Abstract
Arterial stiffening is an independent predictor of cardiovascular diseases, closely associated with hypertension and aging. Periodontitis, a chronic inflammatory disease caused by microbial imbalances, has been linked to systemic inflammation and endothelial dysfunction. This study aims to investigate the association between periodontitis and arterial stiffening in hypertensive individuals. Data utilized in this study were sourced from the 2009–2014 National Health and Nutrition Examination Survey (NHANES). A total of 3165 hypertensive patients aged ≥ 50 years were enrolled. The relationship between the severity of periodontitis and arterial stiffening (PP ≥ 60) was analyzed using multivariate logistic regression model. Moreover, the associations between pocket depth (PD) and clinical attachment loss (CAL) with arterial stiffening were investigated using multivariate logistic regression model and restricted cubic splines. Among the patients, 1223 (39%) exhibited no or mild periodontal disease, while 1447 (46%) and 495 (15%) were diagnosed with moderate and severe periodontal disease, respectively. Moderate and severe periodontitis were associated with higher odds of arterial stiffening compared to no/mild periodontitis in the fully adjusted model (moderate: OR 1.30 [95% CI 1.09–1.55], P = 0.004; severe: OR 1.35 [95% CI 1.05–1.73], P = 0.019; P for trend < 0.001). Higher quartiles of PD and CAL scores were significantly associated with greater odds of arterial stiffening (PD Q4 vs. Q1: OR 1.41 [95% CI 1.12–1.79], P = 0.004; CAL Q4 vs. Q1: 1.31 [95% CI 1.03–1.67], P = 0.030), with evidence of linear dose-response relationships (P non−linear association=0.114 for PD; P non−linear association=0.308 for CAL). Subgroup analyses showed that the association between periodontitis severity and arterial stiffening remained significant in participants without diabetes or chronic kidney disease. In hypertensive patients aged 50 years and over, periodontitis is associated with elevated PP, thus reinforcing the association between periodontitis and arterial stiffening. Screening and treating periodontitis may offer additional clinical benefits.
Similar content being viewed by others
Introduction
Odontogenic infection is a major source of the global disease burden. According to the National Health and Nutrition Examination Survey (NHANES), the prevalence of severe and non-severe periodontitis in adults aged 30–44 was 4% and 25%, respectively, while in adults aged ≥ 65 it was 9% and 51%1.Periodontitis is a chronic multi-factorial inflammatory disease caused by the imbalance of local microbial ecology2. Numerous studies have shown that periodontitis can lead to systemic inflammatory response, immune metabolism changes, and vascular endothelial dysfunction. Effective treatment can reduce the average PPD and the number of periodontal pathogens in patients with periodontitis, and reduce the level of cardiovascular risk mediators3,4. There is increasing evidence linking periodontitis with heart failure, coronary heart disease, atrial fibrillation, and other cardiovascular diseases5,6,7,8.
Arterial stiffening is the hardening of blood vessel walls. It caused by elastin fiber and collagen disorder, oxidative stress, mineral metabolism disorder, and low-grade inflammation, and is closely associated with hypertension and aging9. Arterial stiffening is an early marker of structure and functional changes in the vessel walls and an independent predictor of cardiovascular diseases10,11. Arterial stiffening can be measured by a variety of non-invasive techniques. Pulse pressure (PP) is a marker of arterial stiffening that can predict adverse cardiovascular events and effectively indicate arterial stiffening in the elderly12.
Chronic low-grade systemic inflammation caused and maintained by periodontitis may be associated with an increased risk of hypertension and ASCVD in patients with severe periodontitis13,14. Studies have shown that the degree of arterial stiffening is significantly correlated with white blood cell count, neutrophil/lymphocyte ratio, C-reactive protein, adhesion molecules, fibrinogen and other inflammatory markers15. Periodontitis can induce a systemic inflammatory response, endothelial dysfunction, and other pathological changes associated with arterial stiffening, thus prompting further investigation into the relationship between periodontitis and arterial stiffening. At present, the research results on the association between periodontitis and arterial stiffening are inconsistent, and the sample size of existing studies is relatively small, so the evidence supporting the research results is insufficient16. This study used the NHANES data from 2009 to 2014 to observe the association between periodontitis and arterial stiffening in hypertensive patients, which may further guide the improvement of arterial stiffening by periodontitis treatment.
Methods
Study design and participants
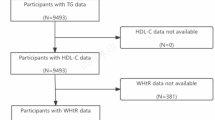
This study utilized data obtained from the NHANES 2009–2014 cycles. The NHANES is a cross-sectional survey aimed at assessing the nutritional and health status of the U.S. population. Among the initial sample of 5,589 participants aged 50 years or older with hypertension, we excluded individuals who had not undergone a comprehensive oral health examination (n = 2,077) and those without recorded blood pressure measurements or SBP < 60/DBP < 30 (n = 420), resulting in a sample of 3,389 participants. After further excluding individuals with incomplete covariates data (n = 224), our study ultimately included 3,165 participants (Fig. 1). The data collection protocol was approved by Ethics Review Board of the National Center for Health Statistics, and all participants signed informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Blood pressure measurement
The measurement of arterial blood pressure (BP) was conducted by trained physicians at mobile examination centers (MECs). After the participants rested for about 5 min, consecutive measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP) are taken three times, with SBP and DBP calculated as the mean of the three readings. PP, defined as the difference between SBP and DBP, was calculated, and a PP value ≥ 60 mm Hg indicated arterial stiffening as described in previous studies17,18,19,20.
Periodontal examination
The periodontal examination comprised a comprehensive assessment of pocket depth (PD) and clinical attachment loss (CAL) for each tooth at six sites. The severity of periodontal disease was classified based on the periodontal monitoring scheme recommended by American Academy of Periodontology. Participants with at least 2 interproximal CAL ≥ 3 mm, and at least 2 interproximal PD ≥ 4 mm (not on the same tooth) or 1 PD ≥ 5 mm were classified as having mild periodontitis. Participants with at least 2 interproximal CAL ≥ 4 mm (not on the same tooth) or at least 2 interproximal PD ≥ 5 mm (not on the same tooth) were classified as having moderate periodontitis. Participants with at least 2 interproximal CAL ≥ 6 mm (not on the same tooth) and at least 1 interproximal PD ≥ 5 mm were classified as having severe periodontitis. Participants not meeting the above criteria were classified as having no periodontitis.
Covariates
The following covariates were considered: age, sex, ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic, and other race), education (less than 11th grade, high-school grade and above), marital status (married/with a partner, and widowed/divorced/other), smoking, body mass index (BMI), total cholesterol to high density lipoprotein cholesterol ratio, diabetes, estimated glomerular filtration rate (eGFR), mean arterial pressure (MAP), white blood cell count, monocyte count, neutrophil count, lymphocyte count, and C-reactive protein (CRP). The eGFR was calculated using the Chronic Kidney Disease Epidemiology equation. MAP was defined as the sum of DBP and one-third of pulse pressure.
Statistical analysis
All statistical analyses were conducted using R version 4.0.3. In the descriptive analysis, continuous variables were presented as means with standard deviations (SD), and categorical variables were presented as count with percentages. Baseline characteristics stratified by periodontitis severity were compared using analysis of variance for continuous variables and chi-square test for categorical variables.
Multivariate logistic regression models adjusted for confounders was used to examine the association between periodontitis severity and arterial stiffening (PP ≥ 60). For all multivariate analysis in this study, 5 models were constructed: following the initial model 1 adjusted for age, sex, and ethnicity, 4 progressively adjusted models were performed (model 2: further including marital status and education; model 3: also including smoking, body mass index, total cholesterol/high density lipoprotein cholesterol, eGFR, and diabetes; model 4: additionally adjustment for mean arterial pressure; model 5: further adjusting for white blood cell count, monocyte count, neutrophil count, and lymphocyte count). Furthermore, the associations between PD/CAL and arterial stiffening were examined using multivariate logistic regression models. We employed restricted cubic splines (using 3 knots at the 10th, 50th, and 90th percentiles of the distribution) to estimate the relationship between PD/CAL and arterial stiffening. Additionally, the association between periodontitis severity and arterial stiffening was evaluated in subgroups stratified by diabetes or chronic kidney disease. A two-sided p-value < 0.05 was considered statistically significant.
Results
During the NHANES survey from 2009 to 2014, a total of 3165 participants aged 50 and above with coexisting hypertension underwent periodontal examination (Fig. 1). Among them, 1223 cases (39%) had no periodontitis or mild periodontitis, while 1447 cases (46%) had moderate periodontitis, and 495 cases (15%) had severe periodontitis. Baseline characteristics of participants recruited based on the severity of periodontitis were shown in Table 1. Overall, individuals with moderate or severe periodontitis tended to be male, Non-Hispanic Black or Other races, unmarried, with lower educational attainment, lower BMI, and a higher diagnosis rate of diabetes. Mean SBP, DBP, MAP, PP, total cholesterol/high density lipoprotein cholesterol, eGFR, white blood cell, monocyte, and neutrophil gradually increased with changes in severity of periodontitis.
Moderate and severe periodontitis were associated with higher odds of arterial stiffening compared to no/mild periodontitis in Model 1 (moderate: OR 1.39 [95% CI 1.17–1.65], P < 0.001; severe: OR 1.58 [95% CI 1.25-2.00], P < 0.001; P for trend < 0.001) (Table 2). The similar associations were also found in full adjusted model 5 (P for trend < 0.001), with a full adjusted OR of 1.30 (95% CI, 1.09–1.55, P = 0.004) for moderate periodontitis, and of 1.35 (95% CI, 1.05–1.73, P = 0.019) for severe periodontitis (Table 2).
Participants in the study were categorized into four groups based on the quartiles of PD (< 1.80, 1.80–2.19, 2.19–2.80, ≥ 2.80 mm) and CAL (< 1.90, 1.90–2.53, 2.53–3.67, ≥ 3.67 mm). The higher quartile of PD scores was associated with the significant greater odds for arterial stiffening compared to the lowest quartile (Q1) in full adjusted Model 5 (Q3 vs. Q1: OR, 1.33, 95% CI, 1.06–1.67, P = 0.013; Q4 vs. Q1: OR, 1.41, 95% CI, 1.12–1.79, P = 0.004; P for trend = 0.001) (Table 3). The same analyses for CAL scores showed similar association with arterial stiffening in full adjusted Model 5 (Q4 vs. Q1: OR, 1.31, 95% CI, 1.03–1.67, P = 0.030; P for trend = 0.035) (Table 3). There appeared to be a linear dose-response relationship between PD (P overall association=0.003; P non−linear association=0.114) and CAL (P overall association=0.030; P non−linear association=0.308) scores with arterial stiffening (Fig. 2).
Restricted cubic spline for associations of PD/CAL with arterial stiffening. Point estimates (solid line) and 95% confidence intervals (dashed lines) were depicted by restricted cubic spline models with knots at the 10th, 50th, and 90th percentiles. All models were adjusted for age, sex, ethnicity, marital status, education, smoking, body mass index, total cholesterol/high density lipoprotein cholesterol, eGFR, diabetes, mean arterial pressure, white blood cell, monocyte, neutrophil, and lymphocyte.
When stratified by diabetes, in the subgroup without diabetes, moderate (OR 1.29, 95% CI 1.04–1.60, P = 0.020) and severe periodontitis (OR 1.40, 95% CI 1.04–1.90, P = 0.029) had significant higher OR for arterial stiffening compared with no/mild periodontitis, whereas no statistical relationship was found among participants with diabetes (Table 4). When stratified by chronic kidney disease, in the subgroup without chronic kidney disease, moderate (OR, 1.40, 95% CI, 1.04–1.90, P = 0.004) and severe periodontitis (OR, 1.48, 95% CI, 1.14–1.93, P = 0.004) had significant higher OR for arterial stiffening compared with no/mild periodontitis, whereas no statistical association was found in the subgroup of chronic kidney disease (Table 4).
Discussion
Arterial stiffening tends to increase significantly with age, particularly after the age of 5021. Additionally, pulse pressure is more likely to indicate the level of arterial stiffening in individuals over 50 years old12,22. Some clinical studies have shown that PP ≥ 60mmHg has good clinical predictive value for cardiovascular adverse events, so it is considered as a clinical indicator of arterial stiffening23,24,25. Blood pressure is an important risk factor for arterial stiffening26, and a series of inflammatory biomarkers have been shown to be associated with increased arterial stiffening and refractory hypertension27. The association between hypertension and periodontitis has been confirmed by many studies28,29. In this study focusing on hypertensive patients aged ≥ 50 years, even after adjusting for the potential impact of hypertension on arterial stiffening by including mean arterial pressure as a covariate, the results still show an independent association between the severity of periodontitis and arterial stiffening. Higher PD and CAL scores were associated with arterial stiffening, with the restricted cubic spline results indicating no evidence of a nonlinear relationship between them. Subgroup analyses showed that the association between periodontitis severity and arterial stiffening remained significant in participants without diabetes or chronic kidney disease.
This study found significant differences in the levels of inflammatory cells among groups with varying degrees of periodontitis. Porphyromonas gingivalis (Pg) is considered a key pathogen promoting the development of severe periodontitis and can produces a variety of virulence factors30. Pg has been detected in vascular tissues and heart valves31,32. The gingival protease produced by Pg can impact the vascular permeability of endothelial cells, leading to edema, chronic inflammation, and vascular injury33. These evidences suggest that periodontitis may be an important factor in the development of CVD. Additionally, a cross-sectional study of 127 patients with ischemic heart disease showed that patients with high Pg-IgG levels had statistically higher pulse pressure than those with low PG-IgG levels, supporting the potential involvement of Pg in arterial stiffening among patients with periodontitis34. In addition, a range of inflammatory biomarkers have been shown to be associated with increased arterial stiffening. In this study, the severity of periodontitis was independently associated with arterial stiffness in hypertensive patients, even after adjustment for leukocytes, monocytes, and neutrophils. Although inflammatory markers such as IL-1, IL-6, TNF, and high-sensitivity CRP were lacking in this study, previous studies have shown that interleukin-6, tumor necrosis factor, and high-sensitivity C-reactive protein are associated with hypertension35,36. Periodontal treatment can reduce the level of CRP3 and reduce the levels of inflammatory factors and arterial stiffening in patients with refractory hypertension37,38. Therefore, inflammatory response may be a potential mechanism for the association between periodontitis and arterial stiffening.
Studies on the association between periodontitis and arterial stiffening are not completely uniform. A cross-sectional study of 269 patients with periodontitis revealed a positive linear association between pulse wave velocity (PWV) and the severity of periodontal disease39. In another cross-sectional study involving 158 dental patients, PWV was significantly higher in patients with severe periodontitis compared with healthy periodontal controls40. However, a cross-sectional study of 291 healthy Japanese men showed that the association between PWV and periodontal disease disappeared after adjusting for systolic blood pressure, age, and smoking41. Nonetheless, this study, which included a large sample of hypertensive individuals, still demonstrated an association between the severity of periodontitis and arterial stiffening even after adjusting for mean arterial pressure.
Diabetes and renal insufficiency are important factors affecting the progression of arterial stiffening. Previous studies have not found a association between periodontitis and arterial stiffening in diabetic patients42. Previous study has shown that the level of advanced glycosylation end products is increased significantly in diabetic patients and correlated with cfPWV independently, independent of age, gender, blood pressure and renal function43. Meta-analysis results also provide evidence that DM is significantly and independently associated with cfPWV44. Additionally, CKD serves as an independent risk factor for arteriosclerosis. In our study, there was no significant association between the severity of periodontitis and arterial stiffening in the subgroup of patients with hypertension combined with diabetes or renal dysfunction.
In recent years, there has been increasing attention on the association between periodontitis and various diseases, some of which have high prevalence rates in the general population, such as cardiovascular disease and diabetes. Effective periodontal treatment can not only improve the quality of life of patients, reduce the levels of inflammatory factors, reduce the risk of arterial stiffening disease, improve metabolic control, and reduce the occurrence of diabetic complications3,45, but also may bring clinical benefits to cardiovascular and metabolic diseases. This underscores the clinical significance of evaluating patients’ periodontal status in comprehensive risk assessments, given that screening and treatment for periodontal disease are readily available and cost-effective46. The additional benefit of oral therapy may increase the willingness and compliance of patients with periodontal disease. Therefore, the cooperation between clinicians and stomatologists should be strengthened to strengthen the screening and treatment of periodontitis.
At present, there is a paucity of research on the association between periodontitis and arterial stiffening, with existing studies having relatively small sample sizes that result in inadequate assessment of arteriosclerosis in patients with periodontitis. This study, for the first time, focused on a larger sample of hypertensive patients, adjusted for confounding factors such as mean arterial pressure, to explore the relationship between the severity of periodontitis and arterial stiffening. Furthermore, this study examined the relationship between components of periodontitis, namely PD and CAL, and arterial stiffening. However, this study may have some limitations. The cross-sectional design precludes inference of causal relationships between variables; thus further research is necessary to confirm any associations identified. Additionally, the absence of other inflammatory markers such as IL-1, IL-6, TNF, and high-sensitivity CRP may limit a comprehensive reflection of inflammation levels within the sample population.
Conclusion
The findings of this cross-sectional study suggest the association between periodontitis and arterial stiffening in hypertensive individuals aged 50 and older. The identification and management of periodontitis may provide additional clinical advantages.
Data availability
The data utilized in this study are sourced from a publicly available NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm), accessible to all through the provided links.
References
Eke, P. I. et al. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009–2014. Journal of the American Dental Association (). 2018;149(7):576–588.e576. (1939).
Lamont, R. J., Koo, H. & Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16 (12), 745–759 (2018).
Isola, G. et al. Effects of minimally invasive non-surgical therapy on C-reactive protein, lipoprotein-associated phospholipase A(2), and clinical outcomes in periodontitis patients: A 1-year randomized, controlled clinical trial. J. Periodontol. 95 (10), 949–962 (2024).
Isola, G. et al. Effect of quadrantwise versus full-mouth subgingival instrumentation on clinical and Microbiological parameters in periodontitis patients: A randomized clinical trial. J. Periodontal Res. 59 (4), 647–656 (2024).
Bui, F. Q. et al. Association between periodontal pathogens and systemic disease. Biomedical J. 42 (1), 27–35 (2019).
Sanz, M. et al. Periodontitis and cardiovascular diseases: consensus report. J. Clin. Periodontol. 47 (3), 268–288 (2020).
Li, Q., Ouyang, X. & Lin, J. The impact of periodontitis on vascular endothelial dysfunction. Front. Cell. Infect. Microbiol. 12, 998313 (2022).
Walther, C. et al. Association between periodontitis and heart failure in the general population. ESC Heart Fail. 9 (6), 4189–4197 (2022).
Boutouyrie, P., Chowienczyk, P., Humphrey, J. D. & Mitchell, G. F. Arterial stiffness and cardiovascular risk in hypertension. Circul. Res. 128 (7), 864–886 (2021).
Erbel, R. et al. ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). European heart journal. 2014;35(41):2873–2926. (2014).
Vlachopoulos, C. et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European society of cardiology working group on peripheral circulation: endorsed by the association for research into arterial structure and physiology (ARTERY) society. Atherosclerosis 241 (2), 507–532 (2015).
Franklin, S. S. Ageing and hypertension: the assessment of blood pressure indices in predicting coronary heart disease. J. Hypertens. Supplement: Official J. Int. Soc. Hypertens. 17 (5), S29–36 (1999).
Demmer, R. T. et al. The influence of anti-infective periodontal treatment on C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. PloS One. 8 (10), e77441 (2013).
Schenkein, H. A. & Loos, B. G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Periodontol. 84 (4 Suppl), S51–69 (2013).
Mozos, I. et al. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front. Immunol. 8 (7), 1058 (2017).
Darnaud, C. et al. Association between periodontitis and pulse wave velocity: a systematic review and meta-analysis. Clin. Oral Invest. 25 (2), 393–405 (2021).
Desai, G. et al. Low-level exposure to lead, Mercury, arsenic, and cadmium, and blood pressure among 8-17-year-old participants of the 2009–2016 National health and nutrition examination survey. Environ. Res. 197, 111086 (2021).
Heffernan, K. S., Jae, S. Y. & Loprinzi, P. D. Estimated pulse wave velocity is associated with residual-specific mortality: findings from the National health and nutrition examination survey. J. Hypertens. 39 (4), 698–702 (2021).
Alkalbani, M., Prabhu, G., Lagbo, J. & Qayyum, R. Serum Klotho and pulse pressure; insight from NHANES. Int. J. Cardiol. 355, 54–58 (2022).
Zeng, H. et al. Exposure to barium and blood pressure in children and adolescents: results from the 2003–2018 National health and nutrition examination survey. Environ. Sci. Pollut. Res. Int. 29 (45), 68476–68487 (2022).
Chirinos, J. A., Segers, P., Hughes, T. & Townsend, R. Large-Artery stiffness in health and disease: JACC State-of-the-Art review. J. Am. Coll. Cardiol. 74 (9), 1237–1263 (2019).
Safar, M. E. Systolic blood pressure, pulse pressure and arterial stiffness as cardiovascular risk factors. Curr. Opin. Nephrol. Hypertens. 10 (2), 257–261 (2001).
Alderman, M. H., Cohen, H. & Madhavan, S. Distribution and determinants of cardiovascular events during 20 years of successful antihypertensive treatment. J. Hypertens. 16 (6), 761–769 (1998).
Benetos, A., Rudnichi, A., Safar, M. & Guize, L. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertens. (Dallas Tex: 1979). 32 (3), 560–564 (1998).
Mulè, G. et al. Relationship of metabolic syndrome with pulse pressure in patients with essential hypertension. Am. J. Hypertens. 20 (2), 197–203 (2007).
Tsai, J. P. & Hsu, B. G. Arterial stiffness: A brief review. Tzu Chi Med. J. 33 (2), 115–121 (2021).
Barbaro, N. R. et al. Increased arterial stiffness in resistant hypertension is associated with inflammatory biomarkers. Blood Press. 24 (1), 7–13 (2015).
Desvarieux, M. et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J. Hypertens. 28 (7), 1413–1421 (2010).
Darnaud, C., Thomas, F., Pannier, B., Danchin, N. & Bouchard, P. Oral health and blood pressure: the IPC cohort. Am. J. Hypertens. 28 (10), 1257–1261 (2015).
Hajishengallis, G., Darveau, R. P. & Curtis, M. A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10 (10), 717–725 (2012).
Oliveira, F. A. F. et al. Molecular analysis of oral Bacteria in heart valve of patients with cardiovascular disease by Real-Time polymerase chain reaction. Medicine 94 (47), e2067 (2015).
Mougeot, J. C. et al. Porphyromonas gingivalis is the most abundant species detected in coronary and femoral arteries. J. Oral Microbiol. 9 (1), 1281562 (2017).
Farrugia, C. et al. Mechanisms of vascular damage by systemic dissemination of the oral pathogen Porphyromonas gingivalis. FEBS J. 288 (5), 1479–1495 (2021).
Hanaoka, Y. et al. Level of serum antibody against a periodontal pathogen is associated with atherosclerosis and hypertension. Hypertens. Research: Official J. Japanese Soc. Hypertens. 36 (9), 829–833 (2013).
Tomiyama, H. et al. The contribution of inflammation to the development of hypertension mediated by increased arterial stiffness. J. Am. Heart Association 6(7), e005729 (2017).
Dumor, K., Shoemaker-Moyle, M., Nistala, R. & Whaley-Connell, A. Arterial stiffness in hypertension: an update. Curr. Hypertens. Rep. 20 (8), 72 (2018).
Vidal, F., Cordovil, I., Figueredo, C. M. & Fischer, R. G. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J. Clin. Periodontol. 40 (7), 681–687 (2013).
Hajishengallis, G. & Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 21 (7), 426–440 (2021).
Kapellas, K. et al. Effect of periodontal therapy on arterial structure and function among aboriginal Australians: a randomized, controlled trial. Hypertens. (Dallas Tex: 1979). 64 (4), 702–708 (2014).
Jockel-Schneider, Y. et al. Arterial stiffness and pulse wave reflection are increased in patients suffering from severe periodontitis. PloS One. 9 (8), e103449 (2014).
Miyaki, K. et al. Periodontal disease and atherosclerosis from the viewpoint of the relationship between community periodontal index of treatment needs and brachial-ankle pulse wave velocity. BMC Public. Health. 6, 131 (2006).
Khumaedi, A. I., Purnamasari, D., Wijaya, I. P., Soeroso, Y. & Marhamah, S. Association of periodontitis and arterial stiffness in type 2 diabetic patients. Acta Med. Indones. 50 (4), 320–327 (2018).
Semba, R. D., Najjar, S. S., Sun, K., Lakatta, E. G. & Ferrucci, L. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am. J. Hypertens. 22 (1), 74–79 (2009).
Yapei, Y. et al. Clinical significance of arterial stiffness and thickness biomarkers in type 2 diabetes mellitus: an Up-To-Date Meta-Analysis. Med. Sci. Monitor: Int. Med. J. Experimental Clin. Res. 21, 2467–2475 (2015).
Holmstrup, P. et al. Comorbidity of periodontal disease: two sides of the same coin? An introduction for the clinician. J. Oral Microbiol. 9 (1), 1332710 (2017).
Solowiej-Wedderburn, J., Ide, M. & Pennington, M. Cost-effectiveness of non-surgical periodontal therapy for patients with type 2 diabetes in the UK. J. Clin. Periodontol. 44 (7), 700–707 (2017).
Acknowledgements
We express appreciation to the data collection team and NHANES administration for facilitating access to the pertinent data through the NHANES website.
Funding
This research has been supported by the China National Key R&D Program during the 13th Five-year Plan Period (Grant No. 2018YFC2000300).
Author information
Authors and Affiliations
Contributions
Hu C and Zhang H designed the study, performed the statistical analysis and drafted the manuscript. Qi G acquired data and participated in drafting the manuscript. Tian W conceived of the study, and participated in its design, helped to draft the manuscript, and provided critical revision for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The survey protocol received approval from the NCHS Ethics Review Board (https://www.cdc.gov/nchs/nhanes/irba98.htm), and all participants have written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, C., Zhang, H., Qi, G. et al. The association between periodontitis and arterial stiffening among the hypertensive middle-aged and elderly U.S. Population. Sci Rep 15, 10498 (2025). https://doi.org/10.1038/s41598-025-92876-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92876-y