Abstract
Microvesicles (MVs) are membrane vesicles secreted by cells and are present in the saliva of healthy individuals. It has various functions and has been reported to be a biomarker for malignant tumors. The changes in saliva levels of MVs associated with disease(s) is unclear. This study aimed to determine the proportion of salivary apoptotic MVs and their association with oral ulcer(s) in patients with non-healing oral ulcer(s) and reported oral cancer. Saliva (5 mL) was collected from patients with non-healing oral ulcer(s) and reported oral cancer (at the time of saliva collection, the participant have an oral ulcer(s) in the oral cavity and have an oral cancer lesion; n = 73) and healthy volunteers with oral ulcer(s) (n = 62). A standard differential centrifugation protocol was used for the purification of MVs. Dynamic light scattering and transmission electron microscopy were used to characterize MVs. Flow cytometry was used to quantify salivary apoptotic MVs. Immunocytochemistry was performed according to a standard protocol. None of patients with oral cancer has smoking and drinking habit. The majority of saliva samples derived from patients with non-healing oral ulcer(s) and reported oral cancer were more positive for the fluorescent dye carboxyfluorescein succinimidyl ester than those of healthy volunteers with oral ulcer(s). Salivary fluid obtained from patients had membrane-limited vesicles that were round and/or slightly elongated in shape, with diameters of 100–1,000 nm. The number of salivary apoptotic MVs was higher in patients with non-healing oral ulcer(s) than in those derived from healthy volunteers with oral ulcer(s) (p < 0.001). There was an association between salivary apoptotic MVs in patients with non-healing oral ulcer(s) and the degree or severity of oral ulcers (p < 0.001). Levels of salivary apoptotic MVs are elevated in patients with non-healing oral ulcer(s) and confirmed oral cancer. Elevated levels of salivary apoptotic MVs are associated with clinicopathological data of patients with oral cancer. Evidence level: IV. Technical efficacy: stage 3.
Similar content being viewed by others
Introduction
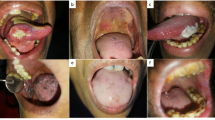
Oral squamous cell carcinoma is the most common head and neck oral cancer may be preceded by a non-healing oral ulcer1. Non-healing oral ulcers can have a wide variety of diagnoses and are not necessarily indicative of oral cancer. Prognosis of non-healing oral ulcer(s) is poor in oral squamous cell carcinoma2, with diminished survival and higher local recurrence rates3. Therefore, a mechanism underlying the non-healing of oral ulcer(s) is required especially for oral squamous cell carcinoma4. Additionally, note that not all non-healing oral ulcers have a poor prognosis.
Microvesicles (MVs) are 100–1,000 nm membrane vesicles secreted by cells5. MVs are present in the saliva of healthy individuals6. It has various functions and has been reported to be a biomarker for malignant tumors7. In oral cancer specifically on oral squamous cell carcinoma, elevated levels of salivary apoptotic MVs and their association with pathological parameters have been reported8,9. In addition to the elevated levels of blood MVs, a high levels of MVs have been reported in the urine of patients with bladder cancer10, ascites in patients with ovarian carcinoma11, and lung cancer patients in multiple fluids12. Changes in the salivary levels of MVs associated with disease(s) are unclear. Tumor cells may directly release MVs in the saliva2. MVs released during apoptosis have different pathophysiological functions2. Suppression of apoptosis is a key mechanism for cell apoptosis in the development of cancer13 and the levels of apoptotic MVs reflect the level of cell apoptosis and the development of cancer14,15,16. The number of apoptotic MVs is higher in the peripheral blood of patients with lung cancer17. The level of apoptotic salivary MVs in the case of non-healing oral ulcer(s) has not yet been revealed.
This study aimed to determine the proportion of salivary apoptotic MVs and their association with oral ulcer(s) in patients with non-healing oral ulcer(s) and reported oral cancer.
Methods
Design, setting, period
A cross-sectional study conducted from September 1, 2017, to October 1, 2021 at the Affiliated Hospital of JiangNan University, Wuxi, Jiangsu, China and the Shanghai East Hospital Affiliated to Tongji University, Shanghai, China.
Inclusion criteria
Patients 18 years and above with non-healing oral ulcer(s) (at the time of saliva collection, the participant have an oral ulcer in the oral cavity and have an oral cancer lesion) and reported oral cancer were included in the study. Oral squamous cell carcinoma and all others type of oral cancers were included in this study (limited to ameloblastoma malignant, oral squamous cell carcinoma, or melanoma). Healthy volunteers 18 years and above with oral ulcer(s) (six months of history) were also included in the study. Both types of patients were taking medications for non-healing oral ulcers as directed by physician. Healthy volunteers are free of oral and systemic medication of oral cancer. They have already had to be biopsied to confirm the disease. The oral cancer cases have been confirmed by histopathology.
Exclusion criteria
Patients with incomplete demographical and clinical data were excluded from the study. Pregnant women were not included in the research. Patients with other systemic diseases (hepatitis, tuberculosis, etc.) were also excluded from the study (because MVs are seen in other systemic diseases).
Saliva collection
The patients were instructed to stop smoking, drinking, and eating for 1 h before sample collection. Saliva (5 mL) was collected from patients with non-healing oral ulcer(s) and reported oral cancer, or healthy volunteers with oral ulcer(s)2. The saliva was obtained from the salivary gland duct. The patients were receiving any treatments for the cancer or oral ulcer prior to saliva collection. Saliva was collected in early morning before brushing. There was collection of one sample from each subject. Xerostomia procedure was considered for collecting saliva from oral cancer patients. Sampling method was purposive. Five investigators were involved in saliva collection and they were blinded.
Purification of MVs
Salivary apoptotic MVs were purified from saliva of patients using a standard differential centrifugation protocol (speed: 100,000 rpm; creating centrifugal speed forces: 800,000–1,000,000 g at 4 °C for 30 min)8,18,19.
Characterization and quantification of MVs
Dynamic light scattering and transmission electron microscopy were used to characterize the MVs. Flow cytometry was used to quantify salivary apoptotic MVs8,18. The concentration of the MVs was calculated using Eq. (1):
Fluorescence minus one (FMO) control and isotype-, isoclonic, and negative controls were preferred to overcome a high coincidence rate when measuring sub-micron particles by flow cytometry.
Immunocytochemistry
Salivary samples were mixed with 4% paraformaldehyde and embedded in paraffin. Immunocytochemistry was performed according to a standard protocol20,21,22. The saliva debris was analyzed in immunocytochemistry. Vascular endothelial growth factor C was used as marker for immunocytochemical staining.
Collection and testing of endothelial growth factor C
Venous blood was collected and Dynex™ DSX™ Automated ELISA system (Fisher Scientific, Waltham, MA, USA) was used to evaluate endothelial growth factor C23. Pathologist collected blood samples and the process was blinded.
Statistical analysis
InStat 3.01 GraphPad, software Inc., (San Diego, CA, USA) was used for the statistical analysis. The study is a primitive cross-sectional study. Therefore, the sample size was not calculated. The normality of continuous variables was checked using Kolmogorov–Smirnov test. Unpaired Student’s t-test or Mann–Whitney test was used for statistical analysis of normal continuous variables with homogeneous of variances or non-normal continuous variables and the Fisher exact test or Chi-square test (χ2-test, in the four-grid table, when the total number of cases was n ≥ 40 and the theoretical frequency of all grids was greater than or equal to 5) was used for statistical analysis of constant variables. Levene’s test was performed for homogeneity of variances. The Yate’s continuity correction was used when the any of the expected frequencies smaller than 10. Spearman’s rank correlation test was performed for clinicopathological data and MVs levels23. All results were considered statistically significant at p < 0.05.
Results
Study population
From September 1, 2017, to October 1, 2021, a total of 78 patients with non-healing oral ulcer(s) and oral cancer and 65 healthy volunteers with oral ulcer(s) were available and provided saliva samples at the Affiliated Hospital of JiangNan University, Wuxi, Jiangsu, China and the Shanghai East Hospital Affiliated to Tongji University, Shanghai, China. Among them, the complete data of five patients with non-healing oral ulcer(s) and reported oral cancer and the complete data of three healthy volunteers with oral ulcer(s) were not available. Therefore, these data (eight samples) were excluded from analysis. A summary of this study is shown in Fig. 1.
Demographic and clinical conditions
None of patients with oral cancer has smoking and drinking habit. Healthy volunteers with oral ulcer(s) were oral disease free and no systemic diseases. There were no significant differences in sex, age distribution, ethnicity, and comorbidities among patients with non-healing oral ulcer(s), reported oral cancer, and healthy volunteers with oral ulcer(s) (p > 0.05 for all, Table 1).
Characterization of MVs
Salivary fluid obtained from the patients had membrane-limited vesicles that were round and/or slightly elongated (Fig. 2) and 100–1,000 nm in diameter. There was no significant difference in the size of membrane-limited vesicles among derived from patients with non-healing oral ulcer(s), reported oral cancer, and healthy volunteers with oral ulcer(s) (Table 2).
There was no significant difference in the zeta potential of salivary fluid among derived from patients with non-healing oral ulcer(s), reported oral cancer, and healthy volunteers with oral ulcer(s) (p = 0.39, Fig. 3; Table 3, unpaired Student’s t-test).
The majority of saliva samples derived from patients with non-healing oral ulcer(s) and reported oral cancer were more positive for the fluorescent dye carboxyfluorescein succinimidyl ester than those derived from healthy volunteers with oral ulcer(s).
Quantification of MVs
The number of salivary apoptotic MVs was higher in saliva derived from patients with non-healing oral ulcer(s) than in those derived from healthy volunteers with oral ulcer(s) (p < 0.001, Mann–Whitney test, Fig. 4; Table 4).
Events values of microvesicles. Flow cytometry results. The middle horizontal line in the box is represents median value. Box represents quartile values. Upper whisker: maximum value, upper box line: third quartile value, x: median value, lower box line: first quartile value, lower whisker: minimum value.
Immunocytochemistry results were also in line with the results of flow cytometry (p < 0.001, Fig. 5, Mann–Whitney test) which shows the distribution of salivary apoptotic MVs and apoptotic events, mentions expression level of vascular endothelial growth factor C as one parameter.
Association of severity of oral ulcer(s) and salivary apoptotic MVs
Spearman’s rank correlation test showed an association between salivary apoptotic MVs in patients with non-healing oral ulcer(s) and degree or severity (events of apoptotic MVs) of oral ulcer(s) (p < 0.001, r = 0.9905, 95% confidence interval: 0.9838 to 0.9944; Fig. 6; Table 5).
The association between salivary apoptotic microvesicles of patients with non-healing oral ulcer(s) and degree or severity of oral ulcer(s) (expression level of vascular endothelial growth factor C). Point far from the right side (above 0.246 on the x-axis) was removed from the figure, because it occurred to be an outlier (numbers of points used: 61). It only represents the distribution of vascular endothelial growth factor C expression and apoptotic events.
The results of assumption tests are presented in Table 6.
Discussion
In the current study, the levels of salivary apoptotic MVs derived from patients with non-healing oral ulcer(s) were higher than those of salivary apoptotic MVs derived from healthy volunteers with oral ulcer(s). In addition, there is an association between salivary apoptotic MVs in patients with non-healing oral ulcer(s) and the degree or severity (based on clinical parameters) of oral ulcer(s). The available literature reports that 100–1,000 nm-sized MVs are released by cells upon apoptosis or activation due to the development of malignant tumors11,24,25. A previous ex-vitro study on association of tissue factor with blood coagulation6 reported an association between salivary apoptotic MVs and the severity of oral cancer; however, the detailed association between salivary apoptotic MVs and the severity of oral cancer has not been revealed. The current study suggests salivary apoptotic MVs as biomarkers for patients with non-healing oral ulcer(s) for oral cancer. However, the mechanisms leading to elevated levels of salivary apoptotic MVs in patients with non-healing ulcers and oral cancer is required to explore. The biological pathways linking MVs to oral cancer progression is required to elaborate.
The study showed an association between salivary apoptotic MVs in patients with non-healing oral ulcer(s) and the expression level of vascular endothelial growth factor C. The expression level of vascular endothelial growth factor C is a major pro-lymphangiogenic growth factor that upregulates the density of lymphatic vessels and promotes metastasis to the lymphatic node of tumor cells16,26. The results of the current study are in line with those of previous studies2,27. Apoptotic MVs are functionally involved in lymph node metastasis2. Salivary apoptotic MVs are associated with lymph node metastasis.
An unusual observation noted was that none of the patients with oral cancer had smoking and drinking (alcohol drinking) habits, which is concerning given the established correlation between these habits and oral cancer in much of the existing literature. This anomaly may indicate the presence of selection bias and can potentially affect the external validity of the findings. This was so because clinicians instructed to the patients to stop smoking and drinking. The changes that this may have affected the saliva concentration. Moreover, smoking has a high influence on oral mucosa conditions, especially in relation to apoptosis28. Thus, to stop these factors that may affect the findings.
In the study the patients with the non-healing ulcer(s) were included in the healthy group. However, a group of people without ulcer(s) should be added as the healthy group. The possible justification for the same is that if we used a group of people without ulcer(s) in the healthy group then it would be not clear whether the cancer or the ulcer(s) induced the increase in apoptotic MVs.
The study is based on a particular pathological data. The study identified a clear difference in the number of MVs between patients suffering from non-healing oral ulcer(s) and oral cancer. However, in this study, the possible mechanism underlying the increase in the association between salivary apoptotic MVs in patients with non-healing oral ulcer(s) and the expression level of vascular endothelial growth factor C was not evaluated (it does not analyze biological mechanisms). There is also a lack of depth in understanding the possible mechanisms behind the observed increase in salivary apoptotic MVs, creating a knowledge gap. The samples were from a small group of patients, limiting the results’ generalizability. Geographic parts of the oral cavity are not defined for non-healing oral ulcer(s). The study does not specify which geographic regions of the oral cavity were affected by non-healing ulcer(s), introducing potential variability in results. Additionally, the selection of the control group, consisting of healthy volunteers with oral ulcer(s), raises questions. They might not be the ideal comparator for patients with non-healing ulcer(s) and diagnosed oral cancer. Lastly, the duration of the study, spanning from September 2017 to October 2021, could introduce confounders, given the possible evolution in diagnostic and therapeutic methodologies over the period. Besides oral squamous cell carcinoma all others type of oral cancers were included in this study. Different types may vary in their proportions, which could affect the outcomes.
Conclusions
Levels of salivary apoptotic microvesicles are elevated in patients with non-healing oral ulcer(s) and confirmed oral cancer. Elevated levels of salivary apoptotic microvesicles are associated with clinicopathological data of patients with oral cancer. The study suggested salivary apoptotic microvesicles as biomarkers for patients with non-healing oral ulcer(s) due to oral cancer. In addition, more investigation about the clinical outcomes must be conducted to consider the microvesicles as a biomarker in future. To support such a claim, it would be essential to have a stronger evidence base, ideally provided by larger, multi-center studies that can more comprehensively and robustly examine the association. The idea of the study is important but if the oral cancer patients could be evaluated pathologically and staged and their MVs findings could be correlated the results will be so valuable and it will aid more and more in cancer biologic research in the future. The study sufficiently discussed the clinical significance of this biomarker and how it compares to existing diagnostic methods. In addition, a comparative analysis would enhance review of current biomarkers.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- MVs:
-
Microvesicles
- χ2-test:
-
Chi-square test
- r:
-
Sperman rank constant
- SD:
-
Standard deviation
- FMO:
-
Fluorescence minus one
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 65, 5–29. https://doi.org/10.3322/caac.21254 (2015).
Zhong, W. Q. et al. Increased salivary microvesicles are associated with the prognosis of patients with oral squamous cell carcinoma. J. Cell. Mol. Med. 23, 4054–4062. https://doi.org/10.1111/jcmm.14291 (2019).
Zhong, L. P. et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J. Clin. Oncol. 31, 744–751. https://doi.org/10.1200/JCO.2012.43.8820 (2013).
Ashara, K. C. et al. Vesicular drug delivery system: a novel approach. Mintage J. Pharm. Med. Sci. 3(3), 1–14 (2014).
Loyer, X., Vion, A. C., Tedgui, A. & Boulanger, C. M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 114, 345–353. https://doi.org/10.1161/CIRCRESAHA.113.300858 (2014).
Berckmans, R. J., Sturk, A., van Tienen, L. M., Schaap, M. C. & Nieuwland, R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood 117, 3172–3180. https://doi.org/10.1182/blood-2010-06-290460 (2011).
Hoefer, I. E. et al. Novel methodologies for biomarker discovery in atherosclerosis. Eur. Heart J. 36, 2635–2642. https://doi.org/10.1093/eurheartj/ehv236 (2015).
Ren, J. G. et al. Elevated level of circulating platelet-derived microparticles in oral cancer. J. Dent. Res. 95, 87–93. https://doi.org/10.1177/0022034515592593 (2016).
Ren, J. G. et al. Clinical significance and roles in angiogenesis of circulating microparticles in oral cancer. J. Dent. Res. 95, 860–867. https://doi.org/10.1177/0022034516641037 (2016).
Smalley, D. M., Sheman, N. E., Nelson, K. & Theodorescu, D. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J. Proteome Res. 7, 2088–2096. https://doi.org/10.1021/pr700775x (2008).
Press, J. Z. et al. Microparticles from ovarian carcinomas are shed into ascites and promote cell migration. Int. J. Gynecol. Cancer. 22, 546–552. https://doi.org/10.1097/IGC.0b013e318241d9b9 (2012).
Park, J. O. et al. Identification and characterization of proteins isolated from microvesicles derived from human lung cancer pleural effusions. Proteomics 13, 2125–2134. https://doi.org/10.1002/pmic.201200323 (2013).
Cotter, T. G. Apoptosis and cancer: the genesis of a research field. Nat. Rev. Cancer. 9, 501–507. https://doi.org/10.1038/nrc2663 (2009).
Rak, J. Microparticles in cancer. Semin Thromb. Hemost. 36, 888–906. https://doi.org/10.1055/s-0030-1267043 (2010).
Tseng, C. C. et al. Levels of circulating microparticles in lung cancer patients and possible prognostic value. Dis. Markers. 35, 301–310. https://doi.org/10.1155/2013/715472 (2013).
Sugiura, T. et al. VEGF-C and VEGF-D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: implications for use as a prognostic marker. Int. J. Oncol. 34, 673–680. https://doi.org/10.3892/ijo_00000193 (2009).
Wang, C. C. et al. Circulating endothelial-derived activated microparticle: a useful biomarker for predicting one-year mortality in patients with advanced non-small cell lung cancer. Biomed. Res. Int. 2014, 173401. https://doi.org/10.1155/2014/173401 (2024).
Chen, G. et al. Transformation of cell-derived microparticles into quantum-dot-labeled nanovectors for antitumor SiRNA delivery. Angew Chem. Int. Ed. Engl. 54, 1036–1040. https://doi.org/10.1002/anie.201410223 (2015).
Zhao, J. Y. et al. Ultrasmall magnetically engineered Ag2Se quantum Dots for instant efficient labeling and whole-body high-resolution multimodal real-time tracking of cell-derived microvesicles. J. Am. Chem. Soc. 138, 1893–1903. https://doi.org/10.1021/jacs.5b10340 (2016).
Zhong, W. Q. et al. Down-regulation of connexin43 and connexin32 in keratocystic odontogenic tumours: potential association with clinical features. Histopathology 66, 798–807. https://doi.org/10.1111/his.12569 (2015).
Zhong, W. Q. et al. M2-polarized macrophages in keratocystic odontogenic tumor: relation to tumor angiogenesis. Sci. Rep. 5, 15586. https://doi.org/10.1038/srep15586 (2015).
Liu, H. et al. Overexpression of macrophage migration inhibitory factor in adenoid cystic carcinoma: correlation with enhanced metastatic potential. J. Cancer Res. Clin. Oncol. 39, 287–295. https://doi.org/10.1007/s00432-012-1330-z (2013).
Almasy, E. et al. The diagnostic and prognostic role of vascular endothelial growth factor C in sepsis and septic shock. J. Crit. Care Med. 6(3), 152–158 (2020).
Muralidharan-Chari, V., Clancy, J. W., Sedgwick, A. & D’Souza-Schorey, C. Microvesicles: mediators of extracellular communication during cancer progression. J. Cell. Sci. 123, 1603–1611. https://doi.org/10.1242/jcs.064386 (2010).
Roca, E. et al. Detection of EpCAM-positive microparticles in pleural fluid: a new approach to mini-invasively identify patients with malignant pleural effusions. Oncotarget 7, 3357–3366. https://doi.org/10.18632/oncotarget.6581 (2016).
Siriwardena, B. S. et al. VEGF-C is associated with lymphatic status and invasion in oral cancer. J. Clin. Pathol. 61, 103–108. https://doi.org/10.1136/jcp.2007.047662 (2008).
Żmigrodzka, M., Guzera, M., Miśkiewicz, A., Jagielski, D. & Winnicka, A. The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression. Tumour Biol. 37, 1391–14401. https://doi.org/10.1007/s13277-016-5358-6 (2016).
Goyal, G. Comparison of salivary and serum alkaline phosphates level and lactate dehydrogenase levels in patients with tobacco related oral lesions with healthy subjects—a step towards early diagnosis. Asian Pac. J. Cancer Prev. 21(4), 983–991 (2020).
Acknowledgements
The authors are thankful to the medical and non-medical staff of the Affiliated Hospital of JiangNan University, Wuxi, Jiangsu, China and the Shanghai East Hospital Affiliated to Tongji University, Shanghai, China.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All the authors have read and approved the manuscript for publication. JZ was the project administrator who contributed to the methodology, software, validation, literature review, and data curation of this study. YD contributed to the conceptualization, literature review, formal analyses, software, and visualization of the study. SS contributed to the investigation, literature review, formal analyses, data curation, and methodology of this study. HY contributed to visualization, literature review, software, methodology, and validation of the study. LT contributed to the software, literature review, and data curation of the study. All authors contributed to the drafting and editing of the manuscript for intellectual content. All authors agree to be accountable for all aspects of the work, ensuring its integrity and accuracy.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The designed protocol (approval number: TU1524 dated 17 August 2017) was approved by the Affiliated Hospital of JiangNan University review board and the Shanghai East Hospital Affiliated to Tongji University review board and the Chinese Society of Clinical Oncology. The study followed the law of China and the v2008 Declarations of Helsinki. Written informed consent was obtained from all participating patients regarding participation in the study.
Consent for publication
Written informed consent was obtained from all participating patients regarding publication of manuscript (in one or more form).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, J., Dong, Y., Shi, S. et al. Salivary apoptotic microvesicles as biomarkers for prognostic non-healing oral ulcers and oral cancer: a cross-sectional study. Sci Rep 15, 9297 (2025). https://doi.org/10.1038/s41598-025-93075-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93075-5