Abstract
Mantle cell lymphoma (MCL) exhibits significant biological and clinical heterogeneity, necessitating a refined prognostic model. According to the drawbacks of existing models which do not truly define the complexity of the disease, we used the clinical and molecular data from nine medical centers of China to validate the predictive utility of progression of disease within 24 months (POD24), and also established a novel prognostic risk model to predict the survival outcome of MCL patients. POD24 occurred in 37.7% of evaluable patients, with the median over survival being 21 months (vs. 122 months for those without POD24, P < 0.0001). The POD24-based risk model had the highest sensitivity to predict survival with the most satisfying AUC value for risk score (AUC = 0.869). In conclusion, we confirm the obviously predictive performance of POD24 and established a novel risk model combined POD24 and clinical factors. Our new prognostic model might be helpful in effectively classify MCL patients with high-risk groups in terms of survival rate, which may help in selecting high-risk MCL patients for more intensive treatment at time of relapse.
Similar content being viewed by others
Introduction
Mantle cell lymphoma (MCL) is a unique subcategory of B cell malignancy disease accounting for 6–7% of non-Hodgkin lymphomas (NHL), which is characterized by the overexpression of the cell cycle regulatory protein cyclin D11,2,3. This lymphoma subtype is considered an incurable disease showing a generally aggressive, albeit heterogeneous and clinical course3. The prognosis of MCL after relapse is generally poor, and there is no standard of treatment guidance, with a median survival time of approximately 5 years2. MCL is a disease of older individuals with the median age at diagnosis being 70 years, and is more common in male than female (male/female ratio, 3:1)4. Additionally, MCL has a heterogeneous clinical course, including a subgroup of patients with long survival in different treatment but without a striking unfavorable effect on their outcomes5. The majority of patients are diagnosed with widespread disease and are characterized by frequent recurrences6. Most of MCL patients respond to the current standard of care but often relapse or refractory due to drug resistance7. At time of relapse, no standard of treatment guidance exists and the prognosis after relapse is generally poor despite the increasing novel treatment options, such as BTK inhibitors8.
Well-established prognostic factors such as the MCL International Prognostic Index (MIPI) and the proliferative activity (Ki-67), classify MCL patients into different risk groups, but have not been useful in predicting the prognosis of identifying indolent disease patients9,10. Other relevant factors such as karyotypic complexity, genetic aberrations and DNA methylation are well verified as independent prognostic factors for MCL outcome8. Moreover, in the era of newer therapies such as proteasome inhibitors, immunomodulatory drugs, tyrosine kinase inhibitors and Chimeric Antigen Receptor T (CAR-T) cells, the relevance of these prognostic factors is largely inefficient for the accurate definition of expected response and survival rates for relapsed or refractory (R/R) MCL patients8,11. Therefore, there is an urgent necessity to develop innovative biomarkers for individual prognosis and risk stratification, enabling a precise estimation of survival outcomes.
POD within 2 years (POD24) of diagnosis has been identified as a robust predictor of poor survival in lymphoma disease, although the specific diagnosis or induction regimen administered is unknown12,13,14. POD24 can assist in selecting second line treatments in relapsed patients, which defines high-risk groups of patients in whom further study is warranted in both directed prospective clinical trials of biology and treatment15. Likewise, the observation that patients with relapse over two years have prolonged survival expectation in the novel treatment era seems of great importance in the clinical management of MCL patients13,15.
Due to its rarity and the great diversity in clinical behavior of MCL, it has been difficult to predict the prognosis and the heterogeneity of clinical of MCL16. The aim of this study was to describe the clinical features and clinical outcomes of MCL patients in China from nine cancer centers, while confirming the prognostic impact of progression of disease within 24 months (POD24) and highlighting the relevance of other clinical biomarkers in predicting the survival of MCL patients. More importantly, a first-ever prognostic model based on POD24 and clinical factors has been provided to stratify MCL patients into different survival outcomes, which might be helpful for allowing individualized, risk-adapted treatment decisions in patients with advanced stage of MCL.
Results
Clinical features and characteristics
Of 979 total patients with MCL, 369 patients were in POD ≤ 24 months group and 610 patients in POD>24 months (Non-POD24) group. Clinical features and characteristics for different POD groups were shown in Table 1.
In the POD24 cohort, 283 (77%) were male. In POD 24 patients with quantifiable MIPI, 218 (66%) and 110 (34%) had low/intermediate and high-risk MIPI respectively. In the Non-POD24 cohort, 464 (76%) were male. In Non-POD24 patients with quantifiable MIPI, 482 (82%) and 103 (18%) had low/intermediate and high-risk MIPI respectively. POD24 patients were more likely to have B symptoms (p = 0.003), elevated LDH (p<0.0001), splenomegaly (p<0.0001), Ki67 expression (p<0.0001), Anti-CD20 with induction (p<0.0001), novel agent with induction (p = 0.042), post-induction rituximab maintenance (p<0.0001) and autologous transplant (p = 0.037) and as expected, were more likely to have a worse performance status (p = 0.005).
Patient outcomes
Follow-up time was available for all patients with a median follow-up of 31 months, with ranging from 1 to 210 months. The median PFS was 30 months (95% CI: 26.73–33.28, range: 1–194 months), and the median OS was 75 months (95%CI: 66.17–85.83, range: 1–210 months). The 5-year PFS and OS estimates for all patients were 30.5% and 56.1%, respectively (Fig. 1A and B).
Overview the survival outcome of all patients and confirm POD24 as a prognostic marker for outcome. Kaplan-Meier analysis of (A) overall survival and (B) progression-free survival of all patients with mantle cell lymphoma. (C) Spline curve showing the predicted hazard ratio of time to POD from start of frontline treatment as a prognostic marker. (D) Time-dependent receiver operating characteristic (ROC) curve for predicting 3-years survival of POD12 and POD24. POD24: Progression of disease within 24 months; POD12: Progression of disease within 12 months.
RCS models showed an approximately linear association between month to disease progression and risk of death, while it became flat thereafter, with a point of inflection located around 24 months. The hazard ratio for death decreased rapidly with POD during the 24–36 months (Fig. 1C). Subsequently, the ROC curve analysis also confirmed POD24 presented a good performance in survival prediction with the highly reliable AUC being 0.797, and the AUC for POD12 was 0.754 (Fig. 1D). Thus, 24 months seemed a reasonable cut-off which showed the most significant prognostic effects in predicting OS.
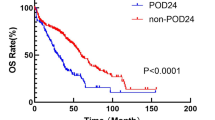
Risk prognostic factors associated with overall survival in patients with MCL were presented in Fig. 2. There were significant differences in median OS between POD24 groups and Non-POD24 groups. The median OS for Non-POD24 patients (mOS: 122 months) was significantly longer compared with those of POD24 patients (mOS: 24 months) (P<0.0001, Fig. 2G). In the time-dependent Cox model, POD24 was associated with poor sub-sequent OS (HR = 2.345; 95% CI, 1.678–3.276; P < 0.001). These data demonstrated that POD24 was significantly associated with inferior survival of MCL patients.
Risk prognostic factors associated with overall survival in patients with MCL. Kaplan-Meier curves of OS for MCL patients according to (A) the age, (B) the LDH, and (C) the MIPI score, (D) the Splenomegaly, (E) the Ki67, (F) the MIPI score and (G) the POD24. MCL: Mantle cell lymphoma; POD24: Progression of disease within 24 months; LDH: Lactate dehydrogenase; MIPI: mantle cell lymphoma international prognostic index, Ki67: cell proliferation marker, POD24: Progression of disease within 24 months.
Univariate analysis and multivariate analysis
In a univariate analysis of all patients, sex, disease stage, extranodal sites and HBV infection were not associated with OS. The identified prognostic factors included age, ECOG performance status, LDH level, MIPI score, B symptoms, splenomegaly, Ki67 expression, ASCT, Anti-CD20 with induction, post-induction rituximab maintenance and POD24 (Table 2).
Variables factors included in the multivariate analysis were those clinically relevant or statistically significant in univariate analysis. Among these factors, age, LDH level, MIPI score, splenomegaly, Ki67 expression, ASCT and POD24 remained significant for OS in multivariate analysis (Table 2). These results indicated that those clinical factors and POD24 were significantly related with the prognosis of MCL patients.
Construction of the prognostic nomogram
In an exploratory analysis, we developed a nomogram to quantitatively evaluate the impact of progression of disease within 24 months (POD24) alongside other overall survival (OS)-related clinical factors within a predictive model for Mantle Cell Lymphoma (MCL) patient survival. Utilizing multivariate Cox regression analysis, we allocated scores to each factor on the nomogram scale, ascertained the individual contribution of each variable, and computed the aggregate risk score for each patient, thereby enhancing the precision of survival prediction. The total score was normalized to a distribution ranging from 0 to 100 and used to calculate the 3- and 5-year estimated OS rates of MCL patients (Fig. 3A). Calibration curves of observed versus predicted probabilities of 3-and 5-year OS demonstrated excellent concordance between the predicted survival rate and the actual survival rate (Fig. 3B). Patients with higher score indicated a poor outcome and POD24 combined with clinical factors contributed most to the survival. These results suggested that the nomogram might reliably predict MCL patient prognoses in clinical practice.
Validation of the POD24 combined with clinical prognostic factors signature
To compare the predictive power of compound nomogram and POD24 or clinical risk factors separately, we then used multivariable ROC to compare the predictive accuracy between nomogram model and individual predictors. The AUC of the POD24 was 0.797 for predicting 3-year OS, which also showed a good predictive effect, but was significantly lower than the POD24-model (AUC 0.797vs. 0.869, p < 0.001, Fig. 4A).Similar results were also observed in predicting 5-year survival (Fig. 4B). To sum up, although POD24 showed an obviously predictive performance, our POD24-clinical nomogram combining the POD24 and clinical risk factors demonstrated high prognostic predictive efficiency than their separation.
Estimate the prognostic accuracy of the POD24 combined with clinical factors signature-based nomogram to predict 3- and 5-years survival in mantle cell lymphoma patients. Time-dependent receiver operating characteristic curve analysis for clinical risk factors only, the POD24, and POD24 combined with clinical risk factors for prediction 3- (A) and 5-years (B) survival of patients.
Discussion
MCL is a rare subtype of hematological malignant disease that comprised 2.5–6% of non-Hodgkin’s lymphomas. The incidence of MCL has been increasing over the years, especially among elderly patients17. Moreover, the complexity of its clinical presentations, variety in disease pathophysiology and the high incidence of disease progression/recurrence pose a major challenge to developing personalized precise therapeutic strategy8,17. Although various of chemotherapeutic regimens show significant activity in MCL patients, no regimen has been found to be superior, and no standard treatment has been identified for4,18. Over the years, the therapeutic options have been gradually evolving from combination chemotherapy to combination novel treatment options4. A number of targeted therapies are approved for R/R MCL patients in a surprisingly short time, including immunomodulatory agents, proteasome inhibitors and Bruton kinase inhibitors11,19,20. Despite currently available therapies show significant activity in MCL patients, prognosis remains poor after progression on these novel agents21. Treating aggressive variants of this disease is a serious challenge for clinicians and researchers18. Therefore, there is an urgent need to identify new prognostic and risk-stratified biomarkers to distinguish MCL prognostic subsets and guide therapeutic decisions.
In this study, we summarized 979 cases of MCL from nine medical centers of China, which is the a largest retrospective study on MCL in China. The present study presents similar clinical outcome as those reported in previous reports13,15. The proportion POD-24 patients with Low/intermediate MIPI were significantly lower (66% vs. 82%), and the POD-24 patients more likely to have a worse performance status. Also, POD24 could be used as a prognostic predictor of MCL patients, as has been proposed by previous works13,15. Consistent with these results, our study demonstrated that the median OS for Non-POD24 patients was significantly longer than those of POD24 patients (122 months vs. 24 months), which demonstrates that POD24 was significantly associated with inferior survival of MCL patients. Additionally, there was nonlinear dose-response relationship between POD and survival outcome of MCL, with POD was highly prognostic with a cut-off around 24 months. POD24 was associated with markedly reduced OS with a hazard ratio (HR) of 5.373 (95% CI, 3.984-7.247) in multivariable cox regression analysis and POD24 was most powerful factor associated with an elevated risk of death, more powerful than age, LDH level, MIPI score, splenomegaly, Ki67 expression and ASCT. These results indicated that POD24 was related to worse prognosis outcome for MCL patients.
MCL patient risk stratification and prognosis remain a challenge for clinicians. Mantle cell lymphoma international prognostic index (MIPI) score was established as the first prognostic stratification tool to identify clinical prognostic factors and patient with different risk groups22. The MIPI discriminated three groups of low, intermediate and high risk groups and has been validated by numerous studies10,22,23. However, the MIPI was derived from patients with advanced stage disease primarily in the pre-rituximab era and therefore presumably does not represent the general patient population of a new era10. Several studies have demonstrated that Ki-67 proliferation index is a strong biologic prognostic factor independent of MIPI score24,25. Thus, a combined biologic index (MIPI-b) that integrated MIPI and the Ki-67 proliferation index was developed and confirmed the prognostic value for patients with MCL10. MIPI-b model discriminates the high-risk group well, but a small low-risk group that overlapping with the intermediate-risk group in overall survival curves10. Therefore, MIPI-b does not adequately reflect the heterogeneity clinical outcome of MCL13. Similarly, our results also confirmed that MIPI and Ki67 do not show a promising performance in 3-years survival prediction with the AUC was 0.626 and 0.572, respectively. Therefore, new risk assessment strategies with relevant biomarkers are crucial for developing precision medicine.
Various of prognostic factors are markedly associated with survival outcome in MCL patients, including TP53 mutation, epigenetic profile variance, SOX11 gene expression profile and POD244,11,26. Several prognostic tools currently allow for risk stratification at diagnosis, but do not truly define the complexity of the heterogeneity of MCL disease12,13. Our findings are consistent with some results of the previously studies from western countries, which have confirmed the impact of POD24 as a prognostic and predictive marker both at diagnosis and at time of relapse in the 2 prospective Nordic MCL trials13. Notably, by using a time-dependent Cox regression model, this study overcame the time-dependency bias commonly found in traditional survival analyses, offering a more precise evaluation of the dynamic impact of POD24. Furthermore, we constructed an encompassed prognostic nomogram combining POD24 with OS-related clinical factors. This nomogram (AUC = 0.869) was superior to the POD24 single factor and other clinical factors. This model significantly improves prognostic accuracy and can be used to personalize patient survival outcomes, with potential for future clinical application.
Additionally, the level of healthcare resources and available treatments in China vary from one country/region to another27,28,29. While some areas may have only basic access to oncology care, some highly urbanized areas may have world-class expertise and newer therapies, some areas may have only basic treatments after being diagnosed with MCL27. Additionally, many Chinese patients commonly have different comorbidities, for example hepatitis B virus or tuberculosis infection. Although, our results did not found the clinically relevant or statistically significant of active hepatitis B virus infection in univariate analysis, chemotherapy may increase threat with hepatitis B virus reactivation and the spread of tuberculosis. Therefore, these should be considered as a serious consideration for the treatment of MCL patients in China27.
While our study has several limitations including its limited quality of the captured data and a heterogeneous patient population, making it difficult to generalize the findings to a broader population. Second, treatment response was not centrally reviewed due to the retrospective design of this study. Third, genetic testing for specific mutations or deletions, such as TP53, was not routinely performed in our clinical practice and therefore was not included in the data analysis. Lastly, it truly reflects a real-world experience in Chinese patients, further validation in different ethnic and geographical contexts is needed.
In summary, we confirm the prognostic impact of the POD24 and highlight the relevance of other known prognostic biomarkers in a retrospective analysis of a real-world cohort in Chinese patients, including a nomogram model of POD24-Clinical that was established. Our study is the first to identify and validate a POD24-based prognostic model with clinical factors in MCL patients, which could serve as a stratification tool to guide patient counseling, treatment decisions, and follow-up scheduling.
Methods
Patients
We conducted a retrospective cohort study of 979 patients diagnosed with MCL from nine medical centers of China between January 17, 2020 and February 1, 2020. All patients received at least one chemotherapeutic regimen of MCL. All patients had biopsy-proven MCL disease and were age 18 or older at time of evaluation at each center. The following data were collected: age, gender, ECOG performance status, stage of disease, serum LDH, extranodal disease, MIPI score, presence of B symptoms, splenomegaly, hepatitis B virus (HBV) infection, initial date of diagnosis, Ki67 expression and use of ASCT. Response to induction treatment was assessed by each participating center through clinical examination, standard biological parameters, computed tomography scan and, when available, bone marrow aspiration/biopsy and positron emission tomography scan. The outcomes were updated in August 2023. The study was approved by the institutional review board of The Affiliated Tumor Hospital of Xiangya Medical School. All procedures were carried out in accordance with the principles of the Helsinki Declaration. The requirement for written informed consent was waived for the retrospective data by institutional review board of The Affiliated Tumor Hospital of Xiangya Medical School.
Statistics
The primary objective of this work was to describe clinical characteristics and survival outcomes of disease progression in MCL patients with POD24 and 24 months after detection, and secondarily to assess prognostic and predictive biomarkers of survival across different groups. We reported differences in baseline characteristics, MIPI score, and prognostic markers between POD groups (≤ 24 and >24 months ) using Fisher Exact and Chi-Square tests as appropriate. Individual risk factors were compared using the χ2 test. Cox proportional hazards regression was used to estimate each hazard ratio (HR) and build the multivariate model. Cox regression analysis was performed, treating POD24 as a time-dependent covariate, to assess the relationship between POD24 and subsequent overall survival (OS).
Overall survival (OS) was calculated from the time of diagnosis to the date of last follow-up or date of death. Progression-free survival (PFS) was defined as the time from the date of diagnosis to either the date of first relapse or first sign of progression after initial treatment. The Kaplan-Meier test was used to analyze the OS and PFS of MCL patients and each subgroup was performed by using a log-rank test.
To evaluate the prognostic impact of POD24, restricted cubic spline (RCS) were applied to detect the possible nonlinear dependency of the relationship between time to POD and risk of death, using 4 knots at prespecified locations according to the percentiles of the distribution of POD.
The descriptive statistics were represented using the proportion and median. P < 0.05 was considered statistically significant. All Statistical analysis were performed using Statistical Package for the Social Sciences (SPSS) 17.0 software (Chicago, IL, USA).
Construction and validation of the prognostic nomogram
Univariate and multivariate analysis of conventional clinical risk factors and POD24 was performed to identify independent prognostic factor of OS in MCL patients. A prognostic nomogram encompassing POD24 and OS-related clinical factors was assessed by “rms” R package. Calibration curves and concordance index (C-index) were used to calculate the accuracy abilities of nomogram between predicted survival and actual survival. Time-dependent receptor operating characteristic (ROC) analysis was conducted by “survival ROC” R package to evaluate the predictive accuracy of the POD24-clinical prognostic model. The Delong test was used to evaluate the difference in AUC between the two different models.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Navarro, A., Beà, S., Jares, P. & Campo, E. Molecular pathogenesis of mantle cell lymphoma. Hematol. Oncol. Clin. North. Am. 34, 795–807. https://doi.org/10.1016/j.hoc.2020.05.002 (2020).
Jain, P. & Wang, M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am. J. Hematol. 94, 710–725. https://doi.org/10.1002/ajh.25487 (2019).
Flinn, I. W. et al. First-Line treatment of patients with indolent Non-Hodgkin lymphoma or Mantle-Cell lymphoma with Bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-Year Follow-Up study. J. Clin. Oncol. 37, 984–991. https://doi.org/10.1200/jco.18.00605 (2019).
Jain, P., Dreyling, M., Seymour, J. F. & Wang, M. High-Risk mantle cell lymphoma: definition, current challenges, and management. J. Clin. Oncol. 38, 4302–4316. https://doi.org/10.1200/jco.20.02287 (2020).
Jain, A. G., Chang, C. C., Ahmad, S. & Mori, S. Leukemic Non-nodal mantle cell lymphoma: diagnosis and treatment. Curr. Treat. Options Oncol. 20, 85. https://doi.org/10.1007/s11864-019-0684-8 (2019).
Nadeu, F. et al. Genomic and epigenomic insights into the origin, pathogenesis, and clinical behavior of mantle cell lymphoma subtypes. Blood 136, 1419–1432. https://doi.org/10.1182/blood.2020005289 (2020).
Jung, D., Jain, P., Yao, Y. & Wang, M. Advances in the assessment of minimal residual disease in mantle cell lymphoma. J. Hematol. Oncol. 13, 127. https://doi.org/10.1186/s13045-020-00961-8 (2020).
Roue, G. & Sola, B. Management of drug resistance in mantle cell lymphoma. Cancers (Basel). 12 https://doi.org/10.3390/cancers12061565 (2020).
Hoster, E. et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 111, 558–565 (2008).
Hoster, E. et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-Cell lymphoma: results from randomized trials of the European mantle cell lymphoma network. J. Clin. Oncol. 34, 1386–1394. https://doi.org/10.1200/JCO.2015.63.8387 (2016).
Zhang, L. et al. Metabolic reprogramming toward oxidative phosphorylation identifies a therapeutic target for mantle cell lymphoma. Sci. Transl. Med. 11 https://doi.org/10.1126/scitranslmed.aau1167 (2019).
Moccia, A. A. et al. Prognostic value of POD24 validation in follicular lymphoma patients initially treated with chemotherapy-free regimens in a pooled analysis of three randomized trials of the Swiss group for clinical Cancer research (SAKK). Br. J. Haematol. 192, 1031–1034. https://doi.org/10.1111/bjh.17045 (2021).
Eskelund, C. W. et al. Detailed Long-Term Follow-Up of Patients Who Relapsed After the Nordic Mantle Cell Lymphoma Trials: MCL2 and MCL3. Hemasphere 5, e510, (2021). https://doi.org/10.1097/HS9.0000000000000510
Yamaguchi, M. et al. Early disease progression in patients with localized natural killer/T-cell lymphoma treated with concurrent chemoradiotherapy. Cancer Sci. 109, 2056–2062. https://doi.org/10.1111/cas.13597 (2018).
Visco, C. et al. Time to progression of mantle cell lymphoma after high-dose cytarabine-based regimens defines patients risk for death. Br. J. Haematol. 185, 940–944. https://doi.org/10.1111/bjh.15643 (2019).
Visco, C. et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: results from the MANTLE-FIRST study. Leukemia 35, 787–795. https://doi.org/10.1038/s41375-020-01013-3 (2021).
Maddocks, K. Update on mantle cell lymphoma. Blood 132, 1647–1656. https://doi.org/10.1182/blood-2018-03-791392 (2018).
Ladha, A., Zhao, J., Epner, E. M. & Pu, J. J. Mantle cell lymphoma and its management: where are we now? Experimental hematology & oncology 8, 2, (2019). https://doi.org/10.1186/s40164-019-0126-0
Le Gouill, S. et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial. Blood 137, 877–887. https://doi.org/10.1182/blood.2020008727 (2021).
Dobrovolsky, D. et al. Bruton tyrosine kinase degradation as a therapeutic strategy for cancer. Blood 133, 952–961. https://doi.org/10.1182/blood-2018-07-862953 (2019).
Wang, M. et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory Mantle-Cell lymphoma. N. Engl. J. Med. 382, 1331–1342. https://doi.org/10.1056/NEJMoa1914347 (2020).
Hoster, E. et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 111, 558–565. https://doi.org/10.1182/blood-2007-06-095331 (2008).
Hoster, E. et al. Confirmation of the mantle-cell lymphoma international prognostic index in randomized trials of the European Mantle-Cell lymphoma network. J. Clin. Oncol. 32, 1338–1346. https://doi.org/10.1200/JCO.2013.52.2466 (2014).
Chihara, D. et al. Ki-67 is a strong predictor of central nervous system relapse in patients with mantle cell lymphoma (MCL). Annals Oncology: Official J. Eur. Soc. Med. Oncol. 26, 966–973. https://doi.org/10.1093/annonc/mdv074 (2015).
Determann, O. et al. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL network and the German low grade lymphoma study group. Blood 111, 2385–2387. https://doi.org/10.1182/blood-2007-10-117010 (2008).
Mohanty, A. et al. Regulation of SOX11 expression through CCND1 and STAT3 in mantle cell lymphoma. Blood 133, 306–318. https://doi.org/10.1182/blood-2018-05-851667 (2019).
Yoon, D. H. et al. Treatment of mantle cell lymphoma in Asia: a consensus paper from the Asian lymphoma study group. J. Hematol. Oncol. 13 https://doi.org/10.1186/s13045-020-00855-9 (2020).
Kang, B. W. et al. Clinical features and treatment outcomes in patients with mantle cell lymphoma in Korea: study by the consortium for improving survival of lymphoma. Blood Res. 49, 15–21. https://doi.org/10.5045/br.2014.49.1.15 (2014).
Chuang, S. S., Huang, W. T., Hsieh, P. P., Tseng, H. H. & Chang, H. M. Striking male predominance of mantle cell lymphoma in Taiwan. J. Clin. Pathol. 59, 780. https://doi.org/10.1136/jcp.2005.035071 (2006).
Acknowledgements
We thank the patients and doctors at the participating institutes for their invaluable contributions to this cooperative study.
Funding
This study was supported by grants from the National Natural Science Foundation of China [no. 82000200], grants from clinical trial agreement which is Signed by Xi ‘an Janssen Pharmaceutical Co., Ltd. and Hunan Cancer Hospital[no:54179060MCL4007]. Hunan Provincial Natural Science Foundation of China (grant numbers 2022JJ30026 and 2025JJ50527). Clinical Research Center For Lymphoma In Hunan Province, Hunan Provincial Clinical Medical Research Center for Lymphatic tumor (grant number 2021SK4015),Project of Health Commission of Hunan Province (grant numbers 202203045455), Xisike-Xinda Clinical Oncology Research Foundation(grant number Y-XD202002-0104).
Author information
Authors and Affiliations
Contributions
Conceptualization: Hui Zhou and Ling Xiao; Formal analysis: Qingqing Cai, Dehui Zhou, Huilai Zhang, Rong Liang, Dongfeng Zeng, Haige Ye, Yun Liang and Xiuhua Sun. Writing - Original draft: Yizi He Writing - review & editing: Caiqin Wang, Tao Pan, The work reported in the article has been performed by the authors, unless clearly specified in the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statements
According to ethical policies of the Affiliated Tumor Hospital of Xiangya Medical School Ethics Committee, clinical data can be analyzed and used under the precondition of without revealing the identity of patients.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, Y., Wang, C., Pan, T. et al. POD24-Based prognostic signature enables personalized risk stratification in mantle cell lymphoma. Sci Rep 15, 8687 (2025). https://doi.org/10.1038/s41598-025-92963-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92963-0