Abstract
This study investigates the characteristics and performance of ceramic coatings fabricated on 2024 aluminum alloy surfaces using the scanning plasma electrolytic oxidation (SPEO) process. The effects of various scanning parameters, including discharge gap, scanning frequency, scanning speed, and overlap rate, on coating properties were systematically examined. The coatings were characterized using potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), microhardness testing, roughness analysis, and thickness measurement. The results reveal that optimizing scanning parameters significantly enhances coating performance. The SPEO process enables efficient localized coating deposition through progressive scanning, achieving performance comparable to that of conventional full-surface PEO. Although the high current density associated with the SPEO process generates more surface pores and microcracks, which slightly affect hardness, roughness, and corrosion resistance, the differences are minimal and acceptable for most applications. Furthermore, the SPEO process demonstrates high efficiency in repairing large or complex components, highlighting its significant potential for industrial applications.
Similar content being viewed by others
Introduction
Aluminum and its alloys are widely utilized in aerospace, automotive manufacturing, and medical device industries due to their excellent specific strength, thermal conductivity, and corrosion resistance1,2. Among them, 2024 aluminum alloy, a high-strength and low-density wrought alloy, is particularly well-suited for aerospace and medical devices applications owing to its superior fatigue strength and fracture toughness. However, 2024 aluminum alloy is highly susceptible to environmental corrosion, especially in chloride-containing environments, where it is prone to pitting and crevice corrosion, significantly shortening its service life. To enhance its surface properties and extend its operational lifespan, surface modification technologies have become a focal point of research.
Plasma electrolytic oxidation (PEO), also known as micro-arc oxidation (MAO), is a relatively new surface treatment technique that has garnered significant interest for fabricating ceramic coatings on valve metals such as aluminum, magnesium, and titanium alloys to enhance their functional properties3,4,5. The PEO technique is an electrochemical surface process in a plasma environment on these metals by spark micro-discharges, without using strong acid solutions, in contrast with conventional anodization6,7,8. It is featured by high productivity, ecological friendliness, economic efficiency, good wear and corrosion resistance, high hardness, and excellent bonding strength with the substrate, due to the characterization of in-situ growth of the coating9,10,11.
Although the PEO process offers significant advantages in enhancing surface properties, its application to large-scale components faces considerable challenges, severely limiting its widespread adoption in engineering. Traditional PEO techniques require complete immersion of the workpiece in an electrolyte solution, which poses substantial difficulties in handling and processing large components. This approach also leads to increased power loads and electrolyte consumption. To improve processing efficiency, researchers have explored covering untreated areas of the workpiece with insulating layers. However, this method is only suitable for components with simple geometries and proves ineffective for complex structures. Furthermore, in practical engineering applications, surface modification is typically required only for specific functional areas, such as those needing wear resistance or corrosion protection. Treating non-functional areas not only wastes energy but also reduces processing efficiency. In order to overcome these shortcomings for traditional PEO, some researches have been conducted to control treated area. Timoshenko et al.12. put forward a means to produce PEO coating for large and intricate workpieces, by immersing them gradually, which enables the PEO process to be performed under relatively low electric current. However, these ways do not break the limit that workpieces should be immersed in electrolyte. Therefore, it is urgent to develop a PEO technology that can efficiently and accurately handle specific areas on the surface of complex workpieces.
To address the aforementioned challenges, scanning plasma electrolytic oxidation (SPEO) technology has emerged as a research focus due to its flexibility and efficiency. This technique confines the discharge area to a small, localized region and incrementally covers the substrate surface by following a controlled trajectory, enabling precise treatment of specific areas. Such a localized processing approach significantly reduces electrolyte and energy consumption while also minimizing the handling and processing difficulties associated with large workpieces. Additionally, SPEO technology mitigates localized overheating and offers high coating efficiency13. However, the dynamic discharge characteristics and uneven coating formation inherent to the scanning process present new technical challenges, emphasizing the need to optimize process parameters to achieve high-quality coatings.
Notably, the Cu element in 2024 aluminum alloy can participate in oxidation reactions during the PEO process, resulting in antimicrobial coatings that hold great promise for medical applications14. Such coatings are particularly beneficial for thoracic and microsurgical instruments, as they not only significantly enhance corrosion resistance but also effectively reduce the risk of postoperative infections. Furthermore, the SPEO process demonstrates exceptional potential for efficiently treating complex surface structures, making it highly suitable for the repair and functional enhancement of these medical devices.
This study investigates the preparation of ceramic coatings on 2024 aluminum alloy substrates using the scanning plasma electrolytic oxidation (SPEO) technique. The effects of various scanning parameters, including discharge gap, scanning frequency, scanning speed, and overlap ratio, on coating thickness, surface roughness, and microhardness were systematically examined. Scanning electron microscopy (SEM), X-ray diffraction (XRD), and electrochemical impedance spectroscopy (EIS) were employed to analyze the coating growth mechanism and optimize its performance. This study aims to elucidate the regulatory effects of the scanning plasma electrolytic oxidation (SPEO) process on coating properties, offering a feasible and efficient solution for surface modification of complex structures in medical applications and large-scale metal components in industrial sectors such as aerospace and automotive engineering.
Materials and methods
Preparation of SPEO coating
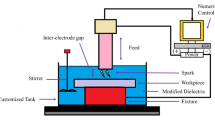
Samples of 2024 Al alloy (0.50% Si plus Fe, 4.2% Cu, 0.50% Mn, 1.40% Mg, 0.30%Zn, balance Al) were used in these ex-periments. The samples were polished with #1000 SiC abra-sive paper, rinsed in distilled water, cleaned ultrasonically in alcohol and finally dried in air. Figure 1 shows the composition and working principle of SPEO system.
The SPEO process was carried out with a 10 kW homemade power supply equipped with a stirring system and a cooling system. A water pump was used to eject electrolyte to sample surface through a stainless steel tube with an inner diameter of 1.25 mm. The specimen was fixed on a three-axis motion platform controlled by a CNC system, which ensured the PEO process performed in any position. The distance between the tube and specimen was 2.0 mm. The scanning speed was fixed at 0.25 mm/s. The stainless steel tube and the specimen were used as cathodic electrode and anodic electrode, respectively. The current, frequency, duty ratio, overlapping factor and scanning speed were fixed at 1 A, 500 HZ, 50%, 15% and 0.25 mm/s. The electrolyte used during PEO process was composed of 1 g/L KOH and 8 g/L Na2SiO3. The temperature of the electrolytic solution was kept below 35 °C.Based on these parameters, two kinds of SPEO coatings were prepared: line coating produced by rectilinear movement of tube and area coating by route movement depicted in Fig. 1b, respectively. For comparison, conventional PEO coatings were also prepared using the same process parameters as those employed for SPEO coatings.
Coating analysis
X-ray diffraction (XRD) was carried out using a D/max-rB to examine the composition of the SPEO coatings. The specimens were scanned using CuKα radiation. Diffraction data were obtained over scattering angle 2θ from 10° to 90°with a step of 0.04° and acquisition time of 1s/step. Scanning electron microscope (SEM, FEI, SIRION) was used to examine the surface and the cross-sectional morphologies of the SPEO coating. Electrochemical tests were conducted using an electrochemical workstation (Reference 600+, Gamry) conducted with a standard three-electrode system. A platinum sheet served as the counter electrode, an Ag/AgCl electrode was used as the reference electrode, and the sample, with an exposed surface area of 1 cm², functioned as the working electrode. Following a 30-minute stabilization period in 3.5 wt% NaCl solution, potentiodynamic polarization tests were carried out at a scan rate of 1 mV/s, ranging from − 0.2 V to 0.1 V relative to the open circuit potential (OCP). Corrosion current density (Icorr) and corrosion potential (Ecorr) were obtained using Tafel extrapolation. EIS tests were obtained with 10 mV sinusoidal perturbation at open circuit potential. Measurement frequencies ranged from 100,000 Hz to 0.1 Hz. Corrosion test results were analyzed using NOVA 2.1 software. The hardness of the coatings was measured using a microhardness tester (EM-810, FUTURE-TECH, Japan) with an applied load of 4.9 N and a loading time of 15 s. Surface roughness was measured using a surface roughness tester (SJ-210, Mitutoyo, Japan). Coating thickness was measured using an eddy current thickness gauge (MiniTest 600 B-N, EPK, Germany). Hardness, roughness and thickness were measured in three different areas. SPSS 19.0 software was used for statistical analysis. Data were expressed as mean ± standard deviation (SD).
Results and discussion
Effect of scanning process parameters on the performance of SPEO coatings
Scanning micro-arc oxidation ceramic coating is completed by scanning the electrode line by line scanning, its discharge characteristics and film formation characteristics are different from conventional micro-arc oxidation. The discharge gap, the number of scans, the scanning speed and the scanning overlap rate are its unique process parameters. These process parameters have a critical influence on the thickness, surface roughness and hardness of the ceramic coating, as shown in Fig. 2.
During the scanning micro-arc oxidation (SPEO) process, variations in the discharge gap lead to changes in the inter-electrode voltage. The micro-arc discharge and film formation processes indicate that the inter-electrode voltage influences the micro-arc discharge, which, in turn, affects the performance of the micro-arc oxidation ceramic coating. As shown in Fig. 2a, as the discharge gap increases, the thickness and microhardness of the scanning micro-arc oxidation ceramic coating first increase and then decrease, reaching their maximum values at a 2 mm discharge gap, with values of 17 μm and 252 Hv, respectively. Based on the discharge and film formation mechanisms of micro-arc oxidation, the inter-electrode current and voltage are key factors determining the coating performance. In constant current mode, when the discharge gap is smaller than 2 mm, the lower stable voltage results in insufficient micro-arc discharge energy and lower film formation efficiency, limiting the coating thickness and hardness. On the other hand, when the discharge gap exceeds 2 mm, although the inter-electrode voltage increases, the rise in equivalent resistance distributes some of the voltage, reducing the effective voltage for micro-arc discharge, leading to weakened discharge intensity and decreased film formation efficiency. Therefore, when the discharge gap exceeds 2 mm, both the coating thickness and microhardness significantly decrease.
To enhance the thickness and microhardness of the scanning micro-arc oxidation (SPEO) ceramic coating, repeated scanning along the original scanning trajectory is necessary. This approach increases the coating thickness and microhardness through multiple micro-arc discharges. As shown in Fig. 2b, the coating thickness increases with the number of scans, but the incremental increase gradually decreases. After the 6th scan, the film formation efficiency significantly decreases, and after the 9th scan, the coating thickness reaches 50.2 μm and stabilizes. During the micro-arc oxidation process, plasma discharge mainly occurs at the bottom of the micropores. As the coating thickens, the micropore depth increases, making discharge more difficult and reducing film formation efficiency. Simultaneously, the microhardness of the coating increases with the number of scans, with the increment first increasing and then decreasing. At the 9th scan, the microhardness reaches 826 Hv and stabilizes. The increase in hardness is primarily due to the increase in coating thickness and the phase transformation of Al₂O₃ from the γ phase to the α phase, especially the formation of a dense high-hardness layer during deep discharge and buried arc discharge processes. As the thickness further increases, the discharge intensity weakens, and the heat is insufficient to drive the phase transformation, preventing further increases in hardness. In practical applications, a coating hardness of 500 Hv is sufficient to meet requirements. Six scans are adequate to meet both film formation efficiency and hardness requirements.
The selection of scanning speed directly affects the actual oxidation time of the treated area, thereby indirectly influencing the performance of the scanning micro-arc oxidation (SPEO) ceramic coating. As shown in Fig. 2c, scanning speed has a significant effect on the thickness of the SPEO ceramic coating. At low scanning speeds, the coating thickness is larger; as the scanning speed increases, the thickness gradually decreases in a linear relationship. When the scanning speed reaches 0.54 mm/s, the effect becomes less pronounced; after exceeding 0.9 mm/s, the thickness remains nearly constant. The variation in scanning speed determines the coating thickness by affecting the actual processing time of the treated area. At lower scanning speeds, the discharge time is longer, which helps with deep discharge, improves film formation efficiency, and leads to thicker coatings. At higher scanning speeds, the discharge stays on the surface, reducing film formation efficiency and resulting in thinner coatings. Figure 2c also shows that scanning speed has a similar effect on surface roughness. At lower scanning speeds, the surface roughness is higher; as the scanning speed increases, the surface roughness gradually decreases and stabilizes at 0.54 mm/s. During the micro-arc oxidation process, as the coating thickness increases, discharge becomes more difficult, and discharge energy concentrates on a single spark, causing the spark size to increase. The larger spark size increases the diameter and depth of micropores on the coating surface, significantly affecting surface roughness, consistent with the trend of coating thickness changes. Considering film formation efficiency and coating performance, the optimal scanning speed is 0.54 mm/s.
To ensure uniformity in thickness and consistent performance of the scanning micro-arc oxidation (SPEO) ceramic coating, it is necessary for the ceramic coating on adjacent tracks to partially overlap. As shown in Fig. 2d, increasing the overlap rate significantly improves the coating thickness. When the overlap rate is below 20%, the thickness increases linearly, but it accelerates once the overlap rate exceeds 20%. Figure 2d also indicates that the surface roughness first decreases and then increases with the overlap rate. The minimum roughness occurs at a 15% overlap rate. The discharge characteristics of micro-arc oxidation lead to a decrease in coating thickness from the center of the track towards the edges. After overlapping adjacent tracks, the discharge is repeated, resulting in increased coating thickness and reduced thickness variation, which improves surface roughness. When the overlap rate is 0%, the roughness is maximum, and at 15%, the roughness is minimum; beyond 15%, the roughness increases. The increase in average coating thickness is mainly attributed to the overlapping discharge in the edge areas, reflecting the overall improvement in coating thickness. Increasing the overlap rate helps to improve coating thickness and corrosion resistance. However, an excessively high overlap rate reduces processing efficiency, extends processing time, and leads to increased coating roughness. Therefore, an optimal overlap rate of 15% is selected in this experiment.
Overall, the scanning process parameters have a significant impact on coating performance. The optimal process parameters are as follows: discharge gap: 2 mm, scan number: 6 times, scan speed: 0.54 mm/s, and overlap rate: 15%.
Voltage-time response
Figure 3 shows the voltage-time response during the SPEO and PEO processes. Generally, four stages are divided during PEO process: general anodization, sparking anodization, micro-arc oxida-tion and arc oxidation15. During general anodization stage, a passivation layer is formed on the surface of the Al alloy and the voltage increases abruptly. After the voltage reaches the breakdown voltage, sparking anodization stage occurs. In this stage, the voltage increases continually, but the voltage-time response slope decreases. As the oxidized process contin-ues, a steady-state sparking is established on metal surface and the voltage reaches a relatively stable value, which indi-cates the micro-arc oxidation stage, the main stage for coating formation16,17,18,19. Usually, the fourth stage arc oxidation stage is not needed. However, the voltage-time response of SPEO does not conform to that of traditional PEO. In term of SPEO, two stages are divided. In the initial stage, the anodic voltage increased extremely fast and after the voltage reached about 520 V at about 7 s, the process turned into a stable stage, which was the main process of SPEO.
During the initial stage, as no ceramic coating was formed on the surface of substrate, the general anodization and sparking anodization for traditional PEO were dominant20. Since the major oxidizing area was very small (Fig. 1b), in which most of the power was consumed, the current density was as high as about 8149 A/dm2 (Eq. (1)), which is hundreds of times higher than that of traditional PEO. Under such a high current density, ceramic coating was formed so fast that the initial stage lasted only about 7 s, which is usually several minutes for traditional PEO21,22,23.
where DI is the current density of in oxidizing area, i is the current during SPEO process, d is the diameter of the ejecting tube.
After the voltage reached about 520 V, the process turned into the second stage, stable stage. In this stage, all the first three stages for traditional PEO, general anodization, sparking anodization and micro-arc oxidation, existed at the same time in different place of oxidizing area and changed as the tube moved continuously. Since the area in the front of the water spot mainly consisted of uncoated substrate, general anodization and sparking anodization were dominant in this area. In contrast, the area in the back of the water spot was mainly composed of oxidized coating and thus, in this area, micro-arc oxidation was dominant. In sum, from the front to the back of the water spot, as the oxidizing time became longer, these three stages changed: micro-arc oxidation became more dominant; general anodization and sparking anodization became less dominant.
Coating composition analysis
The XRD patterns of the SPEO and PEO coating samples are shown in Fig. 4. Strong Al peaks in the pattern correspond to the metal substrate. According to the XRD analysis, The coating produced by SPEO is mainly composed of γ-Al2O3 and a relatively minor amount of α-Al2O3, which accords with traditional PEO coating. Based on the relative intensities of the γ-Al2O3 and α-Al2O3 diffraction peaks, the PEO coating contains a slightly higher proportion of α-Al2O3. Consistent with this, the hardness of the SPEO coating is slightly lower.
As the tubular cathode moved continuously, the average oxidizing time for individual point was only less than 10 s in the major SPEO area (Eq. (2)), much shorter than that of traditional PEO, which usually lasts between 3 and 60 min23,24. Therefore, the growth of SPEO coatings in such a short time is mainly due to the extremely high current density.
where dC is the distance from the central line, dS is the distance that the main SPEO area passes, at certain point dC away from the central line, r is the radius of the main SPEO area, v is scanning speed of ejecting tube, tO is oxidizing time, at certain point, dC away from the central line.
Surface morphology
Figure 5a shows the pictures of the line coating and area coating prepared by SPEO on 2024 aluminum alloy, and Fig. 5b shows the scanning trajectory of area coating. From Fig. 5a, it can be seen that there are three obvious zones in the line coating, from the centerline of the scanning trajectory to the coating, the growth stopping edge of the micro-arc oxidation zone, anodic oxidation zone, and passivation zone, in that order. The zone coatings are gradually generated by continuous line scanning with “S” shaped trajectories, as shown in Fig. 5b. Moreover, due to the flexibility of the CNC system, any area that needs to be coated can be reached. SPEO, therefore, is proved to be an applicable way to produce or repair ceramic coating, extremely fit for large work-pieces.
Figure 6 shows the SEM images of the single-pass coating surface, at different distances from the scan trajectory center, and for conventional PEO coatings. The ceramic coatings prepared by SPEO exhibit a porous and loose surface, consistent with conventional PEO coatings. A large number of lamellar spherical fragments are randomly distributed across the surface, resembling volcano tops, which is attributed to discharge activities and gas release during the PEO process. The micropores near the scan centerline are uneven in size, indicating non-uniform discharge phenomena. Additionally, due to the rapid solidification of alumina in the discharge channels, some microcracks appear on the coating surface, caused by thermal stress25. Figure 6g presents the SEM image of a conventional PEO coating, showing distinct micropores and molten material ejected from the micro-pools after cooling. At 0 mm from the scan centerline in the SPEO coating (Fig. 6b), compared to the PEO coating, the surface has larger and denser micropores with higher surface roughness. At 6 mm from the centerline, the surface morphology is similar to that of the PEO coating, with comparable surface roughness. In comparison to the PEO process, the rapid voltage rise and higher current density in the SPEO process exacerbate surface discharge bursts, creating more large-pore micropores and increasing surface roughness.
As is the basic factor of various area coating, line coating is mainly investigated in this work. Largest roughness appears near the central line of scanning route of water spot. As the distance away from central line increases, the roughness decreases from Fig. 6b–e. This result is owing to the decrease of oxidizing time, with the increase of the distance away from central line in major SPEO area (Eq. 2). After the distance sur-passes the boundary of major SPEO area, ceramic coating becomes not obvious, which was generated under very low current density and very short time in minor SPEO area (Fig. 6f). In this area, extremely smooth surface and small holes are found, indicating that general anodization and sparking anodization are dominant. Moreover, along the scanning route, coating morphology appears consistent, due to the same oxidizing condition, except for the oxidizing area in initial stage. For area coating, the roughness stays the same in the main SPEO area but becomes higher in the boundary of the major SPEO area and minor SPEO area, which was oxidized twice.
(a) Overall surface characteristics of the line scan coating; (b) Surface characteristics at 0 mm from the scan trajectory centerline; (c) Surface characteristics at 0.2 mm from the scan trajectory centerline; (d) Surface characteristics at 0.4 mm from the scan trajectory centerline; (e) Surface characteristics at 0.6 mm from the scan trajectory centerline; (f) Surface characteristics at 0.8 mm from the scan trajectory centerline; (g) Surface characteristics of the PEO coating.
Figure 7a–f shows the cross-sectional images of SPEO coatings prepared on 2024 Al alloy at different distances from the centerline of the scanning path, and Fig. 7f shows the cross-sectional image of PEO coatings. Porous layer and compact layer are identified in these images (Fig. 7a–c), in conformity with traditional PEO coating. The largest thickness appears in the central line of scanning route, which is as large as 25 μm, due to the longest oxidizing time. As distance from central line increases, the thickness decreases, as a result of the de-crease of oxidizing time. After surpassing the boundary line of major SPEO area, coating is extremely thin, since the current density was very small and oxidizing time was very short. During SPEO process, general anodization and sparking anodization were dominant in this area and thus only thin oxidized layer, not typical structure of PEO coating, was formed (Fig. 7e).
(a) Cross-sectional characteristics at 0 mm from the scan path centerline; (b) Cross-sectional characteristics at 0.2 mm from the scan path centerline; (c) Cross-sectional characteristics at 0.4 mm from the scan path centerline; (d) Cross-sectional characteristics at 0.6 mm from the scan path centerline; (e) Cross-sectional characteristics at 0.8 mm from the scan path centerline; (f) Cross-sectional characteristics of the PEO coating.
The corrosion properties test
The corrosion properties of the 2024 alloy and the SPEO and PEO coating produced in sodium silicate aqueous solution were evaluated using potential dynamic polarization tests in a 3.5 wt% NaCl solution and electrochemical impedance spectroscopy (EIS) tests. The results are shown in Fig. 8; Table 1. The parameters related to the corrosion properties, the corrosion potential (Ecorr) and corrosion current density (icorr) were meas-ured from the potential dynamic polarization curves. Expressing the coating’s corrosion performance in terms of corrosion rate, the table results indicate that all coatings exhibit significantly lower corrosion rates compared to the substrate (0.1851 mm/year). Notably, the SPEO coating shows a substantially reduced corrosion rate of 0.0095 mm/year, nearly 20 times lower than that of the substrate. These findings confirm the superior corrosion resistance of the SPEO coating.
As shown in Fig. 8a, the untreated aluminum alloy exhibits a relatively low corrosion potential (Ecorr = -0.61 V) and a high corrosion current density (Icorr = 6.0 × 10⁻⁶ A/cm²). After SPEO treatment, the Ecorr increases to 0.15 V, and icorr drops to 8.1 × 10⁻⁷ A/cm². The corrosion potential of the treated sample increases by approximately 760 mV, indicating a significant reduction in the thermodynamic tendency for corrosion. The corrosion current density of the SPEO coating is reduced by nearly an order of magnitude compared to the aluminum alloy substrate, indicating a marked decrease in corrosion rate. Additionally, the corrosion potential and current of the SPEO coating are similar to those of the PEO coating, although the PEO coating exhibits a slightly higher corrosion potential and a slightly lower corrosion current.
EIS testing further confirms the corrosion resistance of the SPEO and PEO coatings, as shown in Fig. 8b–d. In the Nyquist plot, all samples exhibit incomplete capacitive arcs, with larger arc diameters indicating better corrosion resistance26. The PEO coating has the largest capacitive arc diameter, followed by the SPEO coating, and then the substrate. In Fig. 8c, the phase angle at low frequency (0.01 Hz), |Z|0.01 Hz, can serve as a semi-quantitative indicator of the coating’s corrosion resistance. The values of |Z|0.01 Hz for the substrate, SPEO, and PEO coatings are 1336.75Ω, 8546.92Ω, and 14628.6Ω, respectively, with maximum phase angles of 57.3°, 63.5°, and 69.7°. A higher phase angle can delay the corrosion reaction rate at the interface.
The EIS data were fitted using the equivalent circuit shown in Fig. 8d1–d2. In the circuit, Rs, Rc, Rct, W, CPE1, and CPEdl represent electrolyte resistance, coating resistance, charge transfer resistance, Warburg impedance, constant phase element (CPE), and double-layer capacitance, respectively. Table 1 summarizes the electrical parameters of the substrate, SPEO, and PEO coatings obtained from the EIS data analysis. The total resistance of the substrate (denoted as Rt = Rc + Rct) is 2062.36 Ω cm², while the total resistance for the SPEO and PEO coatings is 13,455 Ω cm² and 124,508 Ω cm², respectively. The total resistance of the SPEO coating is 6.5 times that of the substrate, although there is still a gap compared to the PEO coating. The EIS test results show that the corrosion resistance of the SPEO coating is significantly improved compared to the substrate, though it is slightly inferior to the PEO coating. This is primarily due to the high current density in the SPEO process, which increases the number of pores and microcracks, providing more pathways for the corrosion medium to penetrate, thereby weakening the shielding effect of the coating and slightly reducing its corrosion resistance. In summary, the potentiodynamic polarization tests show that the SPEO treatment significantly improves the corrosion resistance of 2024 aluminum alloy.
Microhardness, roughness and thickness
The microhardness, surface roughness, and thickness of the SPEO and PEO coatings are shown in Fig. 9. The microhardness of the SPEO coating is 554 HV, which is 206.85 HV lower than that of the PEO coating. This difference is primarily due to the coating formation mechanism, as the hardness is significantly influenced by the applied voltage. At a high voltage of 520 V, the increased heat from micro-arc discharge promotes the formation of the hard α-Al₂O₃ phase in the coating, thereby enhancing its hardness. Regarding surface roughness, the SPEO coating has a roughness of 3.131 μm, which is 0.461 μm higher than that of the PEO coating. The maximum surface roughness of the line coating is observed near the centerline of the discharge area, with roughness decreasing as the distance from the centerline increases. In comparison to the line coating, the area coating is formed by the connection of multiple line coatings, resulting in a slight reduction in the roughness in the overlapping regions and a more uniform roughness distribution across the area coating surface. In terms of thickness, the SPEO coating has a thickness of 48.59 μm, which is similar to the 49.25 μm thickness of the PEO coating. With an increase in the number of scans, the thickness of the SPEO coating may exceed that of the PEO coating. Additionally, increasing the number of scans can further enhance the properties of the SPEO coating, though it will reduce the workpiece repair efficiency.
Overall, the SPEO coating, due to its higher current density, tends to form more pores and cracks, which slightly reduces the coating’s performance. However, the performance difference between SPEO and PEO coatings is minimal, and the SPEO coating meets the requirements of most applications. Furthermore, the SPEO process has distinct advantages in handling large-sized workpieces, such as flexibility and electrolyte savings. Therefore, the SPEO process demonstrates significant potential for practical engineering applications.
Conclusion
This study investigates the characteristics and performance of the scanning plasma electrolytic oxidation (SPEO) process for ceramic coating preparation on aluminum alloys, and compares it with the conventional full-surface PEO process. The results demonstrate that the SPEO process enables efficient and precise localized coating treatment through stepwise scanning, making it particularly suitable for large or complex workpieces. The study also optimized scanning process parameters, including discharge gap, scanning frequency, scanning speed, and overlap rate, significantly enhancing the coating properties.
The SPEO process consists of an initial and a stable stage. In the initial stage, the voltage rises rapidly to a stable value, and anodic oxidation and spark oxidation dominate. In the stable stage, anodic oxidation, spark oxidation, and micro-arc oxidation occur simultaneously. Compared to conventional PEO, the SPEO process offers a clear advantage in film-forming efficiency, although the coating performance is slightly inferior. The surface and cross-sectional morphology of SPEO coatings are similar to those of PEO coatings, with the coating thickness from a single scan being greatest at the centerline of the scanning path and gradually decreasing outward.
Despite the higher current density of the SPEO process, which leads to the formation of pores and microcracks on the coating surface and affects the coating’s hardness, roughness, and corrosion resistance, the performance difference is minimal, and the SPEO coating still meets the requirements of most applications. Overall, the SPEO process accelerates coating formation through rapid voltage rise and higher current density in small area treatments, although it may impact coating quality. Future research should focus on optimizing the voltage and current density rise rates to improve coating quality. The SPEO process demonstrates significant potential for applications, particularly in repair efficiency and the treatment of large workpieces.
Data availability
All data generated or analysed during this study are included in this published article.
References
Al-qawabeha, U. et al. Properties and structures of micro-arc oxide coatings on airplane aluminum alloys. J. Mech. Sci. Technol. 1–6 (2025).
Pezzato, L. et al. Effect of different types of glass powders on the corrosion and wear resistance of Peo coatings produced on 6061 aluminum alloy. Met. Mater. Int. https://doi.org/10.1007/s12540-024-01786-7 (2024).
Wu, T., Blawert, C., Serdechnova, M. & Zheludkevich, M. L. Dissimilar metal joints on macro-and micro scales: impact on PEO processing-A review. J. Mater. Sci. Technol. (2024).
Jadhav, P., Bongale, A. & Kumar, S. A review of process characteristics of plasma electrolytic oxidation of aluminium alloy. J. Phys. : Conf. Ser. 1854, 012030 (2021).
Pezzato, L., Gennari, C., Franceschi, M. & Brunelli, K. Influence of silicon morphology on direct current plasma electrolytic oxidation process in AlSi10Mg alloy produced with laser powder bed fusion. Sci. Rep. 12, 14329 (2022).
Dudareva, N. Y., Kruglov, A. & Gallyamova, R. Structure and thermophysical properties of coatings formed by the method of microarc oxidation on an aluminum alloy AK4-1. Solid State Phenom. 284, 1235–1241 (2018).
Markov, M. et al. Study of the microarc oxidation of aluminum modified with silicon carbide particles. Russ. J. Appl. Chem. 91, 543–549 (2018).
Fattah-Alhosseini, A., Keshavarz, M. K., Molaei, M. & Gashti, S. O. Plasma electrolytic oxidation (PEO) process on commercially pure Ti surface: Effects of electrolyte on the microstructure and corrosion behavior of coatings. Metall. Mater. Trans. A 49, 4966–4979 (2018).
Nikoomanzari, E. et al. Impressive strides in antibacterial performance amelioration of Ti-based implants via plasma electrolytic oxidation (PEO): A review of the recent advancements. Chem. Eng. J. 441, 136003 (2022).
Yao, W. et al. Recent advances in protective coatings and surface modifications for corrosion protection of Mg alloys. J. Mater. Res. Technol. (2024).
Yan, H. et al. Effects of Micro-arc oxidation process parameters on Micro-structure and properties of Al2O3 coatings prepared on sintered 2024 aluminum alloy. J. Mater. Eng. Perform. 1–12 (2023).
Timoshenko, A. V. & Rakoch, A. G. Method for microplasma electrolytic processing of surfaces of electroconductive materials. (2001).
Wang, J. et al. Effect of ultra accurate control of electrolyte temperature on the performance of micro Arc oxidation ceramic coatings. Ceram. Int. 49, 33236–33246 (2023).
Li, Q., Liu, C., Yang, W. & Liang, J. Growth mechanism and adhesion of PEO coatings on 2024Al alloy. Surf. Eng. 33, 760–766 (2017).
Dai, W. et al. Influence of duty cycle on fatigue life of AA2024 with thin coating fabricated by micro-arc oxidation. Surf. Coat. Technol. 360, 347–357 (2019).
Hao, Y. et al. Construction and growth of black PEO coatings on aluminum alloys for enhanced wear and impact resistance. Ceram. Int. 49, 30782–30793 (2023).
Sieber, M. et al. Wear-resistant coatings on aluminium produced by plasma anodising—A correlation of wear properties, microstructure, phase composition and distribution. Surf. Coat. Technol. 240, 96–102 (2014).
Nominé, A., Martin, J., Henrion, G. & Belmonte, T. Effect of cathodic micro-discharges on oxide growth during plasma electrolytic oxidation (PEO). Surf. Coat. Technol. 269, 131–137 (2015).
Kasalica, B. et al. The mechanism of evolution of microdischarges at the beginning of the PEO process on aluminum. Surf. Coat. Technol. 298, 24–32 (2016).
Dmitruk, A. et al. Elsevier,. PEO-coated aluminum alloys with good thermal conductivity for TES applications. in Advanced Ceramic Coatings for Energy Applications 213–247 (2024).
Sobolev, A., Kossenko, A., Zinigrad, M. & Borodianskiy, K. Comparison of plasma electrolytic oxidation coatings on al alloy created in aqueous solution and molten salt electrolytes. Surf. Coat. Technol. 344, 590–595 (2018).
Tillous, K., Toll-Duchanoy, T., Bauer-Grosse, E., Hericher, L. & Geandier, G. Microstructure and phase composition of microarc oxidation surface layers formed on aluminium and its alloys 2214-T6 and 7050-T74. Surf. Coat. Technol. 203, 2969–2973 (2009).
Hussein, R. O., Northwood, D. O. & Nie, X. The effect of processing parameters and substrate composition on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloys. Surf. Coat. Technol. 237, 357–368 (2013).
Hussein, R. O., Northwood, D. O. & Nie, X. Coating growth behavior during the plasma electrolytic oxidation process. J. Vacuum Sci. Technol. A 28, 766–773 (2010).
Zhang, Y. et al. Micro-structures and growth mechanisms of plasma electrolytic oxidation coatings on aluminium at different current densities. Surf. Coat. Technol. 321, 236–246 (2017).
Wang, Z., Li, J., Wang, Y. & Wang, Z. An EIS analysis on corrosion resistance of anti-abrasion coating. Surf. Interfaces 6, 33–39 (2017).
Acknowledgements
The authors would like to thank the Materials Heat Treatment Laboratory, School of Mechanical Engineering, Dalian University, for helpful discussions on topics related to this work. The authors also gratefully acknowledge the support of the “Dalian University Discipline Construction—Interdisciplinary Project (DLUXK-2023-ZD-003)” for the innovative research and development of minimally invasive thoracic surgical instruments (2023-2024).
Funding
This work was supported by the “Dalian University Discipline Construction—Interdisciplinary Project (DLUXK-2023-ZD-003)” for the innovative research and development of minimally invasive thoracic surgical instruments (2023–2024).
Author information
Authors and Affiliations
Contributions
X.X. and P.L. drafted the main manuscript text. W.J., T.Y., and Q.D. prepared Figs. 1, 2 and 3. Y.M. and Z.W. contributed to the experimental setup and data collection. Z.Z. provided technical guidance and analysis. P.L. supervised the project and reviewed the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, X., Jia, W., Yin, T. et al. Characterization of the ceramic coating formed on 2024 al alloy by scanning plasma electrolytic oxidation. Sci Rep 15, 8044 (2025). https://doi.org/10.1038/s41598-025-93028-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93028-y