Abstract
This study examined the effects of high-definition transcranial direct current stimulation (HD-tDCS) on social impairment in children with autism spectrum disorder (ASD), focusing on those with and without sensory processing abnormalities. A randomized double-blind sham-controlled trial involved 72 children with ASD, divided into three groups based on sensory integration status. A post-hoc analysis of 51 children aged 4–8 years who received true HD-tDCS was conducted, categorizing them into hypo-tactile, hyper-tactile, and typical tactile sensitivity groups. Therapeutic efficacy was compared across these groups. (1) The randomized cntrolled Trial: The typical sensory integration group showed significant improvements in social awareness (t = 5.032, p < 0.000) and autistic mannerisms (t = 3.085, p = 0.004) compared to the sensory integration dysfunction group. (2)The result of the post-hoc analysis: The hypo-tactile and typical tactile sensitivity groups exhibited notable improvements in social awareness, cognition, communication, autistic mannerisms, and total SRS scores. In contrast, the hyper-tactile group only had a significant reduction in social communication (t = 2.385, p = 0.022) post-intervention. HD-tDCS effectively improved social impairment symptoms in children with ASD, particularly those with typical sensory integration and either typical or hypo-tactile responsiveness.
Similar content being viewed by others
Introduction
Surveys conducted over the past 20 years have shown an increasing prevalence of Autism spectrum disorder (ASD), with global percentages between 1.0% and 1.5%1. Recent analyses also indicate a rising global prevalence of ASD, with estimates ranging from 0.72% to 1.18% based on comprehensive data sources, including registries and administrative databases2,3. ASD is a neurodevelopmental disorder characterized by social impairment, and approximately 45% − 96% of patients exhibit abnormalities or dysregulations in sensory perception or sensory processing, which in turn significantly impacts their communication and social interaction abilities4,5.
The etiology of Autism Spectrum Disorder (ASD) remains unclear, with its development attributed to a combination of genetic and environmental factors. And there are challenges in its treatment, including costs, limited efficacies of single interventions, and low recoveries6. Currently, conventional rehabilitation therapies, such as behavioral analysis therapy and language and cognitive training, are primarily used to treat social impairments in children with ASD7. Additionally, Cognitive behavioural therapy is also effective in improving social impairment in children with autism8. However, these approaches often have limited efficacy and require long-term intervention. In recent years, noninvasive brain stimulation techniques, such as transcranial direct current stimulation (tDCS), have gained attention due to their low-intensity stimulation and minimal adverse effects9,10.
The tDCS involves use of weak direct current by placing electrodes on the scalp, which indirectly induces spontaneous brain activity11,12. The tDCS has emerged as a noninvasive neuroregulation technique that interacts with excitatory and inhibitory neurotransmitters, facilitating neural regulation. The tDCS can modulate cortical excitability, enhance synaptic plasticity, alter local cerebral blood flow, regulate cortical excitatory-inhibitory (E/I) balance, modulate connectivity between cortical areas, and affect neurotransmitters in the brain13,14. And the tDCS can regulates EEG rhythms, promotes neuroplasticity and repair, and improves cerebral blood flow15,16. As a result, it can improve symptoms such as communication difficulties in children with ASD. The use of tDCS in children with ASD has therefore shown positive results and gained positive recognition17,18,19. Moreover, tDCS has shown potential in improving social functioning and cognitive flexibility in children with ASD19, though further studies are needed to evaluate its long-term effects. And it has been widely used in the treatment of various neuropsychiatric disorders, such as depressive disorder, attention-deficit hyperactivity disorder, Parkinson’s disease, stroke, epilepsy, etc.20,21,22,23. The main disadvantage of tDCS is that the spatial location is not precise enough, the stimulation depth is limited, but it generally has no short-term and long-term effects on the patient’s heart rate, blood pressure, respiration, EEG, and brain tissue24,25,26. However, due to the patient’s health condition, individual differences in the human body, and some unforeseen factors, a very small number of patients may experience the following adverse reactions during the treatment process: slight pain or burning sensation on the locally stimulated skin, numbness, dizziness, fatigue, mild excitement, etc.9,27.
High definition transcranial direct current stimulation (HD-tDCS) is an improved version of tDCS. It uses a unipolar current to create a ring-shaped focal stimulation, to enhance penetration of the current. The peak intensity occurs beneath the central electrode, and the polarity within the entire ring is determined by properties of the central electrode. HD-tDCS not only improves the spatial resolution of stimulation but also has the potential to modulate and enhance functional connectivity between cortical regions. By targeting specific brain areas, HD-tDCS can strengthen the connectivity of neural networks involved in sensory integration and social functioning, thereby addressing the part core symptoms of ASD28,29,30,31. Recent studies using neurophysiological techniques have reported that anodal tDCS enhanced neural connectivity within the cortical networks32,33 and increased functional connectivity between brain regions34. Central anodal HD-tDCS may activate the supplementary motor area, paracentral lobule and other relative brain areas, and increase functional connectivity within the social brain network that is anchored in this region35,36, thereby improving the accuracy of social awareness, social recognition, and social interaction in children with ASD. When anodal current stimulates neuronal cell bodies or dendrites, the resting membrane potential decreases, leading to depolarization and subsequent neuronal excitation. HD-tDCS facilitates the activation of neural circuits in the brain, the regulation of functional connectivity between brain regions, and the impact on distal cortical areas, ultimately improving social functioning28,29,30,31.
It is noteworthy that children with ASD often exhibit varying degrees of sensory abnormalities and sensory integration dysfunctions (SIDs)37. Sensory integration (SI) refers to the brain’s ability to recognize, categorize, interpret, and integrate sensory information from various sensory organs, including vestibular, tactile, proprioceptive, visual, auditory, and olfactory inputs38. Based on past experiences, the brain generates adaptive responses to the environment. The ability to perceive and process sensory-motor information is crucial for recognizing and understanding social actions and expressions39. However, when there are abnormalities in sensory processing or difficulties in integrating sensory information, SID can occur. This may interfere with the child’s perception and understanding of social stimuli, thereby impacting his/her social interaction abilities4,40,41. The study shows that there are significant differences in sensory processing patterns among patients with different psychiatric disorders, especially in those with ASD42. For example, hypersensitivity may lead to avoidance of touch, sound, or visual stimuli, making social interactions challenging. In addition, hypo-sensitivity or hypo-reactivity may result in a lack of sensitivity to nonverbal and social cues from others, which also impedes social interactions. Results from 14 studies indicated a significant high difference between ASD and typical groups in the presence/frequency of sensory symptoms, with the greatest difference in under-responsivity, followed by over-responsivity and sensation seeking43. These issues may be associated with abnormalities in neural circuits and synaptic connections related to sensory processing, as well as E/I imbalances in specific brain regions44,44,46. However, there is still a lack of comprehensive knowledge regarding the relationships and mechanisms involving SIDs, sensory abnormalities, and social impairments.
In general, studies have shown that abnormal sensory processing can play an important role in the severity of ASD symptoms as well as in the rate of acquisition of communication skills. The overall sensory score of children with ASD was significantly and positively correlated with the communication skills (P < 0.05)47. Another study showed that in children with ASD, hyporesponsiveness had a significant positive relationship with the severity of socio-communication symptoms48. Based on previous research on the neurobiological and neurochemical mechanisms underlying social impairment and sensory abnormalities in ASD, we hypothesize that there are common pathways between sensory perception and social functioning49,50. SID and sensory abnormalities may therefore affect the reconstruction of social functions.
Research showed that the Cz central anode HD-tDCS may improve the accuracy of perception, recognition, and social interaction of ASD children by activating the paracenter lobule51 and increasing functional connectivity in the social brain network, with the paracenter lobule as the hub52. In our earliest work, based on the functional magnetic resonance data analysis of 6–12-year-old ASD children, the levels of functional connectivity between socially related brain regions and the bilateral precentral gyrus, postcentral gyrus, supplementary motor area, and paracentral lobule in ASD children were lower than those in normal controls53,54. These abnormal brain interregional functional connectivities were related to the severity of ASD social dysfunction. In theory, the decline in these link connectivities in ASD may hinder the specialization of cortical areas involved in motor control, language, and social interaction, and could explain the damage to these functions55,56. In the following study, we found that anodal HD-tDCS targeting the Cz can effectively improve social deficits in children with ASD54. And during process, we found that tactile hypersensitivity may be an important factor determining the compliance of children with ASD with HD-tDCS treatment. Tactile sensitivity is characterized by abnormal perception of pain, temperature, pressure, etc., accompanied by tactile defensiveness. Although touch and pain are distinct sensory modalities, they share some sensory receptors and neural pathways. Abnormal processing of tactile information may result in tactile hypersensitivity or tactile hypo-responsiveness57. Simultaneously, sensory integration and tactile responsiveness are interdependent; the accuracy and efficiency of tactile responses depend on the quality of sensory integration, and vice versa. Sensory integration dysfunction may manifest as either hypersensitivity or hyposensitivity to tactile input58. Firstly, tactile sensation is a crucial component of sensory integration. Receptors in the skin are capable of receiving tactile information from the external environment and transmitting it to the brain. The brain needs to integrate this tactile information with other sensory information, such as visual and auditory inputs, to produce appropriate responses. Additionally, the sensory integration center in the brain is responsible for processing and interpreting information from different senses. The processing of tactile information in the brain not only affects tactile responses but may also influence the interpretation and reaction to other sensory information59.
Anodal HD-tDCS, which provides excitatory stimulation to the brain region, may be more suitable for children with ASD with hypo-reactivity, especially those with tactile hypo-responsiveness. In the present study, we therefore used SID and tactile responsiveness as grouping criteria to determine whether there were differences in the effectiveness of HD-tDCS in improving social impairments in children with ASD with or without sensory integration dysfunctions and sensory abnormalities. This research aimed to provide a theoretical basis for future individualized and precise clinical applications of HD-tDCS in the treatment of ASD patients.

Materials and methods
Participant recruitment and informed consent
The required sample size for this study was calculated using the statistical tool G*Power, setting the significance level α for hypothesis testing at 0.05 and the power 1-β at 0.8. The minimum sample size for each group of participants was determined to be 14. A total of 82 children with ASD were enrolled, including 56 males and 26 females. Inclusion criteria were the following: ① meeting the diagnostic criteria for ASD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)60, the diagnosis was conducted by two associate chief psychiatrists or higher-ranked physicians specializing in child and adolescent psychiatry, ② dual diagnosis of ASD using the Autism Diagnostic Interview-Revised (ADI-R)61 and the Autism Diagnostic Observation Schedule (ADOS) 62, ③ ages between 4 and 12 years, and ④ right handedness; ⑤ Currently not participating in other research projects; ⑥ consent from the guardians to participate in this study. Exclusion criteria were the following: ① clear genetic or metabolic disorders, as well as other neurological conditions, history of traumatic brain injury, or severe physical illness; ② the presence of metal implants in the body, including non-removable dentures, and ③ skin damage at the stimulation site.
This study was approved by the Ethics Committee of Nanjing Brain Hospital, Nanjing Medical University (2022-KY022-01), with a clinical trial registration number: ChiCTR2200062132 (Date of Registration: 24/07/2022), and obtained informed consent from the parents or legal guardians of the participants. Children were selected from the ASD specific disease cohort at the Child Mental Health Research Center, the Affiliated Brain Hospital of Nanjing Medical University (Study recruitment was conducted from August 2022 to December 2023). And all methods were performed in accordance with the relevant guidelines and regulations.
Measurement tools
Comprehensive assessment and data collection of all participants were conducted by trained psychologists or psychiatrists at the Child Mental Health Research Center. Assessments included the following: ① a self-compiled “General Information Questionnaire” to gather demographic data, including the participants’ demographic information, developmental history, clinical information related to their condition, medical history, and family history; ② the Wechsler Intelligence Scale for Children, Fourth Edition, Chinese Version (WISC-IV)63 was used to assess the intelligence of children with ASD aged 8 − 12 years; ③ the Griffiths Developmental Scales, Chinese Version64 were used to evaluate the developmental level of children with ASD aged 4 − 7 years, ④ the Children Sensory Integration Rating Scale (CSIRS)65 was used to assess sensory integration abilities. The raw scores were converted into standard T-scores, and a score below 40 indicated SID. Note that for children with ASD aged 4–12 years, certain items related to “learning ability” and “issues specific to older age” were removed from the scale, and the evaluation focused on vestibular balance, proprioception, and tactile defensiveness. For children with ASD aged 4–8 years, the Sensory Processing and Self-Regulation Checklist (SPSRC)66 was used to assess tactile responses. The total score of tactile items was compared to the normative values of the checklist to calculate the Z-score [(k − mean)/standard deviation]. A Z-score less than − 1 indicated tactile abnormalities. By reviewing the scale,, the child’s tactile responses were classified as hypo-tactile response, hyper-tactile response, and typical tactile sensitivity. ⑤ Autism Treatment Evaluation Checklist (ATEC)67,68 was used to assess the overall improvement of the participants before and after the intervention. The Social Responsiveness Scale (SRS)69 was used to evaluate the participants’ social interaction and communication abilities before and after the intervention.
Study design
This study was a randomized double-blind sham-controlled trial, where assessors, participants, and parents were unaware of the true or sham stimulation. Among them, 26 children with ASD were undergoing Applied Behaviour Analysis (ABA) and other rehabilitation training. Participants were equally randomized across all groups.
Grouping
To avoid confounding effects, we employed the paired randomization method. The participants were grouped based on matching factors such as sex, age, intellectual or developmental level, baseline scores on ADI-R and ADOS, and the type and duration of rehabilitation training for children with ASD. And all participants were randomly assigned to different groups. The personnel implementing the stimulation extracted the labels for true/false stimulation; they did not participate in data analysis. The evaluators, the parents of the subjects, and the data analysts were unaware of the true/false stimulation. Some participants were excluded from matching due to inability to complete the process. The final grouping results were as Table 1.
Based on the assessment results from the CSIRS, the children with ASD aged 4–12 years were divided into three groups: the typical sensory integration group (n = 20), the sensory integration dysfunction group (n = 42), and the sham stimulation group (n = 20, with an equal distribution of 10 children with sensory integration dysfunction and 10 with normal sensory integration.
A post-hoc analysis was conducted on the data of 4–8-year-old children with ASD after intervention with true HD-tDCS. Based on the assessment results from the SPSRC and comprehensive evaluation, the participants were further categorized into three groups: the hypo-tactile response group (n = 18), the hyper-tactile response group (n = 21), and the typical tactile sensitivity group (n = 17).
High definition 5-channel transcranial direct current stimulation
Instrument parameters
The HD-tDCS device (Wogao Model VTS-801D; Nanjing Baibang Medical Equipment, Nanjing China) used circular electrodes with a diameter of less than 12 mm. When placing the electrodes, one central electrode was positioned in the stimulation area, while the remaining electrodes were placed around it. Prior to stimulation, it was necessary that each patient correctly wear a high precision electrode cap. Then, using a syringe, the cap was gently lifted to allow for the application of conductive gel on the scalp. The high precision electrodes were placed into the base and secured with a tight cap (Note: Electrode No. 5 must be positioned in the central location, while there were no specific requirements for the other electrodes). This device had the capability to set parameters for sham stimulation, thereby meeting the requirements of clinical double-blind studies.
Stimulation parameters
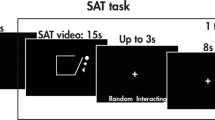
The Cz electrode (based on the 10–20 EEG system) was positioned as the central anode, with surrounding electrodes placed at FC1, FC2, CP1 and CP2 (based on the 10–20 EEG system). The current was set at 2 mA, and delivered as continuous direct current. Each stimulation session lasted for 20 min, and each participant received treatment for 3 consecutive weeks, with five sessions per week in the first 2 weeks (once a day from Monday to Friday, with weekends off), and four sessions in the third week, totaling 14 sessions. For the sham stimulation group, electrode placement and parameter settings were the same as for the active stimulation group. However, a sham stimulation mode was selected, where the current initially increased to the predetermined 2 mA and then quickly decreased to 0 within 30 s. This created a contrast effect to mimic the real stimulation process. The same procedure was followed at the end of each treatment session. Please refer to Fig. 1 for details.
During treatment sessions, the participants’ attention was diverted by watching the same pro-social animated videos “Journey to the West.” Additionally, there was no change in the ongoing rehabilitation training that the participants were undergoing while receiving HD-tDCS interventions.
Safety assessment
During the intervention process, we used a homemade HD-tDCS treatment log for experimental recording and safety assessment. This log included the participants’ basic information (name, sex, date of birth, target symptoms, current rehabilitation training), treatment dates, electrode polarity, efficacy, adverse reactions, and other items. Before each intervention, we meticulously recorded the efficacy and adverse reactions reported by the participants’ parents. After each intervention, we checked the targeted area of the head for any signs of redness, swelling, or burns. The assessment of any residual effects and safety evaluation after the final intervention was conducted during the follow-up period.
Follow-up period
All the parents were followed-up by telephone at 1, 2, and 3 months after treatment. The contents of the follow-ups included the maintenance of social improvements and adverse reactions. First, parents spontaneously reported the status of the subjects since the end of the treatment; second, staff asked questions about social interaction, linguistic communication [please refer to the first 19 items in the Social Communication Questionnaire-lifetime70, and side effects such as physical discomfort and social regression; and third, the telephone records were analyzed and summarized by two child psychiatrists.
Statistical analysis
Statistical analysis was conducted using SPSS statistical software for Windows, version 22.0 (SPSS, Chicago, IL, USA). Continuous data are expressed as the mean ± standard deviation (\(\overline{X}\) ± SD). Independent sample t-tests were used for within-group comparisons, while one-way analysis of variance (ANOVA) was used for between-group comparisons (F-test). To eliminate intergroup differences caused by heterogeneity among children with ASD, a normal distribution test (Kolmogorov–Smirnov test) was performed on the sex, age, intelligence or developmental level, ADI-R, and ADOS scores of each group. All variables showed a normal distribution. One-way ANOVA was used to compare differences between groups in terms of age, WIS-IV and Griffith (GQ) scores, ADI-R and ADOS scores, CSIRS, and SPSRC scores. Factors showing significant differences were controlled as covariates in subsequent tests. Sex comparisons were performed using the chi-square test (X2 test). A significance level of P < 0.01 indicated statistical significance for differences before and after intervention. Independent sample t-tests were used to analyze differences in the average total score and scores of the five subscales of the SRS, before and after interventions, Meanwhile independent sample t-tests were used to compare the mean differences between different groups, and to control for the false positive rate due to multiple comparisons, we applied the Bonferroni correction, adjusting the significance level to 0.05 divided by the number of comparisons.
Results
Due to adverse events one participant was excluded from data analysis and another dropped out of the study, while the remaining participants completed all scheduled HD-tDCS sessions (i.e., 3 weeks/14 sessions; retention = 99%). Additionally, due to noncompliance in completing the questionnaires, such as only completing the pre-treatment questionnaire, inconsistency in caregivers completing the pre- and posttreatment questionnaires(father, mother, grandfather or grandmother), and incomplete questionnaire responses, 10 participants were excluded from all groups. Additionally, we conducted the experiment according to a randomized double-blind sham-controlled study design; however, we did not specifically check the integrity of blinding during the process, which is a limitation of the study.
Participants who experienced the aforementioned adverse reactions during the intervention reported no such discomfort in the first, second, or third month post-intervention, nor did they report any other adverse reactions. Participants who did not experience the aforementioned adverse reactions during the intervention also reported no adverse reactions in the first, second, or third month post-intervention. Those participants who benefited socially during the intervention continued to maintain these benefits in the first, second, and third months post-intervention; however, whether these benefits can be sustained in the long term requires further follow-up work.Overall, HD-tDCS physical therapy was well-tolerated with no reports of severe adverse reactions, and it was generally accepted by the children and their parents.
The five channel HD-tDCS used a concentric ring design, where the electric current was confined within the ring-shaped area. This design provided good stimulation focus, with the peak intensity occurring beneath the central electrode. The polarity of the entire ring-shaped area was only influenced by properties of the central electrode. When placing the electrodes, there was no need to consider a return electrode.
Demographic data and clinical scale scores at baseline for children with ASD in different groups
According to the CSIRS assessment results, a total of 19 children with ASD aged 4–12 years were included in the typical sensory integration group, with 35 in the SID group, and 18 in the sham stimulation group (with equal numbers of individuals with SID and typical sensory integration, 9:9).
Based on the SPSRC assessment results, a total of 16 children with ASD aged 4–8 years were included in the hypo-tactile response group, 20 in the hyper-tactile response group, and 15 in the typical tactile sensitivity group.
There was no statistically significant difference among the groups in terms of age, sex, WISC-IV and Griffith (GQ) scores, ADI-R total scores, social and language module total scores, ADOS total scores, and communication and social interaction total scores (p > 0.05) (Tables 2 and 3).
Comparison of the therapeutic effects of HD-tDCS on social impairments in children with ASD in different groups
ASD with or without sensory integration dysfunction
Based on the ATEC results (as shown in Fig. 2), overall, after HD-tDCS intervention, compared to the sham stimulation group, the Cz center anode true stimulation group showed significant improvement in social impairment. Compared to the SID group, the typical sensory integration group also showed significant changes in social awareness and autistic mannerism dimensions before and after intervention. Among them, the typical sensory integration group showed statistically significant reductions in SRS total score (t = 4.078, p < 0.000), social awareness (t = 5.032, p < 0.000), social cognition (t = 4.151, p < 0.000), social communication (t = 2.862, p = 0.007), social motivation (t = 2.085, p = 0.045), and autistic mannerisms (t = 3.085, p = 0.004) after interventions. The SID group showed statistically significant reductions only in the SRS total score (t = 4.078, p < 0.000), social cognition (t = 3.117, p = 0.004), and social communication (t = 2.287, p = 0.028) after interventions. The sham stimulation group with a Cz center anode showed no statistically significant difference in SRS total scores and individual subscale scores before and after intervention (t = 0.023, 0.000, 0.069, − 0.075, − 0.114, 0.073, p > 0.05) (Table 4).
Comparison of ATEC score reduction before and after treatment in three groups: ASD with and without Sensory Dysfunction. ①the typical sensory integration group; ②the sensory integration dysfunction group; ③the sham stimulation group. ATEC consists of four subtest scales: Scale I (speech/language/communication; has 14 items. Scores can range from 0 to 28), Scale II (sociability; has 20 items. Scores can range from 0 to 40), Scale III (sensory/cognitive awareness; has 18 items. Scores can range from 0 to 36) and Scale IV (Health/Physical/Behaviour; has 25 items). Each subcategory of the ATEC scale is scored using a 0–3 point scale as follows: 0 = not a problem, 1 = minor problem, 2 = moderate problem and 3 = serious problem.
We defined the changes in measurement parameters by subtracting baseline scores from scale scores reported after HD-tDCS interventions, with positive values indicating improvements. We compared difference in scores before and after treatment for each subscale of the SRS between groups, as shown in Table 5. In terms of improving social impairment in children with ASD, we analyzed whether clinically non-sensory integration dysfunction patients were more suitable for HD-tDCS. We also constructed violin plots, as shown in Fig. 3, to show that the typical sensory integration group had more significant improvements in social awareness (F = 26.328, p < 0.000), social cognition(F = 17.778, p < 0.000), social communication(F = 35.905, p < 0.000), social motivation(F = 18.688, p < 0.000), and autistic mannerisms(F = 15.910, p < 0.000) dimensions, when compared with the SID group. Additionally, there was no significant difference in scores for social awareness, social motivation, and autistic mannerisms dimensions before and after interventions in the SID group. Furthermore, we calculated the average percentage change in scores and conducted paired t-tests for HD-tDCS before and after interventions, when compared with baseline (Fig. 4).
Comparisons of intergroup differences in score differences of social responsiveness scale subscale items before and after high definition transcranial direct current stimulation intervention. The X-axis represents the social responsiveness scale subscale items, while the Y-axis represents the score change calculated by subtracting post-intervention scores from baseline scores, with positive values indicating improvements.
ASD with or without tactile sensory abnormalities
In general, significant changes were observed in social awareness, social cognition, social communication, autistic mannerisms dimensions, and total SRS scores for both the hypo-tactile response and typical tactile sensitivity groups before and after HD-tDCS intervention. However, the hyper-tactile response group showed less satisfactory therapeutic effects. Specifically, the hypo-responsive group exhibited statistically significant reductions in SRS total score (t = 5.339, p < 0.000), social awareness (t = 6.890, p < 0.000), social cognition (t = 5.063, p < 0.000), social communication (t = 3.724, p = 0.001), and autistic mannerisms dimensions (t = 3.673, p = 0.001) after intervention. The hyper-tactile response group showed a statistically significant reduction only in social communication (t = 2.385, p = 0.022) after intervention. The typical tactile sensitivity group showed statistically significant reductions in SRS total score (t = 5.835, p < 0.000), social awareness (t = 5.178, p < 0.000), social cognition (t = 4.988, p < 0.000), social communication (t = 3.273, p = 0.003), and autistic mannerisms dimensions (t = 4.088, p < 0.000) after intervention (Table 6).
Similarly, we defined measurement parameter changes by subtracting the baseline scores from scale scores reported after HD-tDCS intervention. We then compared the score differences before and after intervention for each subdomain of the SRS across different groups (Table 7). In terms of improving social difficulties in children with ASD, we analyzed whether children with hypo-tactile and typical tactile responses were more suitable for HD-tDCS intervention. Violin plots were used to illustrate the results (Fig. 5). The hypo-tactile and typical tactile response groups showed more pronounced improvements in social awareness (F = 13.747, p < 0.000), social cognition (F = 12.077, p < 0.000), social communication (F = 4.020, p < 0.000), and autistic mannerisms (F = 12.300, p < 0.000) dimensions, when compared with the hyper-tactile response group. We also calculated the average percentage changes in scores before and after HD-tDCS interventions, when compared to the baseline, and then performed paired t-tests (Fig. 6).
Comparison of intergroup score differences of social responsiveness scale subscale items before and after high definition transcranial direct current stimulation intervention. The X-axis represents the social responsiveness scale subscale items, while the Y-axis represents the score change calculated by subtracting post-intervention scores from baseline scores, with positive values indicating improvements.
Adverse events
During the intervention process, almost all children with ASD experienced scalp erythema at the site of the anode after HD-tDCS, which resolved within 30 min. Out of the 72 children with ASD who received HD-tDCS for the first time, 13 children experienced discomfort at the site of the anode and could not tolerate it. The current was reduced to 1 mA and then gradually increased by 0.2 mA every two minutes until it reached 2 mA within 10 min. This slow increase in current was continued for 2 consecutive days until the children gradually adapted to it, and subsequent sessions were conducted at the designated current of 2 mA. The specific information is as Table 8:
Among them, a child with ASD was unable to tolerate the pain for 5 consecutive days, which was accompanied by mild nausea and dizziness without vomiting. Subsequently, the researchers arranged for him to withdraw from the study. The review of the data shows that the subject’s tactile responses were excessively strong. Additionally, seven children with ASD exhibited symptoms of excitement, irritability, and night terrors during the first week of HD-tDCS interventions, as reported by their caregivers. The research team did not make any special interventions, and the symptoms resolved on their own. In the second week of treatment, these children did not experience any discomfort and completed the study. The review of the data indicates that among the participants, six had excessively strong tactile responses, while one had insufficient tactile responses. However, a child with ASD exhibited persistent excitatory behaviors such as screaming, running, jumping, and night terrors until the seventh day of intervention. Considering the participant’s well-being, the child was withdrawn from the study and unblinded. The initial stimulation was anodal, but after consultation with the caregiver, it was switched to cathodal stimulation. The child did not report any pain or excitatory behaviors thereafter. After intervention, the parent reported that the child’s speech became more fluent, pronunciation was clearer, and the speaking volume was louder. No serious adverse event such as seizures, hearing problems, or visual impairments were observed in any of the children. The review of the data revealed that the subject’s tactile response was also overly strong.
Discussion
Current studies primarily explore the efficacy of tDCS stimulation, by targeting the positive electrode placement at left DLPFC, on the social and language communication functions of individuals with ASD. Possible mechanisms of action are investigated from the perspectives of electroencephalography (EEG) and neuroimaging71,72. The prefrontal cortex, particularly the left DLPFC, is the preferred target for tDCS intervention due to its crucial role in cognitive functions such as executive control, attention, and working memory9,73. We compared the therapeutic efficacy of the F3 central anode and the Cz central anode in our previous work, and the differences were not statistically significant54. Therefore, we are continuing with the previous research. In the present study, we used HD-tDCS, an emerging technique that improves upon traditional tDCS by providing focused stimulation centered around the vertex, without interfering with other brain regions. Compared to conventional tDCS, HD-tDCS offers greater precision in targeting stimulation sites and fewer adverse effects74,75. This makes it more tolerable for ASD individuals, especially those sensitive to pain.
All participants in the true stimulation group showed varying degrees of improvements in social impairments, with an average reduction of 13.78% in ATEC and 12.54% in SRS scores. Due to the small sample size and heterogeneity, we have spare no effort to minimize errors. In addition, since we have previously established the efficacy of anodal HD-tDCS at the Cz vertex, the number of participants in the placebo group for this study was comparatively smaller. The true stimulation group was divided into typical sensory integration and SID, however the sham group included both together in the analysis which may have had an impact on the findings. We will further improve this testing in the upcoming phases of our work.
As mentioned in the section on adverse events, a total of 13 patients were unable to tolerate the pain caused by a 2 mA current, resulting in suboptimal current intensity during the first 10 min of treatment. This may have led to a potential reduction in efficacy. However, existing tDCS studies have not reached a consensus on whether current intensity significantly affects treatment outcomes76. Additionally, although the dosage was reduced for these 13 patients, it still met the treatment duration specified in previous studies and current guidelines77. The need to reduce current intensity due to pain intolerance is an unavoidable issue in both research and clinical practice. We have encountered similar challenges in our previous studies. When using a daily stimulation with a current intensity of 2 mA, a single stimulation duration of 20 min, five times per week, and a total of 14 treatment sessions over 3 weeks, almost all participants demonstrated good tolerability. No severe adverse reactions such as seizures occurred in any of the three groups. The safety assessment results are consistent with previous similar studies10,77,78 and supported the high tolerability and low risk of central anodal HD-tDCS at Cz for the treatment of children with ASD. Compared to traditional 2-channel tDCS, HD-tDCS resulted in fewer reports of burning sensations at the stimulation sites and reduced discomforts. Moreover, the overall retention of participants in the study reached 99%. This indicated widespread acceptance of HD-tDCS as a treatment method, and suggested good compliance when recommending this treatment approach to patients and their families, in clinical practice. Additionally, recent studies on tDCS in children and adolescents with ASD, such as one using 2 mA79 and another using 1.5 mA71, reported no issues with tolerability or the necessity to reduce current intensity. We consider that this difference may be related to the resistance at the stimulation site. Regardless of the technique used, proper electrode placement and preparation are crucial for preventing adverse skin reactions. Future tDCS technology should focus on ensuring that the resistance at the stimulation site is consistent and sufficiently low to enhance patient comfort.
Although pediatric-specific reviews of the safety of non-invasive brain stimulation (NIBS) technologies suggest that tDCS is generally safe for children with underlying neurological or psychiatric conditions80, and the tolerability of 1 mA HD-tDCS is comparable to that of 1 mA conventional tDCS81, evidence from computational current modeling indicates important differences in how stimulation affects children compared to adults82. Specifically, local electric field strength may be up to twice as high in children83,84, and peak electric fields in cortical gray matter may be as much as seven times higher85. These findings highlight the need for careful consideration when selecting stimulation intensities. There is a lack of research on the differences in therapeutic effects produced by different current intensities, and the current intensity was set to 2 mA that was less studied. In this study, we conducted experiments based on preliminary research and guidelines recommending current intensities for tDCS application in children54,86,87. We administered a 2 mA current to modulate cortical neural activity. For participants who initially experienced discomfort, we implemented a gradual increase in current, which aimed to allow them to acclimate to the stimulation, thereby reducing discomfort and ensuring safety. Throughout the intervention, we closely monitored participants for any adverse reactions. While some children experienced mild scalp erythema, these effects were transient and resolved quickly. Importantly, no severe adverse events were reported, and the overall retention rate was 99%. During the three-month follow-up after the intervention, no serious adverse events were reported by the participants’ guardians, indicating that 2 mA is safe and well-tolerated. However, future research needs to continue exploring the optimal current intensity for pediatric populations to enhance safety and efficacy.
Sensory integration ability serves as the foundation for complex psychological and behavioral activities. Previous studies have shown a correlation between sensory integration dysfunction and social impairment, both of which were associated with neurodevelopmental abnormalities22,23,88,89. Currently, the etiology and mechanisms of SID in children remain unclear. Ayres suggested that it may be influenced by genetic and environmental factors90,91. Our study found that anodal HD-tDCS targeting social improvements in children with ASD with typical sensory integration abilities was more significant, when compared with those with SID, both in terms of breadth and depth. This finding is consistent with previous research indicating potential structural and functional interactions between sensory processing pathways and social cognitive networks in the brains of children with ASD92,93. The amygdala is a core region for emotional processing, involved in recognizing and responding to social signals such as subtle nuances in facial expressions and voice, as well as participating in the processing of sensory perceptions94. Furthermore, genetic studies have shown that deletion of genes such as Shank3 or Mecp2 may increase the risk of both sensory processing abnormalities and social impairments95,94,97. Family studies have revealed the clustering of social skills and perceptual traits within families, indicating that these characteristics may share a common genetic basis. DNA methylation and histone modification can affect the expression of genes related to social and perceptual abilities98. Lastly, studies related to the neurotransmitter system have also identified the crucial roles of certain neurotransmitters in both sensory processing and social behavior, such as GABA, 5-HT, oxytocinergic, and glutamate99,98,101. Neurotransmitter levels are closely related to the plasticity of neural circuits, which play a crucial role in the integration of social and sensory information. This plasticity, in turn, enables the brain to perceive external information, adapt to social experiences, and accordingly adjust neurotransmitter levels, such as the activities of the NMDA receptors and the AMPA receptors102. Even the process is cyclical, with neurotransmitters, perception, emotion, and social interaction forming a closed-loop system103..
We therefore speculate that the normal execution of social and sensory integration abilities may occur through shared pathways. When social impairments coexist with SID, disruption of these pathways may be more severe or extensive. During the process of improving social abilities with HD-tDCS, it is necessary to reconstruct severely disrupted pathways. Social improvements may be less comprehensive and less efficient in these cases. However, HD-tDCS may improve social abilities through two pathways: a direct pathway and an indirect pathway. HD-tDCS can directly enhance social improvements by regulating neurotransmitter levels and influencing the strength of functional connections. It can also indirectly improve social functioning by affecting sensory processing pathways that interact with social cognitive networks. Therefore, for children with SID, the indirect pathway is disrupted, resulting in less improvement in social impairment compared to those with typical sensory integration. In the future, we will continue to verify these findings at genetic, neurotransmitter, and brain network levels.
Based on the results from Tables 4 and 5, it can be inferred that there is a statistically significant improvement in the social cognition and social communication domains for the group with sensory integration disorders (SIDs). Furthermore, more than half of the children with autism spectrum disorder (ASD) have comorbid sensory processing disorders. In our previous study on the effectiveness of high-definition transcranial direct current stimulation (HD-tDCS), we found that tactile hypersensitivity may be an important factor influencing the compliance of children with ASD with this therapeutic method. Additionally, sensory perception and sensory integration are closely related. Therefore, we conducted a post-hoc analysis based on the presence or absence of sensory abnormalities, aiming to identify another subgroup of children with ASD who would be suitable for HD-tDCS intervention. Children with ASD primarily exhibited sensory abnormalities characterized by hyper-reactivity, hypo-reactivity, and sensory-seeking behaviors. Tactile hypersensitivity may be associated with neuronal hyper-excitability, abnormal neurotransmitter functioning, enhanced neurotransmitter functions such as dopamine and glutamate, as well as abnormalities in neural networks, including the sensory cortex, limbic system, and motor system104,105,107. In such cases, the body may be in a highly alert “activated state.” Conversely, sensory hypo-reactivity may be related to abnormal sensory signal transmission, reduced neurotransmitter functioning, or weakened functional connections in brain networks106,108, resulting in the body being in an “inhibited state.”
This study found that anodal HD-tDCS had a more significant effect on improving social skills in children with ASD with hypo-reactivity and typical tactile responses, when compared to those with hyper-reactivity. This may be because anodal HD-tDCS induced cortical neuronal excitation, effectively counteracting the “inhibited state” associated with sensory hypo-reactivity. However, for the “activated state” associated with sensory hypersensitivity, the excitatory effect of anodal HD-tDCS may exacerbate the condition. Therefore, we believe that children with ASD with sensory hypersensitivity, especially those with tactile hypersensitivity, who cannot tolerate the burning sensation caused by HD-tDCS stimulation, may not be suitable candidates for this physical intervention, or at least not suitable for anodal HD-tDCS.
During the research process, we encountered a case in which a child exhibited continuous excitatory behaviors such as pain, screaming, running, jumping, and night terrors throughout the 7 day period of using anodal HD-tDCS. Upon reviewing the results of the CSIRS and SPSRC assessments, the child scored 27 on the tactile defensive subscale and the degree of tactile reactivity deviation was -2.72. After switching to cathodal HD-tDCS, the child did not report pain, and the aforementioned excitatory behaviors did not reoccur. Following the completion of intervention, the parents reported that the child’s language expression became more fluent, pronunciation was clearer, and the speech volume was louder. Based on these observations, we speculate that cathodal HD-tDCS may be more suitable for children with ASD with sensory hypersensitivity. Tactile hypersensitivity, or sensory over-reactivity, corresponds to the aforementioned “activated state.” The cathodal electrical stimulation inhibits cortical neuronal excitation, moderating neuronal excitability. Thus, the two factors complement each other. To test this hypothesis, we invited an additional two children with ASD with hyper-tactile response characteristics to participate in cathodal HD-tDCS, and the results were similar to the previous case. Currently, there is limited research on cathodal HD-tDCS, and in the future, more mechanistic and clinical control studies may be necessary to confirm the compatibility of cathodal HD-tDCS with children with ASD who have sensory hypersensitivity.
Sensory integration training, which involves the communication and integration of information between touch, vestibular sense, proprioception, and motor organs, can significantly improve language, social, perceptual, and behavioral abilities in children with ASD, as well as enhance their physical functioning109,107,111. Previous research has also indicated that combining sensory integration training with tDCS produced better outcomes than single rehabilitation training or physical interventions17,112,113. Based on these results, we speculate that when HD-tDCS is combined with sensory integration training in children with ASD, it synergistically induces changes in brain neural excitability, modulates EEG rhythms, promotes neuroplasticity and repair, and improves cerebral blood flow, all within the framework of an excitatory-inhibitory adaptation. These changes are likely to lead to an expansion of the cortical areas associated with coordination and integration functions, making the children more receptive to HD-tDCS and enhancing the effectiveness of HD-tDCS combined with sensory integration training. Consequently, improvements in social impairments may occur more rapidly and significantly.
Our study revealed significant improvements in social impairment among children with ASD following HD-tDCS intervention. However, it is important to note the imbalance in sex/gender distribution within our sample, which may influence the outcomes. Previous research has indicated that sex differences can significantly affect social brain neural responses in autism. Study demonstrated that there are distinct temporal profiles in configural face-processing between sexes, suggesting that males and females may process social information differently114. This finding underscores the necessity of considering sex as a critical variable in autism research.While we statistically addressed the imbalance in our analyses, the implications of these differences should be further explored in future studies. Understanding how sex influences social cognition and neural processing in children with ASD could enhance the precision of interventions like HD-tDCS and inform individualized treatment approaches.
In summary, we believe that for children with ASD, especially those with typical sensory integration, typical or hypo-tactile response, the use of anodal HD-tDCS targeting the Cz central region can be employed to improve their social deficits. Following expert consensus and guideline recommendations, a current of 1.5–2 mA, administered five times a week, with a total of 14–20 sessions per treatment course, appears suitable. Combining this approach with sensory integration training may lead to enhanced therapeutic effects.
Conclusion
This preliminary study suggested that HD-tDCS had greater potential in improving symptoms of social impairment in children with ASD who had typical sensory integration, hypo-tactile response, or typical tactile sensitivity. There may be a common pathway involving social impairments, sensory integration dysfunction, and sensory abnormalities, particularly at the level of neurotransmitters and brain networks. Combining sensory integration training with tDCS may yield better therapeutic effects, when compared to single rehabilitation training or physical interventions. However, it is not yet possible to draw definitive conclusions regarding the underlying mechanisms of its potential efficacy. Furthermore, further research with larger sample sizes is needed to determine which subgroups of children with ASD are more likely to benefit from this treatment.
Limitation
This study has several limitations that should be acknowledged. First, while the trial was designed as a double-blind study, we did not conduct specific checks to confirm the effectiveness of blinding among participants and assessors. Future studies should implement blinding checks to ensure the integrity of the trial. Second, the sample size was relatively small, and the limited number of female participants may affect the generalizability of our findings. And larger-scale studies will provide more robust data. Furthermore, we recommend that future research be pre-registered and include well-defined outcomes to enhance the reliability and reproducibility of the findings. Lastly, while we reported short-term follow-up results, long-term follow-up is necessary to assess the sustainability of the benefits observed.
Data availability
To protect study participant privacy, we don’t share it publicly directly online. But Readers may contact the corresponding author for the original data after the paper is published.
References
Maenner, M. J. et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 70(11), 1–16 (2021).
Talantseva, O. I. et al. The global prevalence of autism spectrum disorder: A three-level meta-analysis. Front. Psychiatry 14, 1071181 (2023).
Wang, J. et al. Global prevalence of autism spectrum disorder and its gastrointestinal symptoms: A systematic review and meta-analysis. Front. Psychiatry 13, 963102 (2022).
Cascio, C. J., Woynaroski, T., Baranek, G. T. & Wallace, M. T. Toward an interdisciplinary approach to understanding sensory function in autism spectrum disorder. Autism Res. 9(9), 920–925 (2016).
Wuang, Y. P., Huang, C. L. & Tsai, H. Y. Sensory integration and perceptual-motor profiles in school-aged children with autistic spectrum disorder. Neuropsychiatr. Dis. Treat 16, 1661–1673 (2020).
Lord, C. et al. Autism spectrum disorder. Nat. Rev. Dis. Primers 6(1), 5 (2020).
Hirota, T. & King, B. H. Autism spectrum disorder: A review. JAMA 329(2), 157–168 (2023).
You, X. R., Gong, X. R., Guo, M. R. & Ma, B. X. Cognitive behavioural therapy to improve social skills in children and adolescents with autism spectrum disorder: A meta-analysis of randomised controlled trials. J. Affect. Disord. 344, 8–17 (2024).
García-González, S. et al. Transcranial direct current stimulation in autism spectrum disorder: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 48, 89–109 (2021).
Luckhardt, C., Boxhoorn, S., Schütz, M., Fann, N. & Freitag, C. M. Brain stimulation by tDCS as treatment option in autism spectrum disorder-a systematic literature review. Prog. Brain Res. 264, 233–257 (2021).
Kang, J. et al. Transcranial direct current stimulation modulates EEG microstates in low-functioning autism: A pilot study. Bioengineering (Basel) 10(1), 1 (2023).
Pavlova, E. L. et al. Transcranial direct current stimulation in neurology and psychiatry. Zh Nevrol Psikhiatr. 120(12), 123–130 (2020).
Herrera-Melendez, A. L., Bajbouj, M. & Aust, S. Application of transcranial direct current stimulation in psychiatry. Neuropsychobiology. 79(6), 372–383 (2020).
Kenney-Jung, D. L. et al. Transcranial direct current stimulation: Mechanisms and psychiatric applications. Child Adol. Psych. Cl. 28(1), 53–60 (2018).
Hertenstein, E. et al. Modulation of creativity by transcranial direct current stimulation. Brain Stimul. 12(5), 1213–1221 (2019).
To, W. T., Hart, J., De Ridder, D. & Vanneste, S. Considering the influence of stimulation parameters on the effect of conventional and high-definition transcranial direct current stimulation. Expert Rev. Med. Dev. 13(4), 391–404 (2016).
Costa, T. L., Lapenta, O. M., Boggio, P. S. & Ventura, D. F. Transcranial direct current stimulation as a tool in the study of sensory-perceptual processing. Atten. Percept Psychophys. 77(6), 1813–1840 (2015).
Gómez, L. et al. Non-invasive brain stimulation for children with autism spectrum disorders: A short-term outcome study. Behav. Sci. (Basel) 7(4), 63–75 (2017).
Han, Y. M. Y. et al. Neurophysiological and behavioral effects of multisession prefrontal tDCS and concurrent cognitive remediation training in patients with autism spectrum disorder (ASD): A double-blind, randomized controlled fNIRS study. Brain Stimul. 15(2), 414–425 (2022).
Lee, J. C., Kenney-Jung, D. L., Blacker, C. J., Doruk Camsari, D. & Lewis, C. P. Transcranial direct current stimulation in child and adolescent psychiatric disorders. Child Adolesc. Psychiatr. Clin. N. Am. 28(1), 61–78 (2019).
Orrù, G., Cesari, V., Conversano, C. & Gemignani, A. The clinical application of transcranial direct current stimulation in patients with cerebellar ataxia: A systematic review. Int. J. Neurosci. 131(7), 681–688 (2021).
Zhao, N. et al. Effects of transcranial direct current stimulation on poststroke dysphagia: A systematic review and meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 103(7), 1436–1447 (2022).
Zhao, X. et al. Abnormalities of gray matter volume and its correlation with clinical symptoms in adolescents with high-functioning autism spectrum disorder. Neuropsychiatr. Dis. Treat 18, 717–730 (2022).
Ko, M. H. Safety of Transcranial Direct Current Stimulation in Neurorehabilitation. 14(1), e9 (2021).
Moliadze, V. et al. Ten minutes of 1 mA transcranial direct current stimulation was well tolerated by children and adolescents: Self-reports and resting state EEG analysis. Brain Res. Bull. 119(Pt A), 25–33 (2015).
Zhao, H. et al. Modulation of brain activity with noninvasive transcranial direct current stimulation (tDCS): Clinical applications and safety concerns. Front. Psychol. 8, 685 (2017).
Bikson, M. et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 9(5), 641–661 (2016).
Lu, H. et al. Modulation of repeated anodal HD-tDCS on Attention in Healthy Young Adults. Front. Psychol. 11, 564447 (2020).
Ostrowski, J., Svaldi, J. & Schroeder, P. A. More focal, less heterogeneous? Multi-level meta-analysis of cathodal high-definition transcranial direct current stimulation effects on language and cognition. J. Neural. Transm. (Vienna) 129(7), 861–878 (2022).
Parlikar, R. et al. High definition transcranial direct current stimulation (HD-tDCS): A systematic review on the treatment of neuropsychiatric disorders. Asian J. Psychiatr. 56, 102542 (2021).
Turski, C. A. et al. Extended multiple-field high-definition transcranial direct current stimulation (HD-tDCS) is well tolerated and safe in healthy adults. Restor. Neurol. Neurosci. 35(6), 631–642 (2017).
Kang, J. et al. Transcranial direct current stimulation (tDCS) can modulate EEG complexity of children with autism spectrum disorder. Front. Neurosci. 12, 201 (2018).
Zhou, T., Kang, J., Li, Z., Chen, H. & Li, X. Transcranial direct current stimulation modulates brain functional connectivity in autism. Neuroimage Clin. 28, 102500 (2020).
Qiu, J. et al. Transcranial direct current stimulation (tDCS) over the left dorsal lateral prefrontal cortex in children with autism spectrum disorder (ASD). Neural. Plast. 2021, 6627507 (2021).
Auvichayapat, N. et al. Brain metabolite changes after anodal transcranial direct current stimulation in autism spectrum disorder. Front. Mol. Neurosci. 13, 70 (2020).
Zemestani, M., Hoseinpanahi, O., Salehinejad, M. A. & Nitsche, M. A. The impact of prefrontal transcranial direct current stimulation (tDCS) on theory of mind, emotion regulation and emotional-behavioral functions in children with autism disorder: A randomized, sham-controlled, and parallel-group study. Autism Res. 15(10), 1985–2003 (2022).
Posar, A. & Visconti, P. Sensory abnormalities in children with autism spectrum disorder. J. Pediat-Brazil. 94(4), 342–350 (2017).
Kuno-Fujita, A. et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 13(5), 741–750 (2020).
Jussila, K. et al. Sensory abnormality and quantitative autism traits in children with and without autism spectrum disorder in an epidemiological population. J. Autism Dev. Disorder 50(1), 180–188 (2020).
Hernandez, L. M. et al. Social attention in autism: Neural sensitivity to speech over background noise predicts encoding of social information. Front. Psychiatry 11, 343 (2020).
Van de Cruys, S., Perrykkad, K. & Hohwy, J. Explaining hyper-sensitivity and hypo-responsivity in autism with a common predictive coding-based mechanism. Cogn. Neurosci. 10(3), 164–166 (2019).
Frank van den B, et al. Sensory processing difficulties in psychiatric disorders: A meta-analysis. J Psyc Res, 2022, 173-180, (2022).
Lee Masson, H. & Isik, L. Rapid processing of observed touch through social perceptual brain regions: An EEG-fMRI fusion study. J. Neurosci. 43(45), 7700–7711 (2023).
Hoffman, T. et al. Indifference or hypersensitivity? Solving the riddle of the pain profile in individuals with autism. Pain 164(4), 791–803 (2023).
Khan, A. J., Nai, A. & Keown, C. L. Using resting-state functional connectivity to identify abnormal connectivity patterns in autism spectrum disorder: A systematic review. Front. Neurosci. 13, 22 (2019).
Supekar, K. et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 5(3), 738–747 (2013).
Khaledi, H. et al. The relationship between communication skills, sensory difficulties, and anxiety in children with autism spectrum disorder. Middle East Curr. Psychiatry. 29(1), 1 (2022).
Watson, L. R. et al. Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. J. Speech Lang. Hear. R. 54(6), 1562–1576 (2011).
Riemersma, I. W. et al. Suppression of Cofilin function in the somatosensory cortex alters social contact behavior in the BTBR mouse inbred line. Cereb. Cortex. 34(4), 1 (2024).
Uono, S., Yoshimura, S. & Toichi, M. Eye contact perception in high-functioning adults with autism spectrum disorder. Autism. 25(1), 137–147 (2020).
Li, G. et al. Sex differences in neural responses to the perception of social interactions. Front. Hum. Neurosci. 14, 565132 (2020).
Rizzo, G. et al. The limbic and sensorimotor pathways of the human amygdala: A structural connectivity study. Neuroscience 385, 166–180 (2018).
Wang, Y. et al. Social Brain Network of children with autism spectrum disorder: Characterization of functional connectivity and potential association with stereotyped behavior. Brain Sci. 13(2), 1 (2023).
Wang, Y. et al. High definition transcranial direct current stimulation of the Cz improves social dysfunction in children with Autism Spectrum Disorder: A randomized, sham, controlled study. Autism Res. https://doi.org/10.1002/aur.3018 (2023).
Hui, K., Katayama, Y., Nakayama, K. I., Nomura, J. & Sakurai, T. Characterizing vulnerable brain areas and circuits in mouse models of autism: Towards understanding pathogenesis and new therapeutic approaches. Neurosci. Biobehav. Rev. 110, 77–91 (2020).
Wang, W. et al. Altered resting-state functional activity in patients with autism spectrum disorder: A quantitative meta-analysis. Front. Neurol. 9, 556 (2018).
Ortiz Rubio, A. et al. Pain experiences of people diagnosed with autism spectrum disorder: A systematic review of case-control studies. Am. J. Occup. Ther. 77(2), 7702185020 (2023).
Villena-Gonzalez, M. Caresses, whispers and affective faces: A theoretical framework for a multimodal interoceptive mechanism underlying ASMR and affective touch: An evolutionary and developmental perspective for understanding ASMR and affective touch as complementary processes within affiliative interactions. BIOESSAYS 45(12), e2300095 (2023).
Tran, H. T. et al. Sensory processing impairments in children with developmental coordination disorder. Children 9(10), 1 (2022).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Publishing, 2013).
Lord, C., Rutter, M. & Le Couteur, A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 24(5), 659–685 (1994).
Lord, C., Luyster, R., Gotham, K., & Guthrie, W. Autism Diagnostic Observation Schedule, 2nd ed. Torrence, CA: Western Psychological Services. (ADOS-2) Manual (Part II): Toddler Module (2012).
Zhang, H. Revision of the Chinese version of the Wechsler Intelligence Scale for Children Fourth Edition (WISC-IV). Psychol. Sci. 32(05), 155–157 (2009).
Luiz, D. M., Barnard, A., & Knoesen, N. P. et al. Griffiths Mental Development Scales - Extended Revised (GMDS - ER) [Administration Manual]. Amersham, UK: ARICD (2006).
Ren, G., Wang, Y., Gu, B. & Shen, Y. Testing report of the children’s sensory integration assessment scale. Chin. J. Ment. Health 4, 145–147 (1994).
Lai, C. Y. Y., Yung, T. W. K., Gomez, I. N. B. & Siu, A. M. H. Psychometric properties of sensory processing and self-regulation checklist (SPSRC). Occup. Ther. Int. 2019, 8796042 (2019).
Netson, R. et al. A comparison of parent reports, the mental synthesis evaluation checklist (MSEC) and the autism treatment evaluation checklist (ATEC), with the childhood autism rating scale (CARS). Pediatric Rep. 16(1), 174–189 (2024).
Rimland, B., & Edelson, S. Autism treatment evaluation checklist: Statistical analyses. Autism Research Institute (2000).
Aldridge, F. J., Gibbs, V. M., Schmidhofer, K. & Williams, M. Investigating the clinical usefulness of the Social Responsiveness Scale (SRS) in a tertiary level, autism spectrum disorder specific assessment clinic. J. Autism Dev. Disord. 42(2), 294–300 (2012).
Liu, J. & Xu, X. Application of social communication questionnaire in primary screening of autism spectrum disorder. Sci. China. Life Sci. 8, 4 (2015).
Han, Y. M., Chan, M. M., Shea, C. K., Mo, F. Y., Yiu, K. W., Chung, R. C., et al. Effects of prefrontal transcranial direct current stimulation on social functioning in autism spectrum disorder: A randomized clinical trial. Autism 13623613231169547 (2023).
Xiao, L. et al. A bibliometric analysis of global research status and trends in neuromodulation techniques in the treatment of autism spectrum disorder. BMC Psychiatry 23(1), 183 (2023).
Khaleghi, A., Zarafshan, H., Vand, S. R. & Mohammadi, M. R. Effects of non-invasive neurostimulation on autism spectrum disorder: A systematic review. Clin. Psychopharmacol. Neurosci. 18(4), 527–552 (2020).
Hill, A. T., Rogasch, N. C., Fitzgerald, P. B. & Hoy, K. E. Effects of prefrontal bipolar and high-definition transcranial direct current stimulation on cortical reactivity and working memory in healthy adults. Neuroimage 15(152), 142–157 (2017).
Villamar, M. F. et al. Technique and considerations in the use of 4x1 ring high-definition transcranial direct current stimulation (HD-tDCS). J. Vis. Exp. 14(77), e50309 (2013).
Hameed, M. Q. et al. Transcranial magnetic and direct current stimulation in children. Curr. Neurol. Neurosci. Rep. 17(2), 11 (2017).
Salehinejad, M. A., Ghanavati, E., Glinski, B., Hallajian, A. H. & Azarkolah, A. A systematic review of randomized controlled trials on efficacy and safety of transcranial direct current stimulation in major neurodevelopmental disorders: ADHD, autism, and dyslexia. Brain Behav. 12(9), e2724 (2022).
Auvichayapat, P. et al. Long-term effects of transcranial direct current stimulation in the treatment of autism spectrum disorder: A randomized controlled trial. Dev. Med. Child Neurol. 65(6), 811–820 (2023).
Prillinger, K. et al. Multisession tDCS combined with intrastimulation training improves emotion recognition in adolescents with autism spectrum disorder. Neurotherapeutics 21(6), e00460 (2024).
Zewdie, E. et al. Safety and tolerability of transcranial magnetic and direct current stimulation in children: Prospective single center evidence from 35 million stimulations. Brain Stimul. 13(3), 565–575 (2020).
Cole, L. et al. Effects of high-definition and conventional transcranial direct-current stimulation on motor learning in children. Front. Neurosci. 12, 787 (2018).
Hunold, A. et al. Review of individualized current flow modeling studies for transcranial electrical stimulation. J Neurosci Res., 101(4), 405-423 (2023).
Chhatbar, P. Y., Ramakrishnan, V. & Kautz, S. Transcranial direct current stimulation post-stroke upper extremity motor recovery studies exhibit a dose-response relationship. Brain Stimul. 9, 16–26 (2016).
Kessler, S. K., Minhas, P. & Woods, A. J. Dosage considerations for transcranial direct current stimulation in children: A computational modeling study. PLoS ONE 8, e76112 (2013).
Ciechanski, P., Carlson, H. L. & Yu, S. S. Modeling transcranial direct-current stimulation-induced electric fields in children and adults. Front. Hum. Neurosci. 12, 1 (2018).
Antal, A. et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 128(9), 1774–1809 (2017).
Srivastav, A. & Chatterjee, S. Transcranial direct current stimulation to enhance mathematical performance in school going developmental dyscalculic children: A single group pretest-posttest, quasi experimental study. Rev. Pesq Fisio 11(3), 1 (2021).
Hazen, E. P., Stornelli, J. L., O’Rourke, J. A., Koesterer, K. & McDougle, C. J. Sensory symptoms in autism spectrum disorders. Harvard Rev. Psychiatry 22(2), 112–124 (2014).
John, T. S. et al. Characterizing social functioning in school-age children with sensory processing abnormalities. J. Autism Dev. Disord. 52(3), 1361–1373 (2022).
Ayres, A. J. Improving academic scores through sensory integration. J. Lear. Disabil. 5, 336343 (1972).
Ayres, A. J. Sensory Integration and Learning Disorders. Los Angeles: Western Psychological Services, pp 258259 (1972).
Cardon, G. J., Hepburn, S. & Rojas, D. C. Structural covariance of sensory networks, the cerebellum, and amygdala in autism spectrum disorder. Front. Neurol. 8, 615 (2017).
Kessler, K., Seymour, R. A. & Rippon, G. Brain oscillations and connectivity in autism spectrum disorders (ASD): New approaches to methodology, measurement and modelling. Neurosci. Biobehav. Rev. 71, 601–620 (2016).
Dal Monte, O. et al. Widespread implementations of interactive social gaze neurons in the primate prefrontal-amygdala networks. Neuron. 110(13), 2183-2197.e7 (2022).
Balasco, L., Provenzano, G. & Bozzi, Y. Sensory abnormalities in autism spectrum disorders: A focus on the tactile domain, from genetic mouse models to the clinic. Front Psychiatry 10, 1016 (2019).
Orefice, L. L. et al. Targeting peripheral somatosensory neurons to improve tactile-related phenotypes in ASD models. Cell 178(4), 867-886.e24 (2019).
Scott, K. E. et al. Loss of Cntnap2 in the rat causes autism-related alterations in social interactions, stereotypic behavior, and sensory processing. Autism Res. 13(10), 1698–1717 (2020).
Moore, S. R. et al. Epigenetic correlates of neonatal contact in humans. Dev. Psychopathol. 29(5), 1517–1538 (2017).
Han, S. et al. Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature 489(7416), 385–390 (2012).
Persico, A. M. et al. The pediatric psychopharmacology of autism spectrum disorder: A systematic review - Part I: The past and the present. Prog. Neuropsychopharmacol. Biol. Psychiatry 110, 110326 (2021).
Port, R. G. et al. Protocadherin 10 alters γ oscillations, amino acid levels, and their coupling; Baclofen partially restores these oscillatory deficits. Neurobiol. Dis. 108, 324–338 (2017).
Moberg, K. U., Handlin, L. & Petersson, M. Neuroendocrine mechanisms involved in the physiological effects caused by skin-to-skin contact - With a particular focus on the oxytocinergic system. Infant. Behav. Dev. 61, 101482 (2020).
Schneider, E. et al. Affectionate touch and diurnal oxytocin levels: An ecological momentary assessment study. Elife. 12, 1 (2023).
Cheung, P. P. P. & Lau, B. W. M. Neurobiology of sensory processing in autism spectrum disorder. Prog. Mol. Biol. Transl. 173, 161–181 (2020).
Schaffler, M. D., Middleton, L. J. & Abdus-Saboor, I. Mechanisms of tactile sensory phenotypes in autism: Current understanding and future directions for Research. Curr. Psychiatry Rep. 21(12), 134 (2019).
Mikkelsen, M., Wodka, E. L., Mostofsky, S. H. & Puts, N. A. J. Autism spectrum disorder in the scope of tactile processing. Dev. Cogn. Neurosci. 29, 140–150 (2018).
Wood, E.T. et al. Sensory over-responsivity is related to GABAergic inhibition in thalamocortical circuits. Transl Psychiatry, 11(1), 39, (2021).
Takarae, Y. & Sweeney, J. Neural hyperexcitability in autism spectrum disorders. Brain Sci. 7(10), 129 (2017).
Abelenda, A. J. & Rodríguez Armendariz, E. Scientific evidence of sensory integration as an approach to occupational therapy in autism. Medicina (B Aires) 80(Suppl 2), 41–46 (2020).
Kashefimehr, B., Kayihan, H. & Huri, M. The effect of sensory integration therapy on occupational performance in children with autism. OTJR-Occup. Part Heal. 38(2), 75–83 (2018).
Martínez, K. et al. Sensory-to-cognitive systems integration is associated with clinical severity in autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 59(3), 422–433 (2020).
Hariri, R. et al. An overview of the available intervention strategies for postural balance control in individuals with autism spectrum disorder. Autism Res. Treat 2022, 3639352 (2022).
Zmigrod, S. & Zmigrod, L. Zapping the gap: Reducing the multisensory temporal binding window by means of transcranial direct current stimulation (tDCS). Conscious Cogn. 35, 143–149 (2015).
Del Bianco, T. et al. Sex differences in social brain neural responses in autism: temporal profiles of configural face-processing within data-driven time windows. Sci Rep, 18;14(1):14038. (2024).
Acknowledgements
This study was approved by the Ethics Committee of Nanjing Brain Hospital, Nanjing Medical University (2022-KY022-01), with a clinical trial registration number: ChiCTR2200062132, and obtained informed consent from the parents or legal guardians of the participants. Children were selected from the ASD specific disease cohort at the Child Mental Health Research Center, the Affiliated Brain Hospital of Nanjing Medical University. And all methods were performed in accordance with the relevant guidelines and regulations. This study was supported by STI2030-Major Projects 2021ZD0204004 (Grant No. 2021ZD0204004) and the Science and Technology development Foundation,Nanjing Department of Health(YKK21115) for Data Collection. Finally, we thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, Xiaoyan Ke, Gongkai Jiao and Hui Fang; methodology, Yonglu Wang Zhijia Li and Yupei Ye; software, Yun Li and Yupei Ye; validation, Yupei Ye; formal analysis, Yuxin Qian; investigation, Lingxi Xu; resources, Yonglu Wang, Zhijia Li and Yupei Ye; data curation, Luyang Guan and Yue Kong; writing—original draft preparation, Yonglu Wang; writing—review and editing, Zhijia Li and Yupei Ye ; visualization, Hui Fang; supervision, Xiaoyan Ke and Gongkai Jiao; project administration, Xiaoyan Ke; funding acquisition, Hui Fang and Yun Li. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Li, Z., Ye, Y. et al. HD-tDCS effects on social impairment in autism spectrum disorder with sensory processing abnormalities: a randomized controlled trial. Sci Rep 15, 9772 (2025). https://doi.org/10.1038/s41598-025-93631-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93631-z
Keywords
This article is cited by
-
Transcranial direct current stimulation on social communication among children and adolescents with autism spectrum disorder: a systematic review and meta-analysis
Journal of Neurodevelopmental Disorders (2026)