Abstract
The aim was to assess the clinical and radiographic peri-implant status among type-2 diabetic and non-diabetic individuals with major depressive disorders (MDD). Participants were divided into four groups; Group-1: patients with type-2 diabetes; Group-2: patients with MDD; Group-3: patients with type-2 diabetes and MDD; Group-4: healthy controls. Demographic data was collected, and medical history including most recent hemoglobin A1c (HbA1c) levels were retrieved from healthcare records. Peri-implant modified plaque and gingival indices (MPI and mGI) and peri-implant probing depth (PPD) were recorded; and crestal bone loss (CBL) was measured. Sample-size was estimated using data from a pilot investigation. Statistical analysis was performed using one way-analysis of variance and Bonferroni Post-hoc adjustment tests. P-vales below 0.05 were considered statistically significant. Thirty, 30, 30 and 30 individuals were included in groups 1, 2, 3 and 4. Mean HbA1c levels were higher in groups 1, 2 and 3 compared with Group-4 (P < 0.05). Thirty-seven, 40, 43 and 36 implants were present in groups 1, 2, 3 and 4, respectively. In groups 1, 2, 3 and 4, the implants were in function for a mean duration of 4.7 ± 2.4, 4.9 ± 1.8, 5.05 ± 1.7 and 10.6 ± 2.2 years, respectively. The mPI, mGI, PPD and CBL were significantly higher in groups 1, 2 and 3 than individuals in Group-4 (P < 0.05). There was significant correlation between peri-implant PD and HbA1c levels among individuals in Group-1 (P < 0.05). Peri-implant soft tissue and osseous statuses are compromised among patients with type-2 DM, and MDD regardless of whether these conditions occur individually or in combination. Clinical relevance: Peri-implant soft tissue and osseous statuses are compromised among patients with type-2 DM, and MDD regardless of whether these conditions occur individually or in combination.
Similar content being viewed by others
Introduction
Dental implants are a transformative solution in the field of prosthetic and restorative dentistry, providing a modern alternative to traditional fixed and removable dental prostheses such as dentures and bridges, respectively1,2,3. In a retrospective cohort study4, the cumulative survival rate of dental implants was nearly 96% after a decade of follow-up. In this study4, the authors concluded that the replacement of a single missing tooth in the maxilla and/or mandible with dental implants is a viable and safe treatment option. Poor routine oral hygiene maintenance is a classical risk-factor of periodontal and peri-implant diseases5,6,7; however, a compromised systemic health status has also been reported to jeopardize the success and survival of dental implants8,9.
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by a partial or absolute insulin insufficiency10; and type-2 DM is its most common form10. Individuals with a poor metabolic control of DM have an increased risk of demonstrating other systemic conditions such as cardiovascular and psychological diseases (e.g. major depressive disorders [MDD])11,12. The MDD is characterized by persistent feelings of sadness and loss of interest, significantly impairing daily functioning. Depression impacts approximately 280 million individuals globally and its prevalence is estimated to be approximately 75% among females from Saudi Arabia13,14. Studies15,16 have shown that patients with poorly-controlled type-2 DM are more likely to demonstrate periodontal and peri-implant diseases in contrast to individuals with well-controlled type-2 DM and non-diabetic subjects. According to Lorean et al.17 the risk of peri-implant crestal bone loss (CBL) is higher among type-2 diabetic individuals with hemoglobin A1c (HbA1c) levels over 8% compared with individuals well-controlled type-2 DM and non-diabetic controls17. Similarly, it has also been reported that the risk of implant failure is higher in patients with MDD using selective serotonin reuptake inhibitors (SSRIs) compared with healthy individuals18. In a recent analytical cross-sectional study, Strooker et al.19 assessed the association between psychological distress and peri-implant diseases. In this study, 230 with 347 implants were evaluated. Univariate analysis showed a significant association between peri-implantitis and MDD19. The authors concluded that presence of MDD is a risk-factor of peri-implantitis19. Likewise, in a retrospective study with 5-years’ follow-up, Deepa et al.20 reported that SSRIs exert a detrimental effect on bone metabolism and enhance osteoclastic activity, which are risk factors of peri-implant CBL and even implant failure. It is worth noting that a bidirectional relationship has been reported between MDD and type-2 DM21. According to Semenkovich et al.21. MDD is present in one of every four individuals with type-2 DM; and type-2 DM elevates the risk of MDD in vulnerable populations21. A careful review of indexed databases showed that to date there are no studies that have evaluated peri-implant soft tissue status and CBL among type-2 diabetic individuals with and without MDD. It is therefore likely that type-2 DM in conjunction with MDD worsens periodontal inflammation in the latter group or vis versa.
The aim of the present study was to assess the clinical and radiographic peri-implant status among type-2 diabetic and non-diabetic individuals with MDD. The present study is based on the null hypothesis that there is no difference in peri-implant clinical parameters (modified plaque index [mPI], modified gingival index [mGI] and peri-implant probing depth [PPD]) and CBL among type-2 diabetic individuals with and without DD.
Materials and methods
Ethical approval
The research study was reviewed and approved by the Institutional Review Board (IRB) at Najran University to ensure the protection of human subjects’ rights and welfare (Approval # 202412-076- 027132–059963). The study was performed in accordance with the regulations and guidelines of the Declaration of Helsinki22,23. Participants were informed that their involvement in the study was completely voluntary, and they were free to decline participation or withdraw at any stage without any consequences. Moreover, participants were made aware of their rights, such as the right to privacy and confidentiality, and the option to contact the IRB if they had any concerns, questions or complaints regarding the study. Individuals that agreed to participate were presented with a consent form and were requested to read and sign it. All participants were invited to ask questions before they signed the consent form.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (a) signing the informed consent form; (b) adult individuals (≥ 18 years); (c) individuals with medically-diagnosed type- 2 diabetic individuals; (d) individuals with medically-diagnosed MDD; (e) self-reported systemically healthy individuals; (f) individuals with at least one dental implant in function in the maxilla and/or mandible. The following exclusion criteria were implemented: (a) Individuals with self-reported systemic diseases other than type-2 DM (such as cardiovascular diseases, overweight/obesity, type-1 DM, prediabetes, renal/hepatic disorders, arthritic diseases, osseous disorders, and HIV/AIDS); (b) nursing and/or pregnant females; (c) individuals that reported to be smoking daily and/or using electronic nicotine delivery systems and smokeless tobacco products; (d) self-reported bisphosphonate users; and self-reported recreational drug users; and (e) third molars, grossly carious teeth and individuals with crowding and/or overlapping dentition; and (f) implants placed in grafted sites.
Study groups
Participants were divided into the following groups based upon their systemic health status: (a) Group-1: individuals with type-2 DM alone; (b) Group-2: individuals with MDD alone; (c) Group-3: type-2 diabetic patients with MDD; (d) Group-4: self-reported systemically healthy individuals.
Questionnaire
A pre-tested semi-structured questionnaire was used to collect information on (a) age in years; (b) gender; (c) duration and mode of management of type-2 DM, (d) duration and mode of management of MDD; (e) family history of psychological disorders and DM; (f) Frequency of daily toothbrushing and flossing; (g) most recent visit to a dentist/dental hygienist. The questionnaire was presented to all participants by one trained investigator.
Evaluation of healthcare records
Digital healthcare charts of all individuals were evaluated to gather the following information: (a) number of implants in function; (b) implant jaw location; (c) implant dimensions (diameter x length); (d) duration of implants in function; (e) implant retention protocol (cement versus-screw retained); (d) most recent HbA1c levels.
Clinical and radiographic periodontal and peri-implant evaluation
One examiner carried out a comprehensive evaluation of the peri-implant soft tissue status in the entire mouth after going through training and reliability tests to ensure consistency, reaching a kappa score of ≥ 0.80. The mPI and mGI were measured on four surfaces (mesial, distal, buccal, and lingual) of each tooth and implant, respectively24,25. The peri-implant PD (PPD) was measured at six sites per implant (mesiobuccal, buccal, distobuccal, mesiolingual, lingual, distolingual) using a plastic periodontal probe calibrated in millimeters (mm)26,27,28. The probe was gently inserted into the peri-implant sulcus until resistance was felt, and the corresponding depth was recorded. Digital bitewing radiographs were taken using the long-cone paralleling technique CBL were measured and recorded in millimeters.
Sample-size estimation
Sample-size estimation (SESE) was performed using the G*Power 3.129 utilizing data from a pilot investigation. A moderate effect size (Cohen’s d) of 0.5 was estimated for differences in peri-implant health indices among patients with and without MDD. The primary outcome variables were PPD and CBL and the desired power of the study was 0.80 (80%) to detect significant differences with an alpha set at 5%. It was estimated that a total of at least 120 participants (30, 30, 30 and 30 in groups 1, 2, 3 and 4, correspondingly) would be required to ensure sufficient power to detect significant differences in peri-implant health between the groups, accounting for potential dropouts.
Statistical analysis
Statistical analysis was done using computer software (SPSS Version 22, Chicago, IL., USA). For descriptive statistics, means and standard deviations were computed; and data normality was assessed using the Kolmogorov-Smirnov test. Group-comparisons were carried out using the one-way analysis of variance and for multiple comparisons, the Bonferroni Post-hoc-adjustment test was done. Logistic regression analysis was done to assess the correlation between peri-implant clinical and radiographic parameters and HbA1c levels, duration of MDD and dosage of SSRIs. P-values which were 0.05 were considered statistically significant.
Results
Participants and general characteristics of the study cohort
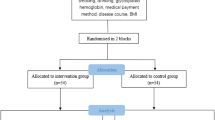
One hundred and sixty-seven individuals were invited to participate in the present study. Twenty-nine females declined participation without providing a reason. Twelve tobacco-smokers and six individuals with self-reported cardiovascular disorders were also excluded. In total, 120 individuals (51 males and 69 females) were included. Thirty, 30, 30 and 30 individuals were included in groups 1, 2, 3 and 4 (Fig. 1). There was no statistically significant difference in the mean ages of individuals in groups 1, 2, 3 and 4. In groups 1, 2, 3 and 4, number of female participants were 13, 21, 20 and 15, correspondingly. In groups 1 and 3, the duration of type-2 DM was 10.2 ± 3.7 and 8.9 ± 2.5 years, respectively (P = 0.14). In groups 2 and 3, the duration of MDD was 7.8 ± 1.9 and 8.3 ± 2.2 years, respectively (P = 0.17). The mean HbA1c levels were significantly higher in groups 1, 2 and 3 compared with Group-4 (P < 0.05). There was no statistically significant difference in the mean HbA1c levels among patients in groups 1, 2 and 3. The family history of DM was more often reported by individuals in Groups 1, 2 and 3 compared with controls (Group-4). A family history of psychological disorders was reported by none of the individuals I Group-4 compared with individuals in groups 1 (33.3%), 2 (40%) and 3 (70%). Toothbrushing twice daily was more often reported by individuals in Group-1 (73.3%) compared with individuals in groups 1 (23.3%), 2 (16.7%) and 3 (30%). Flossing of interproximal spaces was reported by none of the individuals in groups 1, 2 and 3; whereas 33.3% individuals in Group-4 reported that that were flossing once daily. Individuals in groups 1, 2 and 3 had last visited a dentist/dental hygienist nearly 24 months ago in contrast to individuals in Group-4 who had visited an oral healthcare provider nearly 6 months ago (Table 1). As per patients’ medical records, the HbA1c levels were measured within the past 110 ± 12.5 days. All individuals in groups 2 and 4 were using Tablet Fluoxetine (mean dosage 30 mg [range: 20–50 mg]) p.o. once daily since diagnosis of MDD. All patients with type-2 DM were prescribed anti-hyperglycemic medication (Tablet Metformin p.o. 500 mg once daily) and dietary instructions.
Implant related parameters
Thirty-seven, 40, 43 and 36 implants were present among individuals in groups 1, 2, 3 and 4, respectively. Of these, 18, 19, 19 and 20 implants were present in the posterior maxilla and 17, 17, 21 and 16 implants in the posterior mandible among individuals in groups 1, 2, 3 and 4, respectively. All implants were placed at bone level and inserted using a torque ranging between 30 and 35 Ncm. All implants were platform switched and had screw-retained restorations. In groups 1, 2, 3 and 4, the implants were in function for a mean duration of 4.7 ± 2.4, 4.9 ± 1.8, 5.05 ± 1.7 and 10.6 ± 2.2 years, respectively (Table 2).
Peri-implant clinical and radiographic parameters
Scores of mPI, mGI, PPD and mesial and distal CBL were significantly higher in groups 1, 2 and 3 compared with individuals in Group-4. There was no statistically significant difference in the mean scores of mPI, mGI, PPD, and mesial and distal CBL among patients in groups 1, 2 and 3 (Table 3).
Logistic regression analysis
There was statistically significant correlation between peri-implant PD and HbA1c levels among individuals in Group-1 (P < 0.05). There was no correlation between peri-implant PD and HbA1c levels among individuals in groups 2, 3 and 4 (Fig. 2). There was no correlation between age, gender, duration of type-2 DM and MDD, dosage of SSRIs, family history of type-2 DM and MDD, daily oral hygiene maintenance protocols (toothbrushing and flossing) and most recent visit to a dentist/dental hygienist and peri-implant mPI, mGI, PD and CBL in all groups (data not shown).
Discussion
Evidence from indexed scientific literature has shown that a state of hyperglycemia and well as use of SSRIs are independent risk factors of peri-implant diseases including CBL20,30. Persistent hyperglycemia induces a state of oxidative stress in tissues including those of the periodontium and use of SSRIs enhance osteoclastic activity20,31,32,33,34. It was therefore hypothesized that peri-implant soft tissue inflammatory parameters (mPI, mGI and PD) and crestal bone height are compromised to a much greater extent in type-2 diabetic SSRI-users in contrast to non-diabetic SSRI-users and type-2 diabetic individuals. However, results of the present retrospective observational clinical investigation showed otherwise. In summary, there was no statistically significant difference in peri-implant mPI, mGI, PD and CBL among patients in groups 1, 2 and 3; however, individuals in these groups displayed worse peri-implant clinical and radiographic parameters compared with self-reported systemically healthy controls (Group-4). It is however worth noting that the mean HbA1c levels were > 8% among individuals in groups 1 and 3 compared with individuals in Group-4. In other words, all participants in these groups had “poorly-controlled type-2 DM”. Studies35,36 have shown that poorly-controlled increases the production and expression of destructive inflammatory cytokines in the saliva and peri-implant sulcular fluid that enhance soft tissue inflammation as well as bone loss around dental implants. Interestingly, despite being non-diabetic, patients in Group-2 (individuals with MDD alone) showed no statistically significant difference in peri-implant inflammatory parameters when compared with groups 1 and 3. The potential role of SSRIs in augmenting the inflammatory cascade cannot be overlooked, it is pertinent to mention that these individuals had mean HbA1c levels exceeding the normal range (4 to 5.6%)37. In other words, patients in Group-2 were prediabetic or had an impaired glucose tolerance. Studies38,39,40 have shown that prediabetes by itself is a risk factor of poorer periodontal and peri-implant health status. Moreover, it has also been reported that the risk of developing type-2 DM is 60% higher in patients with a diagnosis of depressive disorders compared with individuals without depression41. The authors applaud the results by Nouwen et al.42 according to which, there is a bidirectional association between depressive disorders and DM and the risk of developing DM in patients with than without depressive disorders. It is also notable that in the present investigation, a family history of DM was more often reported by patients with MDD and vis versa as shown in Table 1. It is therefore hypothesized that individuals in Groups 1 and 2 are at a risk of developing MDD and DM (most likely type-2 DM), respectively in the future. In this context, it is proposed that a thorough evaluation of medical health tatus (including HbA1c) should be performed among patients with depressive disorders selected for undergoing future dental implant therapy. Such regimens may facilitate case selection and predict the long-term success and survival of dental implants in patients with depressive disorders.
Regarding treatment of MDD, all participants were prescribed fluoxetine as per their digital healthcare records. Fluoxetine mediates its antidepressant effects by binding to 5-hydroxytryptamine (5-HT) transporter to block the reuptake of 5-HT from the synaptic cleft, which in turn reduces symptoms of MDD43. Moreover, according to Liu et al.44 fluoxetine directly affects pancreatic beta-cells and therefore contributes in regulating glucose homeostasis. The present results are in contradiction to the results reported in the study by Liu et al.44 as mean HbA1c levels among individuals in groups 2 and 3 reflected poor glycemic control. It is demanding to derive an absolute explanation in this regard; however, it is perceived that individuals with MDD were either not taking the medications as prescribed or were not visiting their healthcare providers routinely and/or were not seeking medical attention (via routine yearly or biannual check-ups). This suspicion was based on the frequency of oral health care visits reported by individuals in groups 1, 2 and 3 where the patients had visited an oral healthcare provider nearly 24 months ago.
Poor oral hygiene maintenance is a significant risk factor of periodontal and peri-implant diseases45. In the present investigation, toothbrushing twice daily and flossing of interproximal spaces was more often reported by individuals in Group-4 compared to those in groups 1, 2 and 3. Moreover, as mentioned above, routine dental check-ups were also more prevalent in the former than latter groups. The authors applaud the study by Ball and Darby45, which reported that mental health disorders induce behavioral changes including poor routine oral hygiene maintenance (OHM) practices, which may also induce and aggravate peri-implant diseases in susceptible patients. Interestingly, none of the implants failed in the present study and this could be possibly attributed to the relatively short duration of MDD among patients in groups 2 and 3. Nevertheless, such a statement should be cautiously interpreted as poor oral hygiene, poor compliance towards therapy and irregular dental checkups may also contribute towards future implant complications including implant failure. The authors suggest that healthcare providers should educate patients (particularly those with DM and MDD) about the importance of routine OHM towards the achievement of an overall superior quality of life. Community-based health awareness programs may also contribute to this regard.
One limitation of the present study is that the HbA1c levels that were retrieved from healthcare records were measured over 100 days ago. Therefore, the HbA1c levels of patients during the peri-implant investigation may have varied. The present study was entirely based on peri-implant clinical and radiographic investigations and laboratory-based investigations such as assessment of whole salivary cortisol levels remained uninvestigated. It has been documented that raised salivary CL is associated with severe depression and is a worthy tool for a depression diagnosis. It is hypothesized that in the present patient population, whole salivary CL are higher among patients in Group 3 compared with other groups; however, further studies are needed to test this hypothesis.
Conclusion
Peri-implant soft tissue and osseous statuses are compromised among patients with type-2 DM, and MDD regardless of whether these conditions occur individually or in combination. Furthermore, oral hygiene maintenance and regular medical and dental checkups are often compromised in these patients that further compromises peri-implant soft tissue and osseous status in these individuals.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- 5HT:
-
5-Hydroxytryptaminebone loss
- CBL5:
-
Crestal bone loss
- CL:
-
Cortisol levels
- DM:
-
Diabetes mellitus
- HbA1c:
-
Hemoglobin A1c
- mGI:
-
Modified gingival index
- mPI:
-
Modified plaque index
- MDD:
-
Major depressive disorders
- OHM:
-
Oral hygiene maintenance
- PPD:
-
Peri-implant Probing depth
- SSRI:
-
Selective serotonin reuptake inhibitotrs
References
Aljudaibi, S. M., Alqhtani, M. A. Z., Almeslet, A. S., Aldowah, O. & Alhendi, K. D. S. Retention of mandibular complete overdentures using Mini dental implants (Ø < 3 mm) and standard diameter implants (Ø > 3 mm): A systematic review and Meta-Analysis of randomised controlled trials. Oral. Health. Prev. Dent. 22, 181–188. https://doi.org/10.3290/j.ohpd.b5282167 (2024).
Fleissig, Y., Casap, N., Abu-Tair, J. & Fernandes, R. Long-Term survival of dental implants in irradiated patients. Oral Maxillofac. Surg. Clin. N. Am. 37, 121–131. https://doi.org/10.1016/j.coms.2024.08.001 (2025).
Aljudaibi, S. M. et al. Clinical and radiographic status and PISF levels of PGE2 around cement and screw retained implants. Int. Dent. J. 74, 1378–1385. https://doi.org/10.1016/j.identj.2024.04.026 (2024).
Maló, P., de Araújo Nobre, M., Lopes, A., Ferro, A. & Gravito, I. Single-Tooth rehabilitations supported by dental implants used in an Immediate-Provisionalization protocol: report on Long-Term outcome with retrospective Follow-Up. Clin. Implant Dent. Relat. Res. 17 (Suppl 2), e511–519. https://doi.org/10.1111/cid.12278 (2015).
Lertpimonchai, A., Rattanasiri, S., Arj-Ong Vallibhakara, S., Attia, J. & Thakkinstian, A. The association between oral hygiene and periodontitis: a systematic review and meta-analysis. Int. Dent. J. 67, 332–343. https://doi.org/10.1111/idj.12317 (2017).
Pons, R., Nart, J., Valles, C., Salvi, G. E. & Monje, A. Self-administered proximal implant-supported hygiene measures and the association to peri-implant conditions. J. Periodontol. 92, 389–399. https://doi.org/10.1002/jper.20-0193 (2021).
Rekawek, P. et al. Hygiene recall in diabetic and nondiabetic patients: A periodic prognostic factor in the protection against Peri-Implantitis? J. Oral Maxillofac. Surg. 79, 1038–1043. https://doi.org/10.1016/j.joms.2020.12.032 (2021).
Assery, N. M., Jurado, C. A., Assery, M. K. & Afrashtehfar, K. I. Peri-implantitis and systemic inflammation: A critical update. Saudi Dent. J. 35, 443–450. https://doi.org/10.1016/j.sdentj.2023.04.005 (2023).
Yan, Y. et al. Association between peri-implantitis and systemic inflammation: a systematic review. Front. Immunol. 14, 1235155. https://doi.org/10.3389/fimmu.2023.1235155 (2023).
Taylor, R. Type 2 diabetes: etiology and reversibility. Diabetes Care. 36, 1047–1055. https://doi.org/10.2337/dc12-1805 (2013).
Viigimaa, M. et al. Macrovascular complications of type 2 diabetes mellitus. Curr. Vasc Pharmacol. 18, 110–116. https://doi.org/10.2174/1570161117666190405165151 (2020).
Mukherjee, N. & Chaturvedi, S. K. Depressive symptoms and disorders in type 2 diabetes mellitus. Curr. Opin. Psychiatry. 32, 416–421. https://doi.org/10.1097/yco.0000000000000528 (2019).
Shorey, S., Ng, E. D. & Wong, C. H. J. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br. J. Clin. Psychol. 61, 287–305. https://doi.org/10.1111/bjc.12333 (2022).
Alshardi, A. & Farahat, F. Prevalence and predictors of depression among medical residents in Western Saudi Arabia. J. Clin. Psychol. Med. Settings. 27, 746–752. https://doi.org/10.1007/s10880-019-09667-7 (2020).
Kocher, T., König, J., Borgnakke, W. S., Pink, C. & Meisel, P. Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontol 2000. 78, 59–97. https://doi.org/10.1111/prd.12235 (2018).
Salvi, G. E., Carollo-Bittel, B. & Lang, N. P. Effects of diabetes mellitus on periodontal and peri-implant conditions: update on associations and risks. J. Clin. Periodontol. 35, 398–409. https://doi.org/10.1111/j.1600-051X.2008.01282.x (2008).
Lorean, A., Ziv-On, H., Perlis, V. & Ormianer, Z. Marginal bone loss of dental implants in patients with type 2 diabetes mellitus with poorly controlled HbA1c values: A Long-Term retrospective study. Int. J. Oral Maxillofac. Implants. 36, 355–360. https://doi.org/10.11607/jomi.8476 (2021).
Gupta, B., Acharya, A., Pelekos, G., Gopalakrishnan, D. & Kolokythas, A. Selective serotonin reuptake inhibitors and dental implant failure-A significant concern in elders? Gerodontology 34, 505–507. https://doi.org/10.1111/ger.12287 (2017).
Strooker, H., de Waal, Y. C. M. & Bildt, M. M. Psychological risk indicators for peri-implantitis: A cross-sectional study. J. Clin. Periodontol. 49, 980–987. https://doi.org/10.1111/jcpe.13645 (2022).
Deepa et al. Prognostic implication of selective serotonin reuptake inhibitors in osseointegration of dental implants: A 5-year retrospective study. J. Contemp. Dent. Pract. 19, 842–846 (2018).
Semenkovich, K., Brown, M. E., Svrakic, D. M. & Lustman, P. J. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs 75, 577–587. https://doi.org/10.1007/s40265-015-0347-4 (2015).
World Medical Association Declaration of Helsinki. : ethical principles for medical research involving human subjects. Jama 310, 2191–2194. https://doi.org/10.1001/jama.2013.281053 (2013).
Goodyear, M. D., Krleza-Jeric, K. & Lemmens, T. The declaration of Helsinki. BMJ (Clinical Res. ed.). 335, 624–625. https://doi.org/10.1136/bmj.39339.610000.BE (2007).
Löe, H. The gingival index, the plaque index and the retention index systems. J. Periodontol. 38, 610–616. https://doi.org/10.1902/jop.1967.38.6.610 (1967).
Bawankar, P. V., Kolte, A. P. & Kolte, R. A. Peri-implant soft tissue stability through vestibular extension procedure: a retrospective study. Quintessence Int. 53, 226–235. https://doi.org/10.3290/j.qi.b2407783 (2022).
Armitage, G. C. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 34, 9–21. https://doi.org/10.1046/j.0906-6713.2002.003421.x (2004).
Berglundh, T. et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and Peri-Implant diseases and conditions. J. Clin. Periodontol. 45 Suppl 20, S286–s291. https://doi.org/10.1111/jcpe.12957 (2018).
Salti, L. et al. Estimating effects of craniofacial morphology on gingival recession and clinical attachment loss. J. Clin. Periodontol. 44, 363–371. https://doi.org/10.1111/jcpe.12661 (2017).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A. G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 41, 1149–1160. https://doi.org/10.3758/brm.41.4.1149 (2009).
Jiang, X., Zhu, Y., Liu, Z., Tian, Z. & Zhu, S. Association between diabetes and dental implant complications: a systematic review and meta-analysis. Acta Odontol. Scand. 79, 9–18. https://doi.org/10.1080/00016357.2020.1761031 (2021).
Singh, A., Kukreti, R., Saso, L. & Kukreti, S. Mechanistic insight into oxidative Stress-Triggered signaling pathways and type 2 diabetes. Molecules 27 https://doi.org/10.3390/molecules27030950 (2022).
Sansone, R. A. & Sansone, L. A. SSRIs: bad to the bone? Innov. Clin. Neurosci. 9, 42–47 (2012).
Al-Shammery, D., Michelogiannakis, D., Rossouw, E., Romanos, G. E. & Javed, F. Influence of psychological stress exposure on orthodontic therapy: A comprehensive review. J. Invest. Clin. Dent. 10, e12388. https://doi.org/10.1111/jicd.12388 (2019).
Javed, F., Ahmed, Z. U., Rossouw, P. E. & Romanos, G. E. Can Fluoxetine influence orthodontic tooth movement? A systematic review and meta-analysis of studies on animal models. Int. Orthod. 23, 100960. https://doi.org/10.1016/j.ortho.2024.100960 (2024).
Al-Askar, M., Ajlan, S., Alomar, N. & Al-Daghri, N. M. Clinical and radiographic Peri-Implant parameters and whole salivary Interleukin-1β and Interleukin-6 levels among Type-2 diabetic and nondiabetic patients with and without Peri-Implantitis. Med. Princ Pract. 27, 133–138. https://doi.org/10.1159/000488032 (2018).
Dŏgan, Ş. Evaluation of clinical parameters and levels of Proinflammatory cytokines in the crevicular fluid around dental implants in patients with type 2 diabetes mellitus. Int. J. Oral Maxillofac. Implants. 30, 1119–1127. https://doi.org/10.11607/jomi.3787 (2015).
Ding, L., Xu, Y., Liu, S., Bi, Y. & Xu, Y. Hemoglobin A1c and diagnosis of diabetes. J. Diabetes. 10, 365–372. https://doi.org/10.1111/1753-0407.12640 (2018).
Heji, E. S. et al. Periodontal disease as a predictor of undiagnosed diabetes or prediabetes in dental patients. Eur. J. Dent. 15, 216–221. https://doi.org/10.1055/s-0040-1719208 (2021).
Tan, L., Liu, J. & Liu, Z. Association between periodontitis and the prevalence and prognosis of prediabetes: a population-based study. J. Transl Med. 21, 484. https://doi.org/10.1186/s12967-023-04340-y (2023).
Bencze, B. et al. Prediabetes and poorly controlled type-2 diabetes as risk indicators for peri-implant diseases:a systematic review and meta-analysis. J. Dent. 146, 105094. https://doi.org/10.1016/j.jdent.2024.105094 (2024).
Mezuk, B., Eaton, W. W., Albrecht, S. & Golden, S. H. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 31, 2383–2390. https://doi.org/10.2337/dc08-0985 (2008).
Nouwen, A. et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet. Med. 36, 1562–1572. https://doi.org/10.1111/dme.14054 (2019).
Rudnick, G. Structure/function relationships in serotonin transporter: new insights from the structure of a bacterial transporter. Handb. Exp. Pharmacol. 59–73. 10.1007 (2006). /3-540-29784-7_3.
Liu, B. et al. The selective serotonin reuptake inhibitor Fluoxetine has direct effects on beta cells, promoting insulin secretion and increasing beta-cell mass. Diabetes Obes. Metab. 24, 2038–2050. https://doi.org/10.1111/dom.14791 (2022).
Ball, J. & Darby, I. Mental health and periodontal and peri-implant diseases. Periodontol 2000. 90, 106–124. https://doi.org/10.1111/prd.12452 (2022).
Acknowledgements
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R499), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R499), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
SMA designed the study, wrote the manuscript and revised it prior to submission; MAZA and MT administered the questionnaire and wrote the methods and results; AAA and SMAlq performed the clinical and radiographic examinations and wrote the manuscript; SA and NAA wrote the results and performed the quantitative analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The research study was reviewed and approved by the Institutional Review Board (IRB) at the Najran University to ensure the protection of human subjects’ rights and welfare (Approval # 202412-076- 027132–059963).
Informed consent
All patients read and signed a written informed consent form.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aljudaibi, S.M., Alqhtani, M.A.Z., Tallab, M. et al. Clinical and radiographic peri-implant parameters in type-2 diabetic and non-diabetic individuals with major depressive disorder. Sci Rep 15, 8967 (2025). https://doi.org/10.1038/s41598-025-92869-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92869-x