spain. The study design and conduct were in line with the Good Clinical Practice guidelines for medical device studies (ISO 14155:2020) and conducted in accordance with the Declaration of Helsinki. All approvals by the national regulatory authorities as well as positive opinions by the ethics committees, per local regulations, were obtained prior to study initiation. Written informed consent was collected from each study participant before enrolment. The study was registered at ClinicalTrials.gov under NCT04903977 (27/05/2021).
Study design
The study was designed to enroll a total of approximately 150 women between 18 and 49 years old, primiparous, or multiparous, from the first moments up to 8 weeks after vaginal (spontaneous or assisted) delivery of a singleton, live fetus, in any presentation, in gestational week 34 or higher. All inclusion and exclusion criteria are listed in Supporting Information S1, along with the detailed study plan.
Three study groups were pre-defined, with fixed numbers of participants enrolled as to ensure generation of balanced data from women with and without OASI. Participants were initially enrolled in these groups based on the evaluation made immediately after delivery according to the 4-degree perineal tears scale31: approximately 30 women were planned for group A (no visible perineal tear), approximately 60 for group B (clinically detectable first- or second-degree perineal tear, including episiotomy), and approximately 60 for group C (clinically detectable third- or fourth-degree perineal tear, involving anal sphincters, regardless of primary repair. Specifically, including women with pre-existing primary repairs in Group C was intentional and considered necessary for ML training purposes, as muscle tissue that has been approximated by sutures differs from healthy tissue and should be distinguishable. Furthermore, even after initial sphincter repair, the sphincters’ integrity may remain compromised (in 40–71% of cases) if their continuity is not fully restored32,33.
The study duration for each participant was from 2 days up to 5 weeks and included 3 study visits. The first visit, occurring anytime from the immediate postpartum period up to 8 weeks post-delivery, involved the collection of medical history, including pregnancy and birth details, a comprehensive physical examination with proctological and gynaecological assessment, a 12-lead electrocardiogram (ECG) recording, and an evaluation of clinical FI symptoms using the Wexner score. At this initial visit, three dimensional (3-D) EAUS was also performed, serving as the reference diagnostic method and as the final tool for study group allocation.
Following such allocation (per EAUS-based OASIS classification34,35), participants underwent impedance spectroscopy using the ONIRY system at the second visit, which took place on the same day as the first visit or up to 7 days later. A web-based application was utilized to provide preliminary test interpretation, allowing the operator to experience an immediate OASI detection result from the ONIRY system (indicating either OASI detected or not detected). These preliminary interpretations were generated from an ML model trained on data from previous pilot studies, which could differ from the final interpretation based on the refined ML model trained during Part II of this study.
To explore the reproducibility of impedance measurements, two consecutive measurement runs were conducted per participant. An arbitrary reproducibility criterion was applied, whereby any difference greater than 1 kΩ at the frequency of 1 kHz between the first and second runs was noted as a discrepancy.
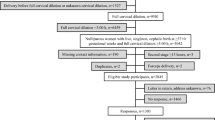
A 12-lead ECG was repeated immediately after impedance measurement with the ONIRY system. Faecal calprotectin levels and blood morphology parameters were also measured to assess any correlation with tissue electrical impedance results as potential confounding factors. At the third study visit, which occurred between 0 and 28 days after the second visit, anal sphincter function was evaluated using high-resolution anorectal manometry. This procedure was optional, depending on availability at each study site. Vital signs were assessed at each study visit to monitor participant safety throughout. The sequence of study procedures is summarized in Fig. 1.
Due to the nature of the study design, no blinding was deemed feasible. However, it was not considered essential for maintaining objectivity of study outcomes, as the preliminary interpretation of impedance measurements displayed by the ONIRY system was generated independently based on the ML model trained in prior pilot studies. This interpretation was thus uninfluenced by the test operator and unaffected by knowledge of the EAUS results at study entry. To further minimize any potential bias associated with using EAUS as the primary reference method, a specific technical control measure ensured that the ONIRY examination could only proceed once the EAUS results and interpretation were finalized and entered into the electronic Case Report Form.
Study endpoints
The diagnostic outcome of the ONIRY examination compared to 3-D EAUS assessed using the OASIS classification was set as primary endpoint and used for the conclusion on the diagnostic performance of the ONIRY system (following the application of the ML algorithms re-trained in Part II of the study). For the EAUS-based Diagnostic Outcome, OASI was considered detected as long as any depth, length, or circumference range of either anal sphincter (external or internal) was captured (score > 2 by OASIS classification). OASI was considered detected if any depth, length, or circumferential involvement of either the external or internal anal sphincter was observed (OASIS classification score > 2).
Secondary endpoints related to diagnostic performance assessed in this study, including Diagnostic Outcomes using other reference methods such as digital rectal examination and high-resolution anorectal manometry, were used for respective ML models construction but are not included in this report.
Adverse Events (AE) were recorded for each participant from the time of enrolment until the last study visit.
Impedance spectroscopy system
Impedance spectroscopy was performed on each study participant using the ONIRY system, which consists of three components: the impedance spectrometer, the endoanal probe, and the ML module. Note that ONIRY is a proprietary name, not an abbreviation. The spectrometer generates a sinusoidal current in the 1–100 kHz frequency range with an amplitude below sensation and pain thresholds, enabling tissue impedance measurement.
The endoanal probe, made from biocompatible, rigid plastic, measures 12 mm in diameter at the electrode site (with a head diameter of up to 19 mm) and contains 8 stainless steel electrodes. These electrodes allow the measurement of impedance modulus, phase shift, resistance, and reactance within the perianal area. The probe also features a handle with a positioning marker to ensure correct placement in the anal canal (see Fig. 2).
During examination, the probe is inserted into the anal canal for approximately one minute, remaining stationary throughout the measurement. The examination is performed with the patient in a supine position or lying on her left side with knees flexed, depending on operator preference (see Fig. 3).
The spectrometer captures raw impedance data through the endoanal probe, which are then processed to determine statistical parameters for various frequency sub-compartments. These processed parameters serve as an input vector for the ML model, which is trained to analyze subtle differences in impedance patterns that distinguish injured tissue from healthy (or repaired) tissue. The ML model processes these patterns across a complex multi-dimensional dataset, refining its analysis with each frequency parameter to classify tissue integrity accurately.
Following the impedance measurement, the ONIRY system, supported by the ML model, determines whether OASI is present and outputs either “PASS” (no OASI detected) or “REFER” (OASI detected). The ML algorithm is also equipped with control protocols that prevent measurement if improper probe placement is detected, including misalignment or incomplete insertion. The complete ONIRY system setup is illustrated in Fig. 4.
Endoanal ultrasound
3-D EAUS, although not typically performed immediately postpartum, remains the only objective, high-precision method for detecting OASI and is thus the gold standard for OASI diagnosis. Despite practical limitations, EAUS was selected as the optimal reference standard for obstetric care in this study to train the ML model and achieve the highest possible diagnostic efficiency for the ONIRY system.
All EAUS examinations were conducted by experts with at least 20 years of experience in perianal imaging. Imaging included assessments of the external and internal anal sphincters as well as the puborectalis muscle. To ensure consistency, the 3-D EAUS procedures followed a standardized study-specific protocol (detailed in a study manual implemented across all centres). The 3-D EAUS results were interpreted according to the OASIS classification32,33 for the primary endpoint.
In addition to the OASIS classification, each EAUS examination was assessed using the Starck scale36 and the Norderval scale37, well-regarded semi-quantitative scoring systems established for EAUS. These alternative assessments allowed for exploratory analyses to determine whether substituting these scores for the OASIS classification could enhance the ONIRY system’s diagnostic performance. Corresponding ML models were also constructed based on these additional scores, allowing for further evaluation in exploratory studies.
Statistical analysis
For calculation of diagnostic performance metrics for ONIRY, the Diagnostic Outcome was determined separately for each performance endpoint, as:
-
1.
Diagnostic Success: presence (True Positive) or absence (True Negative) of OASI consistently detected by the ONIRY examination and the reference diagnostic method, or.
-
2.
Diagnostic Failure: mismatch (False Positive or False Negative) of the OASI detection by the ONIRY examination and the reference diagnostic method, or.
-
3.
Diagnostic Indeterminate: no ONIRY or reference diagnostic method result available or interpretable.
For the primary endpoint, 3-D EAUS result (by OASIS classification) served as the reference method. Evaluation of the exploratory endpoints (diagnostic outcome with 3-D EAUS evaluated with Starck36 or Norderval37 scales) was performed accordingly.
Accuracies were defined as Diagnostic Successes/Total, sensitivities as True Positives/(True positives + False Negatives), and specificities as True Negatives/(True Negatives + False Positives). Also, the F1 score, and Matthew’s Correlation Coefficient (MCC)38 were calculated.
The safety profile of ONIRY system was evaluated using descriptive statistics.
Data analysis (part I) and machine learning modelling (part II)
Details of the algorithm used to interpret impedance examination results in Part I of the study, based on the preliminary ML model trained with data from two prior pilot clinical studies, are provided in Supporting Information S2. In Part II, in silico analyses included exploratory data analysis, dimensionality reduction, ML modeling, and final performance evaluation with 10-fold cross-validation; full methodological details are available in Supporting Information S3.
For the per-patient reproducibility analysis, if a significant discrepancy was found between the two impedance measurement runs from the same participant, the second measurement run was excluded from the analysis dataset used to develop the final algorithm (note: in real-world application, a single measurement run will be performed per patient).
Results
Study population
Of one hundred fifty-three participants screened, 152 were enrolled (1 screening failure). Following 3-D EAUS with OASIS classification, participants were allocated as follows: 30 in Group A (no visible perineal tear and no OASI), 61 in Group B (clinically detectable first- or second-degree perineal tear but no OASI), and 61 in Group C (clinically detectable third- or fourth-degree perineal tear with OASI).
Table 1 presents the key characteristics of the study population by group and overall.
Fifteen participants discontinued the study prematurely: 3 in Group A (10.0%; 3/10), 10 in Group B (16.4%; 10/61), and 2 in Group C (3.3%; 2/61), with all discontinuations occurring between the second and last visits. Thus, as all these participants had undergone both EAUS and impedance examinations, they were included in the Clinically Evaluated Population.
Part I (clinical conduct)
All enrolled participants were evaluable for the primary endpoint as well as safety outcomes. Diagnostic Success or Failure was determined for 147 participants. For the remaining 5 participants (enrolled at 3 study sites), the Diagnostic Outcome was Indeterminate due to electric impedance measurements falling outside the spectrometer’s expected range, unrelated to the anal canal tissue impedance profile. Thus, the primary endpoint was assessed in 60 participants with OASI (Group C) and 87 participants without OASI (Groups A and B).
Using the preliminary ML model trained on data from prior pilot studies, diagnostic performance metrics were observed as follows: sensitivity of 66.7%, specificity of 57.5%, accuracy of 61.2%, F1 score of 0.58, and MCC of 0.24 (see Supporting Information S4 for detailed metrics).
No significant correlation was found between faecal calprotectin levels and the results of wither EAUS or ONIRY examinations.
Part II (in silico machine learning modelling)
The ultimate performance analysis was conducted with an ML model trained on data generated in Part I of this study, incorporating both raw impedance data from ONIRY and OASI detection results from EAUS as a reference standard. A total of 298 impedance measurements meeting quality requirements (see Supporting Information S5) were included: 122 from participants with OASI (group C) and 176 from participants without OASI (group A and B). Following exploratory data analysis, dimensionality reduction, and modelling using artificial neural networks, diagnostic metrics showed marked improvement, achieving sensitivity slightly above 90% and specificity slightly below 85%, as presented in Table 2 (with individual cross-validation statistics available in Supporting Information S6).
The exploratory per-patient reproducibility analysis from Part I revealed discrepancies between the two impedance measurement runs in 19 participants (12.8%). In exploratory analyses using alternative EAUS classification scores, performance metrics based on the Starck36 and Norderval37 classifications were assessed. Using the Starck classification sensitivity was 83.8%, specificity 88.1%, accuracy 86.4%, F1 score 0.83, and MCC 0.73. For the Norderval classification, sensitivity reached 84.3%, specificity 89.3%, accuracy 87.4%, F1 score 0.84, and MCC 0.74.
Safety results
No deaths, serious AE, or AE leading to premature withdrawal from the study were reported during the study conduct. A total of 22 AE were observed in 21 participants: 4 AE in group A, 5 in group B, and 13 in group C. All AE occurred after the ONIRY examinations. Four types of AE were reported by more than one participant, with the most common being nasopharyngitis (2.6%, 4/152) and COVID-19 (2.6%, 4/152).
None of AE were considered related to the ONIRY system, and no adverse device effect were reported.
No trends were observed in ECG parameters when comparing baseline recordings (pre-ONIRY examination) with post-ONIRY recordings. Additionally, no clinically significant cardiovascular abnormalities were recorded as an AE following ONIRY application.
Post-hoc analysis
Following exploratory analyses of per-patient reproducibility in impedance measurements, 19 measurement files (each from the second measurement per relevant patient) were excluded from the final ML model for the ONIRY system, resulting in a “limited dataset” comprising 93.6% (279/298) of the original dataset. The limited dataset included 279 impedance measurements: 117 corresponding to OASI and 162 with no OASI. A post-hoc performance analysis using cross-validations on this refined ML model demonstrated diagnostic metrics with both sensitivity and specificity of ONIRY system at 90.0%, as detailed in Table 3 (individual cross-validation statistics are provided in Supporting Information S6).
Discussion
Key findings
The ML-assisted impedance spectroscopy using ONIRY system demonstrated high diagnostic performance for OASI detection, when compared to EAUS in the study population enriched with OASI cases (40.1%, 61/152 of enrolled women). Following re-training of the ML model in Part II with data generated in Part I, the system achieved an accuracy of 87.0 ± 0.5%, with a sensitivity of 90.6 ± 2.0%, and specificity of 84.6% ± 1.9%. After refining the dataset by excluding 6.4% (19/298) of impedance measurements due to measurement discrepancies, the optimized ML model intended for the final ONIRY system reached an accuracy of 90.0 ± 0.4%, with both sensitivity and specificity at 90% (sensitivity 90.0 ± 1.2%, specificity 90.0 ± 0.7%). This significant improvement, particularly after re-training the ML model with a well-balanced dataset, highlights the importance of balanced and representative training data in machine learning-driven diagnostics.
The initial lower performance observed in Part I of the study (accuracy of 61.2%), where the ML model was trained on smaller, less diverse pilot datasets27,28,29,30, underscores the need for larger, well-balanced training cohorts to enhance model robustness. The improved performance of the final ML model in this study likely reflects the broader variability of raw impedance data collected and the higher representation of OASI cases, which better supported model optimization.
Exploratory analyses using alternative EAUS reference methods (Starck and Norderval classifications with corresponding alternative ML models) did not demonstrate additional diagnostic advantages over the primary performance metrics.
The safety profile of the ONIRY system was also confirmed, with no adverse events related to the device. The few reported adverse events were unrelated to ONIRY, and no device deficiencies were recorded. Future studies in unselected postpartum cohorts will be essential to confirm the real-world applicability of ONIRY.
Clinical implications
Currently, no rapid easy-to-use diagnostic tool is available in maternity care settings for whole obstetric team, beyond digital rectal examination. Although DRE is a standard procedure, recommended in most obstetric guidelines39,40,41,42,43, it has significant limitations due to its subjective nature with sensitivity for detecting OASI heavily dependent on the examiner’s experience44,45,46. Hence the crucial importance of practical training and programmes dedicated to midwives and obstetricians aimed at increasing the OASI detection rate and the effectiveness of its management47,48.
Although EAUS, as the gold standard for detecting OASI, is rarely feasible in the early postpartum period due to resource and operational constraints, as well as challenges to interpret images in the immediate postpartum hours49 - its value in accurately detecting even minor injuries is undeniable. Typically, EAUS is more suitable later in the postpartum period, around 6–8 weeks after delivery, when patients return with symptoms of incontinence or perineal wound healing issues, which are current indications for an EAUS assessment.
As demonstrated in this study, the ML-supported impedance spectroscopy, providing a straightforward interpretation of the perianal tissue impedance results, has shown high diagnostic accuracy, achieving approximately 90% sensitivity and specificity (compared with 3-D EAUS, per OASIS classification, the only available objective reference) during the whole postpartum period (from the first hours up to 8 weeks after delivery).
Unlike DRE, impedance spectroscopy is not examiner-dependent and provides automated, standardized results, reducing variability between different healthcare providers. Unlike EAUS, ONIRY does not require advanced imaging interpretation, making it a practical, accessible diagnostic option for maternity care settings. Given that the entire ONIRY examination—including automated analysis and result generation—takes under a minute, it has the potential for widespread adoption in routine postpartum assessments.
One of the most promising applications of ONIRY is its use within the first 24 h postpartum, when early detection of OASI allows for primary sphincter repair, significantly reducing the risk of long-term FI. A systematic review by Walsh et al.17 highlighted that EAUS performed immediately postpartum, before perineal repair,, before any perineal repair, significantly increases OASI detection rates and improves primary repair outcomes. However, EAUS is rarely available in the maternity setting, leaving most obstetric teams without an effective tool for immediate postpartum OASI diagnosis. ONIRY could fill this critical gap, enabling timely, evidence-based clinical decisions.
Beyond the immediate postpartum period, ONIRY could also serve as a valuable screening tool in the weeks following delivery. Many occult OASI cases remain undiagnosed until women develop incontinence symptoms months or even years later, making early detection critical for long-term pelvic floor health. By bridging the gap until EAUS becomes feasible, ONIRY could identify asymptomatic or underdiagnosed cases, ensuring that at-risk women receive appropriate follow-up care, targeted rehabilitation, or specialist referral before symptoms of FI. This expanded diagnostic window could fundamentally improve the standard of postpartum care, shifting the focus from reactive symptom management to proactive early intervention.
From a cost-effectiveness standpoint, ONIRY represents a practical supplement to standard of care particularly in low-resource or high-volume maternity units, where access to specialized imaging is limited. The implementation of ONIRY could help reduce long-term healthcare costs by preventing chronic FI, minimizing the need for complex surgical interventions, and improving overall maternal quality of life. Future health-economic analyses are warranted to assess the financial benefits of integrating ONIRY into routine postpartum protocols.
What favours EAUS as the diagnostic method is the possibility to separately visualise both external and internal anal sphincters. This enables targeted repairs of the external sphincter alone or combined repair of both sphincters, making EAUS an essential tool for elective diagnosis before any delayed sphincter repair. However, it is not a practical solution for routine OASI screening in maternity settings. ONIRY does not replace EAUS but complements it, serving as an intermediate step between DRE and advanced imaging, ensuring better patient triaging and reducing missed diagnoses.
If impedance spectroscopy proves successful in clinical practice, it could not only enhance postpartum management in maternity care settings but also facilitate timely referrals to specialist surgical units, ultimately improving long-term outcomes for women affected by OASI. Because ONIRY provides automated, rapid interpretation of impedance data in a simple binary output (PASS/REFER), it has potential for routine use by a wider range of maternity care staff, even those with minimal specialized training.
In this study, no device-related safety concerns were identified, and ONIRY was well tolerated by all participants. While no absolute contraindications for ONIRY use were observed, caution is advised in cases involving:
-
Women with implanted pelvic devices or metallic implants, which could theoretically interfere with impedance readings.,
-
Severe perianal malformations, where altered tissue structures may affect measurement accuracy, or.
Patients using electronic medical implants, particularly those generating alternating currents above 1 kHz, to avoid potential electromagnetic interference. These considerations will be addressed in future risk assessments and protocol refinements to ensure the broadest possible applicability of ONIRY in routine obstetric practice.
This study represents the first clinical validation of impedance spectroscopy for OASI detection in a broader postpartum population. Future studies are planned, focusing on:
-
The critical time window within the first few hours postpartum, to further refine the ML model’s efficacy at a point when early OASI detection offers the most benefit—allowing for timely primary repair.
-
Expanding ONIRY validation in an unselected postpartum population, ensuring its effectiveness in diverse maternity care settings.
-
Conducting longitudinal follow-up studies to assess how early OASI detection influences long-term pelvic floor function and FI prevalence.
-
Developing standardized clinical protocols for integrating ONIRY into maternity care guidelines, ensuring routine adoption by midwives and obstetricians.
Limitations and research implications
This study has several limitations.
Firstly, the study population was selectively enriched with OASI cases (Group C, n = 61) to improve the dataset’s internal balance and increase the robustness of the ML model. This approach, common in AI and ML tool development50, ensures higher internal data coherence, enhancing the model’s effectiveness within a controlled dataset structure.
Secondly, the primary endpoint focused on OASI detection rather than a clinically meaningful outcome, such as faecal incontinence (FI) or quality of life measures. The surrogate endpoint was chosen to facilitate a clear comparison between the ONIRY system and 3-D EAUS, the gold standard, within a manageable timeframe. Establishing a clinically meaningful benefit for the ONIRY system would require extended, long-term follow-up studies, spanning years to account for the gradual development of FI in women with OASI. The potential benefit of rapid OASI detection hinges on the availability and prompt implementation of therapeutic measures, notably primary sphincter repair within the optimal 8–12 h window after delivery34,35,39,40,41,42,43.
Another limitation arises from the high proportion of participants in Group C (52 out of 61) who had already undergone primary sphincter repair before study enrolment. This factor may have influenced impedance spectroscopy results toward readings typical of non-injured cases. However, given that the ML models were trained with this dataset, it is plausible that the ONIRY system’s performance could be even greater in real-world use, where OASI detection would ideally occur before repair. Newly injured, unrepaired sphincters are likely to present a clearer contrast in impedance measurements compared to tissues that have already undergone repair.
The difference in median time between delivery and ONIRY examination across groups (28 days in Group C, versus 2 and 11 days in Groups A and B, respectively) reflects challenges encountered in recruiting cases of 3rd and 4th degree perineal tears (Group C) in this challenging study design. This delay also mirrors the lengthy diagnostic and therapeutic pathway typically experienced by OASI patients in current practice.
In addition, the inclusion of participants with previously repaired OASI in Group C captures clinical scenarios where underdiagnosed or inadequately repaired OASIs persist despite initial repair. Literature indicates that primary repair is incomplete in over 30% of cases, often due to the limited experience of the operator or the emergency nature of the procedure itself, usually performed during on-call hours51,52,53. Thus, classifying cases with recent repairs in the “injured” group is methodologically sound and aligns with real-world conditions.
Finally, no detailed data were captured for multiparous women on potentially undetected OASIs from prior deliveries. This could theoretically result in normal impedance readings due to tissue healing, despite persistent EAUS abnormalities. Conversely, abnormal impedance with normal EAUS could indicate fibrosis from a prior injury.
Conclusions
The ML-assisted impedance spectroscopy demonstrated safety and high diagnostic accuracy, achieving approximately 90% sensitivity and specificity in detecting obstetric anal sphincter injuries in women after vaginal birth. This approach could effectively complement digital rectal examination in obstetric settings, supporting timely postpartum care.
Data availability
The data that support the findings of this study are not openly available due to legal and privacy issues. Data requests can be made to the corresponding author.
Abbreviations
- AE:
-
Adverse Events
- EAUS:
-
Endoanal Ultrasound
- FI:
-
Faecal Incontinence
- MCC:
-
Matthew’s Correlation Coefficient
- ML:
-
Machine Learning
- OASIs:
-
Obstetric Anal Sphincter Injuries
References
Sideris, M. et al. Risk of obstetric anal sphincter injuries (OASIS) and anal incontinence: A meta-analysis. Eur. J. Obstet. Gynecol. Reproductive Biol. 252, 303–312. https://doi.org/10.1016/j.ejogrb.2020.06.048 (2020).
Gurol-Urganci, I. et al. Third- and fourth-degree perineal tears among primiparous women in England between 2000 and 2012: Time trends and risk factors. BJOG 120(12), 1516–1525. https://doi.org/10.1111/1471-0528.12363 (2013).
Dudding, T. C., Vaizey, C. J. & Kamm, M. A. Obstetric anal sphincter injury: Incidence, risk factors, and management. Ann. Surg. 247(2), 224–237. https://doi.org/10.1097/SLA.0b013e318142cdf4 (2008).
Orlando, A., Thomas, G. & Murphy, J. I Wsp. A systematic review and a meta-analysis on the incidence of obstetric anal sphincter injuries during vaginal delivery. Colorectal Dis. 26, 227–242 (2024).
Everist, R. et al. Postpartum anal incontinence in women with and without obstetric anal sphincter injuries. Int. Urogynecol. J. 31(11), 2269–2275. https://doi.org/10.1007/s00192-020-04267-8 (2020). Epub 2020 Mar 10.
Richter, H. E. et al. Incidence and predictors of anal incontinence after obstetric anal sphincter injury in primiparous women. Female Pelvic Med. Reconstr. Surg. 21(4), 182–189. https://doi.org/10.1097/SPV.0000000000000160 (2015).
Pollack, J. et al. Anal incontinence after vaginal delivery: A five-year prospective cohort study. Obstet. Gynecol. 104(6), 1397–1402. https://doi.org/10.1097/01.AOG.0000147597.45349.e8 (2004).
Jangö, H., Langhoff-Roos, J., Rosthøj, S. & Sakse, A. Recurrent obstetric anal sphincter injury and the risk of long-term anal incontinence. Am. J. Obstet. Gynecol. 216(6), 610. https://doi.org/10.1016/j.ajog.2017.02.010 (2017).
Nilsson, I. E., Åkervall, S., Molin, M., Milsom, I. & Gyhagen, M. Symptoms of fecal incontinence two decades after no, one, or two obstetrical anal sphincter injuries. Am. J. Obstet. Gynecol. 224(3), 276–e1 (2021).
Guzmán Rojas, R. A., Salvesen, K. Å. & Volløyhaug, I. Anal sphincter defects and fecal incontinence 15–24 years after first delivery: A cross-sectional study. Ultrasound Obstet. Gynecol. 51(5), 677–683. https://doi.org/10.1002/uog.18827 (2018).
Meyer, I. & Richter, H. E. Impact of fecal incontinence and its treatment on quality of life in women. Women’s Health (London) 11(2), 225–238. https://doi.org/10.2217/WHE.14.66 (2015).
Jangö, H. et al. Wexner score and quality of life in women with obstetric anal sphincter injury. Int. Urogynecol. J. 31(6), 1115–1121. https://doi.org/10.1007/s00192-019-04134-1 (2020).
Lo, J. et al. Quality of life in women with postpartum anal incontinence. Obstet. Gynecol. 115(4), 809–814. https://doi.org/10.1097/AOG.0b013e3181d4160d (2010).
Santoro, G. A. et al. State of the art: An integrated approach to pelvic floor ultrasonography. Ultrasound Obstet. Gynecol. 37, 381–396 (2011).
Albuquerque, A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J. Gastrointest. Endosc. 7(6), 575–581. https://doi.org/10.4253/wjge.v7.i6.575 (2015).
Sioutis, D., Thakar, R. & Sultan, A. H. Overdiagnosis and rising rate of obstetric anal sphincter injuries (OASIS): Time for reappraisal. Ultrasound Obstet. Gynecol. 50, 642–647. https://doi.org/10.1002/uog.17306 (2017).
Walsh, K. A. & Grivell, R. M. Use of endoanal ultrasound for reducing the risk of complications related to anal sphincter injury after vaginal birth. Cochrane Database Syst. Rev. 10, CD010826. https://doi.org/10.1002/14651858.CD010826.pub2 (2015).
Ignell, C., Örnö, A. K. & Stuart, A. Correlations of obstetric anal sphincter injury (OASIS) grade, specific symptoms of anal incontinence, and measurements by endoanal and transperineal ultrasound. J. Ultrasound 24(3), 261–267. https://doi.org/10.1007/s40477-020-00485-4 (2021).
Taithongchai, A. et al. Comparing the diagnostic accuracy of 3 ultrasound modalities for diagnosing obstetric anal sphincter injuries. Am. J. Obstet. Gynecol. 221(2), e1–134 (2019).
Wong, K. W., Thakar, R., Sultan, A. H. & Andrews, V. Can transperineal ultrasound improve the diagnosis of obstetric anal sphincter injuries? Int. Urogynecol J. Aug 2. (2022). https://doi.org/10.1007/s00192-022-05290-7
Shiffman, C. A. & Aaron, R. Low-impedance localized measurements using standard bioelectrical impedance analysis instruments. Ann. NY Acad. Sci. 904, 214–217. https://doi.org/10.1111/j.1749-6632.2000.tb06453.x (2000).
Aaron, R. & Shiffman, C. A. Using localized impedance measurements to study muscle changes in injury and disease. Ann. NY Acad. Sci. 904, 171–180. https://doi.org/10.1111/j.1749-6632.2000.tb06443.x (2000).
Seward, B., Rutkove, M. D., Aaron, R. & Shiffman, C. A. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve 25, 390–397. https://doi.org/10.1002/mus.10048 (2002).
Młyńczak, M. & Cybulski, G. Decomposition of the cardiac and respiratory components from impedance pneumography signals. Proc. of the 10th Int. Joint Conf. on Biomedical Engineering Systems and Technologies – Biodevices. 4, 26–33. (2017). https://doi.org/10.5220/0006107200260033
Kushner, R. F. & Schoeller, D. A. Estimation of total body water by bioelectrical impedance analysis. Am. J. Clin. Nutr. 44(3), 417–424. https://doi.org/10.1093/ajcn/44.3.417 (1986).
Tidy, J. A. et al. Accuracy of detection of high-grade cervical intraepithelial neoplasia using electrical impedance spectroscopy with colposcopy. BJOG 120(4), 400–410. https://doi.org/10.1111/1471-0528.12096 (2013). discussion 410-1.
Młyńczak, M., Borycka-Kiciak, K., Uchman-Musielak, M. & Dziki, A. Impedance spectroscopy method to detect pelvic floor muscle Damage - A feasibility study. In: World Congress on Medical Physics and Biomedical Engineering, Springer, Singapore, 875–878. doi: https://doi.org/10.1007/978-981-10-9038-7_161. (2018).
Borycka-Kiciak, K., Młyńczak, M., Kiciak, A., Pietrzak, P. & Dziki, A. Non-invasive obstetric anal sphincter injury diagnostics using impedance spectroscopy. Sci. Rep. 9(7097), 1–9. https://doi.org/10.1038/s41598-019-43637-1 (2019).
Młyńczak, M. et al. Obstetric anal sphincter injury detection using impedance spectroscopy with the ONIRY probe. Appl. Sci. 11, 637. https://doi.org/10.3390/app11020637 (2021).
Borycka, K. et al. Impedance spectroscopy for the diagnosis of obstetric anal sphincter injuries: the pilot experience; In: 15 Congress of the European Society of Gynecology, 2023.
CunninghamFG et al. Williams Obstetrics (23rd Edition) (McGraw-Hill Medical, 2011).
Shek, K. L., Guzman-Rojas, R. & Dietz, H. P. Residual defects of the external anal sphincter following primary repair: An observational study using transperineal ultrasound. Ultrasound Obstet. Gynecol. 44(6), 704–709. https://doi.org/10.1002/uog.13368 (2014).
Gold, S., Paquette, J., Sobel, M. & Alarab, M. Residual defects of the anal sphincter complex following primary repair of obstetrical anal sphincter injuries at a large Canadian obstetrical centre. J. Obstet. Gynaecol. Can. 43(5), 596–600. https://doi.org/10.1016/j.jogc.2021.01.011 (2021).
The Management of Third-. And Fourth-Degree Perineal Tears: a Green-top Guideline No 29 (Royal College of Obstetrician and Gynaecologists, 2015).
Prevention and Management of Obstetric Lacerations at Vaginal Delivery. ACOG practice bulletin 165: American college of obstetricians and gynecologists’ committee on practice bulletins—Obstetrics. Obstet. Gynecol. 128(1), e1–e15. https://doi.org/10.1097/AOG.0000000000001523 (2016).
Starck, M., Bohe, M. & Valentin, L. Results of endosonographic imaging of the anal sphincter 2–7 days after primary repair of third or fourth-degree obstetric sphincter tears. Ultrasound Obstet. Gynecol. 22, 609–615. https://doi.org/10.1002/uog.920 (2003).
Norderval, S., Markskog, A., Rossaak, K. & Vonen, B. Correlation between anal sphincter defects and anal incontinence following obstetric sphincter tears: assessment using scoring systems for sonographic classification of defects. Ultrasound Obstet. Gynecol. 31, 78–84. https://doi.org/10.1002/uog.5155 (2008).
Chicco, D. & Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 21, 1–13 (2020).
Harvey, M. A. et al. Obstetrical anal sphincter injuries (OASIS): Prevention, recognition, and repair. J. Obstet. Gynecol. Can. 37(12), 1131–1148. https://doi.org/10.1016/S1701-2163(16)30081-0 (2015).
Kropshofer, S. et al. December. Management of Third and Fourth-Degree Perineal Tears After Vaginal Birth. Guideline of the DGGG, OEGGG and SGGG (S2k-Level, AWMF Registry No. 015/079, Geburtshilfe Und Frauenheilkunde. 2022;83(02):165–183. (2020). https://doi.org/10.1055/a-1933-2647
Department for Health and Wellbeing. South Australian Perinatal Practice Guideline - Third and fourth degree tear management 2018. Available from: www.sahealth.sa.gov.au/wps/wcm/connect/1faf87004eedec4db635b76a7ac0d6e4/Third+and+Fourth+Degree+Tear+Management_PPG_v5_1.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-1faf87004eedec4db635b76a7ac0d6e4-oc-RC8a
Queensland Clinical Guidelines. Queensland clinical guidelines: Perineal Care 2023. Available from: https://www.health.qld.gov.au/__data/assets/pdf_file/0022/142384/g-pericare.pdf
Danish Association of Obstetrics and Gynaecology. Guideline for obstetric anal sphincter injury (OASIS) 2019. Available from: https://nfog.org/wp-content/uploads/2019/03/190313-obstetric-anal-sphincter-injury.pdf
Jeppson, P. C., Paraiso, M. F. R., Jelovsek, J. E. & Barber, M. D. Accuracy of the digital anal examination in women with fecal incontinence. Int. Urogynecol. J. 23, 765–768 (2012).
Roper, J. C., Sultan, A. H. & Thakar, R. Diagnosis of perineal trauma: Getting it right first time. Br. J. Midwifery 28(10), 710–717 (2020).
Krissi, H., Aviram, A. & Hiersch, L. i wsp. Structured hands-on workshop decreases the over-detection rate of obstetrical anal sphincter injuries. Int. J. Colorectal Dis. 31, 45–50. (2016).
De Meutter, L., van Heesewijk, A. D., van der Woerdt-Eltink, I. & de Leeuw, J. W. Implementation of a perineal support programme for reduction of the incidence of obstetric anal sphincter injuries and the effect of non-compliance. Eur. J. Obstet. Gynecol. Reprod. Biol. 230, 119–123. https://doi.org/10.1016/j.ejogrb.2018.09.021 (2018).
Bidwell, P. et al. A multi-centre quality improvement project to reduce the incidence of obstetric anal sphincter injury (OASI): Study protocol. BMC Pregnancy Childbirth. 18(1), 331. https://doi.org/10.1186/s12884-018-1965-0 (2018).
Huber, M. et al. Use of endoanal ultrasound in detecting obstetric anal sphincter injury immediately after birth. Acta Obstet. Gynecol. Scand. 102, 389–395. https://doi.org/10.1111/aogs.14514 (2023).
Batista, G. E. A. P. A., Prati, R. C. & Monard, M. C. A study of the behavior of several methods for balancing machine learning training data. ACM SIGKDD Explorations Newsl. 6(1), 20–29. https://doi.org/10.1145/1007730.1007735 (2004).
Roper, J. C., Thakar, R. & Sultan, A. H. Under-classified obstetric anal sphincter injuries. Int. Urogynecol. J. 33(6), 1473–1479. https://doi.org/10.1007/s00192-021-05051-y (2022). Epub 2022 Feb 12.
O’Leary, B. D., Kelly, L., Fitzpatrick, M. & Keane, D. P. Underdiagnosis of internal anal sphincter trauma following vaginal delivery. Ultrasound Obstet. Gynecol. 61(2), 251–256. https://doi.org/10.1002/uog.26049 (2023).
Kirss, J., Pinta, T., Böckelman, C. & Victorzon, M. Factors predicting a failed primary repair of obstetric anal sphincter injury. Acta Obstet. Gynecol. Scand. 95(9), 1063–1069. https://doi.org/10.1111/aogs.12909 (2016).
Funding
The study was financed by the Polish National Centre for Research and Development (POIR.01.01.01-00-0726/18).
Author information
Authors and Affiliations
Contributions
Conceptualization: KB, MM, PIData curation: KB, MM, MR, KK, PIFormal analysis: MM, MR, KKFunding acquisition: KB, MMInvestigation: HH, PJ, MUM, ED, EGDMethodology: KB, MM, MR, KK, PIProject administration: KB, MMResources: HH, PJ, MUM, ED, EGDSoftware: MM, MR, KKSupervision: KB, MM, PI, CR, AS Validation: MM, PIVisualization: MR, KKWriting - original draft: KB, MM, MR, KK, PIWriting - review & editing: All co-authors.
Corresponding author
Ethics declarations
Competing interests
K.B. is a founder and board member at OASIS Diagnostics, an author of the related patent and R&D strategy, independent consultant, and trainer of Takeda.M.M., M.R., K.K., and P.I. are researchers at OASIS Diagnostics. A.S. is an independent consultant of Ethicon, Takeda, Pfizer, Sofar. M.UM. is an independent consultant of Regen Lab.H.H., P.J., M.UM., E.D., and E.GD. received remuneration as a study investigator.H.H., C.R., and A.S. are independent consultants and members of the OASIS Diagnostics’ Scientific Advisory Board. Others declare no conflicts of interest.
Ethics approval
The study was approved by the ethics committees respective for each study site: on 19 March 2021 by Ethics Committee of the Institute for Maternal and Child Care (no. 1/19.03.2021), on 27 April 2021 by Ethics Committee for Research with Medicines of the Health Areas of León and Bierzo (no. 2186), on 9 June 2021 by Ethics Committee of the University Hospital of Brno (no. 47/21Zdrav.), on 14 October 2021 by Ethics Committee at the Regional Medical Chamber in Warsaw (no. KB/1362/21) and on 25 July 2022 by Ethics Committee at AGEL Hospital Košice-Šaca (no. ONIRY 3/2/2020).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Borycka, K., Młyńczak, M., Rosoł, M. et al. Detection of obstetric anal sphincter injuries using machine learning-assisted impedance spectroscopy: a prospective, comparative, multicentre clinical study. Sci Rep 15, 7522 (2025). https://doi.org/10.1038/s41598-025-92392-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92392-z
Keywords
This article is cited by
-
Obstetric Anal Sphincter Injuries: A Urogynecologic Perspective on Detection and Diagnosis
International Urogynecology Journal (2025)