Abstract
Sarcoidosis is a systemic granulomatous disease that may differ in clinical presentation, organ involvement and severity among different populations. The primary objective was to evaluate demographic characteristics, disease stage, diagnostic work-up and treatment of patients with sarcoidosis. The sarcoidosis registry was founded in 2022 at the Medical University of Vienna and is intended to record patients from all over Austria who were diagnosed with sarcoidosis. All relevant information was collected at the time of the initial presentation and then on an ongoing basis every 6 months thereafter. Data was available for 199 sarcoidosis patients who were treated at the Medical University of Vienna between 2022 and 2023. The mean age of all patients was 52 ± 13 years. At 57.5%, women were significantly more represented than men were. Of these patients, 44.5% were smokers, and significantly more of them were men (63.8%). Chest X-ray revealed sarcoidosis in lung stages 1, 2, 3 and 4 in 34. 5%, 46%, 9.5% and 6% respectively. Overall, 37.5% (male: 33 (38.8%)) of all patients received oral corticosteroid therapy (OCS) during their illness, the mean duration of treatment with OCS (years, median, IQR) was 5 (3; 7.5). Most patients who received OCS for their disease were in pulmonary stage 4 with 81.8%. Patients were on average overweight with a mean BMI of 28.3 kg/m2 (± 6.5). This study is the first characterization of sarcoidosis patients in Austria.
Similar content being viewed by others
Introduction
Sarcoidosis is an inflammatory granulomatous multisystemic disease of unknown etiology that affects all individuals regardless of sex, race, and age, with a prevalence of about 4.7–64/100,000 people and an incidence of 1–35.5/100,000 people per year worldwide1,2.
The lungs (lymph nodes and lung parenchyma) are involved in most patients, followed by the skin, liver, and eyes. Pulmonary involvement varies from radiographic abnormalities in asymptomatic individuals to a progressive pulmonary disorder causing lung fibrosis and respiratory failure. While dyspnea and cough are the most common organ-related symptoms of sarcoidosis, fatigue is the most common symptom in patients with sarcoidosis, with a broad variety of other constitutional symptoms3,4,5. It was originally assumed that young people aged between 20 and 40 were the age group most affected6. However, there is now evidence that sarcoidosis can be present in older people regardless of gender7,8.
Several studies have been published demonstrating the differences in age at diagnosis, clinical presentation and organ involvement – including in the US9,10,11,12, UK13, Denmark14, Korea15 and Sweden16. The sarcoidosis registry of the Medical University of Vienna was launched to collect long-term follow-up data from a large cohort of patients with sarcoidosis. The overall aim was to present the clinical picture of sarcoidosis, also known as the chameleon of internal medicine due to the disease’s ability to mimic other clinical pictures, in its entirety. In addition, it seems important to be able to follow the course of sarcoidosis, to detect multi-organ involvement in this disease and to characterise the care of patients with proven sarcoidosis.
Methods
Registry
The Sarcoidosis Registry of the Medical University of Vienna was founded in 2022 and enrolls patients from all over Austria at the Department of Pulmonology at Medical University of Vienna with a suspected or confirmed diagnosis of sarcoidosis from February 2022 to March 2023. All patients with confirmed sarcoidosis who were treated at the Department of Pulmonology at Medical University of Vienna from 1 March 2022 onwards were included in this sarcoidosis registry. All patients gave written informed consent before participating in the registry. Patient characteristics are collected on an ongoing basis. This study presents the basic characteristics of the patients with sarcoidosis. In addition, a series of analyses were carried out on these basic data to determine correlations between these characteristics and the course of sarcoidosis. This registry has been approved by the Ethics Committee of the Medical University of Vienna (Ethics Committee Number EK 2246/2021) and was conducted in accordance with the Declaration of Helsinki.
Data collection
Socio-demographic data (age, gender, body mass index (BMI)) and information on concomitant diseases were recorded for all patients at the time of diagnosis and inclusion in this registry. Medical management data including pulmonary function tests (PFT), lung diffusion capacity, chest CT, cerebral MRI, MRI of the heart and diagnostic procedures with biopsies were collected from all patients enrolled in this registry. Patients with cardiac symptoms underwent cardiac MRI. Late enhancement on cardiac MRI was defined as cardiac involvement. Patients with neurological symptoms underwent brain MRI. If pathological changes on MRI were compatible with the presence of neurosarcoidosis and corresponding clinical symptoms, neurological involvement was assumed. Moreover, pharmacological and non-pharmacological therapies, hospitalizations, exacerbations and deaths were recorded to gain a better understanding of the clinical presentation, disease severity, and response to treatment, disease course and mortality. Missing data were not replaced and are addressed when necessary.
Statistical analysis
For categorical parameters, descriptive statistics include relative and absolute frequencies. For metric parameters, the mean and standard deviation, or the median, range and interquartile range (IQR) were calculated. Furthermore, subgroup analyses were conducted to compare characteristics between patients treated with oral corticosteroids and those who were corticosteroid-naïve, and between different stages of pulmonary involvement. Group comparisons were assessed using t-tests or Mann-Whitney-U tests for metric parameters and Chi-square tests for categorical parameters. ANOVA was employed to compare the characteristics between different stages of sarcoid lung involvement. Differences were considered significant if the p-value was less than 0.05. All statistical analyses were conducted using GraphPad Prism version 9 (GraphPad Software, MA, USA).
Results
Clinical characteristics, comorbidities and pulmonary function test
Data were available for 199 Austrian sarcoidosis patients from 2022 to 2023. Table 1 provides an overview of the baseline data. At the time of diagnosis, the median age was 46 years (IQR: 36; 55). Women are more likely to be diagnosed with sarcoidosis than men (men 42.5% vs. women 57.5%, p = 0.0036, x2 test). Patients were on average overweight with a median BMI of 27.76 kg/m2 (24.14–31.64). Almost half of the patients were former or current smokers (44.5%), of whom 63.8% were men (men 63.8% vs. women 34.8%, p = 0.0006, x2 test). Allergies, not specified, were reported as the most common comorbidity in 20% (n = 40), followed by cancer as the second most common comorbidity in 11.5% (n = 23). The most common existing malignancies (n = 23) at the time of inclusion in the registry were gynecological cancers (n = 9), urological cancers (n = 3) and neuroendocrine tumors (n = 3). A total of 15.6% of women had a history of cancer, compared with 7.2% of men. Other comorbidities were diabetes mellitus type 2 (T2DM) in 7.5% and asthma in 5.5%. Clinical features of sarcoidosis are presented in Table 1.
PFT was performed at baseline in 93.5% (n = 186) patients. Airflow obstruction, defined as FEV1/FVC < 70%17 was seen in 26% (n = 52) of patients. Restrictive lung disease, defined by an FVC below under limit of normal (LLN) was found in 8.5% of all patients (n = 17). A diffusion disorder, defined by a DLCO < 70%, was found in 11.5% (n = 23) of all patients in whom a DLCO measurement was performed (Table 1; Fig. 1). KCO was significantly lower in women compared to men (Fig. 1) (mean KCO/SB men: 98% vs. women 82%, p < 0.0001, t-test). Allergic comorbidities at the beginning of the study were present in 40 patients (20%). Median IgE as an expression of a possible allergic component was on average 35.8 U/mL (IQR 11.6; 109.0). The median FeNO was found to be 21 ppb (parts per billion) (IQR 10; 32) (Table 2).
Histological work-up
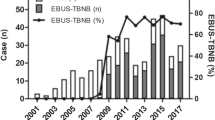
A total of 99.4% (n = 198) of patients had a histologically confirmed diagnosis of sarcoidosis, of which 76.65% (n = 151) were diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and 6.6% (n = 13) by transbronchial biopsy. In 16.75% (n = 33) of all patients, the diagnosis of sarcoidosis was made by biopsy of other tissue, mainly the skin.
Bronchoalveolar lavage (BAL) underwent 50.5% (n = 101) of patients, which revealed a median lymphocyte count 21% (IQR 8; 41) (Table 2). Characteristic BAL findings in sarcoidosis may include a normal or slightly elevated total cell count with lymphocytosis18.
Organ involvement and disease staging
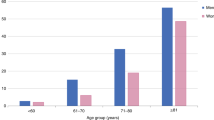
The lungs (mediastinal and hilar lymph nodes and lung parenchyma) were most frequently affected at 96.5%. Radiological assessment with chest CT revealed stage 1, 2, 3 and 4 sarcoidosis in 34.5% (male n = 23 (27.1%)), 46% (male n = 44 (51.8%)), 9.5% (male n = 7 (8.2%)) and 6% (male n = 9 (10.6%)) respectively. In 6% (n = 7) of the patients, no pulmonary involvement could be detected (pulmonary stage 0). Women were more frequently affected in pulmonary stages 0–3 (Table 3; Fig. 2). Only in stage 4 disease, men were significantly more affected (men 10.6% vs. women 2.6%, p = 0.0197, x2 test). The prevalence of skin manifestations was 24% and of ocular manifestation 14%. At the time of enrolment in the sarcoidosis registry, 66.5% of patients underwent MRI of the heart to rule out possible involvement. In 5.5%, late enhancement was a sign of organ involvement. A total of 59% of the patients underwent cerebral MRI that showed cerebral manifestation in 9%. Organ involvement is presented in Table 3.
Laboratory tests
Results from laboratory tests and bronchoalveolar lavage are presented in Table 2. Comparing laboratory results of ACE, IL2 and neopterin between sarcoidosis stages, no significant differences using ANOVA testing were found. Furthermore, no significant differences were found using ANOVA testing in the vital capacity, diffusions capacity and FeNO (a marker of T2 inflammation). Figure 3 visualizes diagnostic results of sarcoidosis stages.
Visualization of selected diagnostic results in patients with histological confirmed sarcoidosis stage (n = 188); stage 0 (n = 7 (6%)); stage 1 (n = 68 (34.0%)); stage 2 (n = 92 (46.0%)); stage 3 (n = 17 (8.5%)); stage 4 (n = 11 (5.5%)); Displayed are results of laboratory and pulmonary function: IL 2 receptor [U/mL]; ACE [U/mL]; Neopterin [U/mL]; VC [%]; TLCO/VA; FeNO [pbb]; Values are reported as median and range;
Figure 4 shows the comparison between single and multi-organ involvement in sarcoidosis in correlation with activity parameters (IL2 receptor, neopterin, ACE). A significant correlation was found between neopterin and multi-organ involvement (Spearman, p = 0.031).
In the comparison between single-organ and multi-organ involvement in sarcoidosis, no correlation was found for IL2 receptor and ACE.
Treatment
Overall, 37.5% (male: 33 (38.8%)) of all patients received OCS while their illness, the median duration of treatment was 5 years (IQR 3; 7.5). (Table 4)
Most patients who received OCS for their disease were in pulmonary stage 4 with 81.8%. (Fig. 5) There were no significant differences in baseline characteristics between OCS treated and OCS naive patients. (Table 4) Among the baseline data such as BMI and nicotine abuse, no significant differences were found between the patients with oral corticosteroid therapy and those without therapy. Similarly, no significant difference in diffusion capacity was found between the two patient groups.
Discussion
Several registries for sarcoidosis exist including the United States of America, Great Britain and Sweden, but they differ immensely in design and data collection, so that direct comparisons are only possible to a limited extent (Supplementary Table 1). This study is the first characterization of sarcoidosis patients in Austria.
Sex aspects and diagnosis
The US Optum health care database identified a total of 29372 adult patients with sarcoidosis, 14700 (55%) of them were over 55 years of age at the time of diagnosis9. Women were two times more likely to have sarcoidosis, the highest prevalence for sarcoidosis was noted in African American women (178.5)9. The data from the sarcoidosis register of the Medical University of Vienna also show a significantly higher number of women (57, 5%) suffering from sarcoidosis. Median age at diagnosis was 46 (IQR 36; 55) and overall median age was 52 (IQR 42; 62).
Data from the Swedish National Patient Register, the British Thoracic Society Sarcoidosis Registry and the Danish National Patient Register (DNPR) showed a slight male predominance with 55%, 58% and 56% respectively14,19. Regarding at the age of diagnosis, 55% of the sarcoidosis patients registered in the US Optum health care database were over 55 years of age and the Swedish National Patient Register found a mean age at diagnosis of 50 years. The National Health Service database identified 6376 individuals with a primary diagnosis of sarcoidosis between 2003 and 201515, with a mean age of 48.8 years. NHLBI-sponsored US studies (ACCESS, A Case Control Etiologic Study of Sarcoidosis and Nurses’ Health Study) have shown that a significant number of patients develop sarcoidosis after the age of 50, which is consistent with previous observations that the incidence may be biphasic9,20,21,22,23,24,25. Data from the Sarcoidosis Register of the Medical University of Vienna, Austria, showed a median age at diagnosis of 46 (36–55) years, significantly younger than the US cohort. In a large multi- center study investigating how demographic differences affect the manifestation of sarcoidosis using the WASOG tool, 1445 patients with sarcoidosis were prospectively reviewed and showed that the median age at diagnosis was 46 years and 61% of patients were female10. However, in the Worldwide Sarcoidosis Research Study (WISE), the median age of more than 1500 patients with was found to be 43 years25.
Therapy
Systemic corticosteroids remain the standard of care. No clear protocol for dose and duration has been validated2. Analyses from an U.S. national health care database showed that the most used treatment for sarcoidosis was prednisone. This is consistent with evidence-based recommendations for the use of glucocorticoids as first-line therapy for symptomatic disease9,26.
About one third (37.5%) of the sarcoidosis patients in this study were treated for the disease. In contrast, in most previously published U.S. studies, researchers have reported that about half of patients achieved steroids19,26,27,28. In a Danish National Patient Register (DNPR), more than half of the patients did not receive systemic corticosteroids14. At the time of presentation to the British Thoracic Society Sarcoidosis Registry, 39% of all patients were being treated with oral corticosteroids13.
Data from the Sarcoidosis Register of the Medical University of Vienna showed that 37.5% of all patients (males: 33, 38.8%) received OCS during the entire course of the disease. This figure also includes data on medical history. Other immunomodulatory drugs were not included in this data collection. The median duration of corticosteroid therapy was 5 years (IQR 3; 7). Most patients who received corticosteroid therapy were in pulmonary stage 4 disease (81.8%). Among the baseline data such as BMI and smoking history, no significant differences were found between the patients with oral corticosteroid therapy and those without therapy. Similarly, no significant difference in diffusion capacity was found between the two patient groups. Corticosteroid therapy has not been shown to have a significant effect on diabetes. A large prospective multi-center study investigating the demographic differences that affect the manifestations of sarcoidosis using the WASOG tool, including 1445 patients with sarcoidosis, 18% of patients did not receive any systemic treatment for sarcoidosis10. The Rheumatology Informatics System for Effectiveness (RISE) registry, which included 3276 patients with sarcoidosis, showed that 59.3% of patients were prescribed glucocorticoids and 24.7% received long-term glucocorticoid therapy12.
Organ involvement and disease staging
Data from the British Thoracic Society Sarcoidosis Registry show pulmonary sarcoidosis in stages 0 (8%), 1 (24%), 2 (29%), 3 (11%), 4 (14%)13, slightly different from our data with 34.5% of patients in radiologically confirmed pulmonary stage 1, 46% of all patients in stage 2, only 9.5% patients of the Austrian cohort were in stage 3 and 6% patients in stage 4. Only 4% (n = 7) of all patients had no radiologically detectable pulmonary manifestation of sarcoidosis. Peculiarities in the gender distribution of pulmonary sarcoidosis stages showed a significant male predominance in stage 4 disease (10.6%). A large prospective multicenter study investigating demographic differences affecting manifestations of sarcoidosis using the WASOG tool in 1445 patients with sarcoidosis showed that women were more likely to have eye or skin involvement, men were more likely to have cardiac involvement10. A US and web-based self-reported questionnaire providing data on demographics, diagnosis, organ involvement and treatment modalities of sarcoidosis included 3835 respondents and compared Hispanic patients with non-Hispanic patients11. The most common organs involved were lung, central and peripheral lymph nodes. Hispanics reported more peripheral nerve and peripheral lymph node involvement than non-Hispanics11.
In the registry of the Medical University of Vienna, the lungs were also the most frequently affected organ (96.5%), the skin was the second most frequently affected organ, significant gender-specific differences in favor of men (n = 8(9.4%)) were only seen in kidney involvement in sarcoidosis, which affected 5% of all patients.
Sarcoidosis was histologically confirmed in 99.4% of patients (n = 198) in the baseline analysis. BAL was performed in 50.5% (n = 101) of all patients (males: 41 (41.4%)) for initial diagnosis. The lymphocyte count in BAL was 21% (IQR 8; 41). The prognostic value of lymphocytes in BAL is questionable. Laviolette M. et al. have already discussed this12. Among the comorbidities examined, 5% of all patients (n = 11) had a history of asthma and allergic comorbidities (requiring a specific IgE, a marker of T2 inflammation) was present in 40 patients (20%) with a specific IgE 35.8 U/mL (IQR 11.6; 109.0). Blood Eosinophils were 2.6% (IQR 1.4; 4.5). FeNO was 21 ppb (IQR 10; 32), so there was no proof of T2 inflammation (eosinophils and FeNO).
FeNO and blood-eosinophils were not elevated on average. The study by Cameli et al. showed that exhaled nitric oxide is not a useful biomarker in the treatment of patients with pulmonary sarcoidosis16. Significant associations between the laboratory parameters neopterin, ACE and s IL2 receptor and pulmonary sarcoidosis stages could not be observed in our study. As expected, the most common manifestation was lung involvement. (96.5%). The most common comorbidities were allergic comorbidities (20%), oncological diseases (11.5%) and diabetes mellitus type 2 (7.5%). Obstructive lung disease was known to be associated with COPD in 2.5% of patients and with asthma in 5.5% of patients. Lung function tests showed airflow obstruction in 26%, restrictive lung disease in 8.5% and diffusion disorder in 11.5%. KCO was significant impaired in women, FEV1 and TLC were significantly lower in women than in men.
In conclusion, the available data and the different approaches to data collection in the different registries, as well as the different case numbers in the international comparison, do not allow any valid statement to be made about relevant differences or correlations. Patients in the Austrian cohort had a different staging distribution than countries such as Great Britain, but the age differences at diagnosis as well as the general average age as well as the general dominance of the female sex did not show any significant differences to the existing data of other registries.
Given the long-term follow-up nature of the registry, future analyses could potentially establish new correlations and help to decipher the still unexplained aetiology of sarcoidosis. As the data originate from standard clinical assessments, a particular challenge (as with all purely observational, non-interventional studies) is the lack of data especially for suitable laboratory and clinical markers for risk assessment.
The initial situation and the various analyses once again illustrate the heterogeneity of sarcoidosis with its many different manifestations, which underlines the importance of a personalised approach to disease management. It is hoped that comparative studies and the observation of a large cohort will overcome the current limitations caused by the small number of cases in individual centres, which have so far made it difficult to draw conclusions about the success of therapy and to determine the dose of the immunomodulatory therapy to be planned in combination with appropriate imaging (PET-CT versus CT/MRI) and serological follow-up examinations. Future research will focus on understanding the causes of sarcoidosis, relevant biomarkers and new effective and targeted therapies for patients with previously refractory sarcoidosis and pulmonary fibrosis.
Strengths and limitations
This is the first register, assessing sarcoidosis patients in central Europe, and offers a decent population size and real-world data. A limiting factor is the small number of patients compared to other registries and the fact that it is an analysis of a single center. Due to the circumstance of the single center and the recruitment modality of the hospital outpatient clinic by means of referrals from the extramural area, there is a not insignificant possibility that sarcoidosis patients remain undetected. Due to the nature of a registry and retrospective observation as well as the fact that real-life data are involved, incomplete data and irregular patient appointments are a limitation that must be considered.
Conclusion
This study is the first characterization of sarcoidosis patients in Austria. It offers new insights in characteristics of this special patient cohort. Based on the data collected, the cohort is characterized by a heterogeneous appearance; in the preliminary analysis of the available baseline characteristics, the basic laboratory values and the lung function, no correlations could be identified regarding clinical severity and diagnostic findings.
The present study is hypothesis-generating and clearly demonstrates the need for further clinical research, particularly in conjunction with radiological findings. The planned long-term follow-up of patients in the sarcoidosis registry at the Medical University of Vienna will show whether the treatment of sarcoidosis can be optimized.
Data availability
The datasets generated and analyzed during the current study are availablefrom the corresponding author on reasonable request.
Abbreviations
- ACE:
-
Angiotensin converting enzyme
- BAL:
-
Bronchoalveolar lavage
- BMI:
-
Body mass index
- CT:
-
Computer tomography
- DLCO or TLCO:
-
Diffusing capacity for carbon monoxide
- EOS:
-
Eosinophil lymphocyte
- FeNO:
-
Exhaled nitric oxide
- FEV:
-
Forced expiratory volume
- FVC:
-
Forced vital capacity
- IgE:
-
Immunoglobulin E
- IL2:
-
Interleukin 2 receptor
- KCO:
-
Carbon monoxide transfer coefficient
- LLN:
-
Lower limit of normal
- MRI:
-
Magnet resonance imaging
- OCS:
-
Oral corticosteroid
- pBNP:
-
Pro brain natriuretic peptide
- PFT:
-
Pulmonary function test
- SD:
-
Standard deviation
- T2DM:
-
Diabetes mellitus type 2
- TLC:
-
Total lung capacity
References
Wijsenbeek, M., Suzuki, A. & Maher, T. M. Interstitial lung diseases. Lancet Lond. Engl. 400 (10354), 769–786 (2022).
Valeyre, D., Prasse, A., Nunes, H., Uzunhan, Y. & Brillet, P. Y. Müller-Quernheim J. Sarcoidosis Lancet Lond. Engl. 383(9923), 1155–1167 (2014).
Jayakrishnan, B., Al-Busaidi, N., Al-Mubaihsi, S. & Al-Rawas, O. A. Sarcoidosis in the middle East. Ann. Thorac. Med. 14(2), 106–115 (2019).
Mihailovic-Vucinic, V. & Jovanovic, D. Pulmonary sarcoidosis. Clin. Chest Med. 29(3), 459–473 (2008).
Sève, P. et al. Sarcoidosis: A clinical overview from symptoms to diagnosis. Cells 10(4), 766 (2021).
Rybicki, B. A., Major, M., Popovich, J., Maliarik, M. J. & Iannuzzi, M. C. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am. J. Epidemiol. 145(3), 234–241 (1997).
Sawahata, M. et al. Age-related and historical changes in the clinical characteristics of sarcoidosis in Japan. Respir Med. 109(2), 272–278 (2015).
Ungprasert, P., Crowson, C. S. & Matteson, E. L. Influence of gender on epidemiology and clinical manifestations of sarcoidosis: A population-based retrospective cohort study 1976–2013. Lung 195(1), 87–91 (2017).
Baughman, R. P. et al. Sarcoidosis in America. Analysis based on health care use. Ann. Am. Thorac. Soc. 13(8), 1244–1252 (2016).
Zhou, Y. et al. The impact of demographic disparities in the presentation of sarcoidosis: A multicenter prospective study. Respir Med. 187, 106564 (2021).
Innabi, A. et al. Sarcoidosis among US Hispanics in a nationwide registry. Respir Med. 190, 106682 (2021).
Hammam, N. et al. Treatment of sarcoidosis in US rheumatology practices: Data from the American college of rheumatology’s rheumatology informatics system for effectiveness (RISE) registry. Arthritis Care Res. 74(3), 371–376 (2022).
Thillai, M. et al. Sarcoidosis in the UK: Insights from British thoracic society registry data. BMJ Open. Respir Res. 6(1), e000357 (2019).
Sikjær, M. G., Hilberg, O., Ibsen, R., Løkke, A. & Sarcoidosis A nationwide registry-based study of incidence, prevalence and diagnostic work-up. Respir Med. 187, 106548 (2021).
Park, J. E. et al. Prevalence, incidence, and mortality of sarcoidosis in Korea, 2003–2015: A nationwide population-based study. Respir Med. 144S, S28–34 (2018).
Cameli, P., Barbagli, E. & Rottoli, P. Exhaled nitric oxide is not increased in pulmonary sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. Off J. WASOG 33(1), 39–40 (2016).
Singh, D. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur. Respir J. 53(5), 1900164 (2019).
Drent, M., Mansour, K. & Linssen, C. Bronchoalveolar lavage in sarcoidosis. Semin Respir Crit. Care Med. 28(5), 486–495 (2007).
Cozier, Y. C. Assessing the worldwide epidemiology of sarcoidosis: Challenges and future directions. Eur. Respir J. 48(6), 1545–1548 (2016).
Anantham, D. et al. Sarcoidosis in Singapore: epidemiology, clinical presentation and ethnic differences. Respirol. Carlton Vic. 12(3), 355–360 (2007).
Byg, K. E., Milman, N. & Hansen, S. Sarcoidosis in Denmark 1980–1994. A registry-based incidence study comprising 5536 patients. Sarcoidosis Vasc. Diffuse Lung Dis. Off J. WASOG. 20(1), 46–52 (2003).
Dumas, O., Abramovitz, L., Wiley, A. S., Cozier, Y. C. & Camargo, C. A. Epidemiology of sarcoidosis in a prospective cohort study of U.S. Women. Ann. Am. Thorac. Soc. 13(1), 67–71 (2016).
Morimoto, T. et al. Epidemiology of sarcoidosis in Japan. Eur. Respir J. 31(2), 372–379 (2008).
Yigla, M., Badarna-Abu-Ria, N., Goralnik, L., Rubin, A. H. E. & Weiler-Ravell, D. Sarcoidosis in residents of Northern Israel of Arabic and Jewish origin: A comparative study. Respirol. Carlton Vic. 11(5), 586–591 (2006).
Gerke, A. K., Judson, M. A., Cozier, Y. C., Culver, D. A. & Koth, L. L. Disease burden and variability in sarcoidosis. Ann. Am. Thorac. Soc. 14(Supplement_6), S421–S428 (2017).
Baughman, R. P. et al. Presenting characteristics as predictors of duration of treatment in sarcoidosis. QJM Mon J. Assoc. Phys. 99(5), 307–315 (2006).
Baughman, R. P. et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am. J. Respir Crit. Care Med. 164(10 Pt 1), 1885–1889 (2001).
Gottlieb, J. E., Israel, H. L., Steiner, R. M., Triolo, J. & Patrick, H. Outcome in sarcoidosis. The relationship of relapse to corticosteroid therapy. Chest 111(3), 623–631 (1997).
Funding
No special funds were provided for this study.
Author information
Authors and Affiliations
Contributions
C.G.-D.: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Project administration, Writing original draft & editing. M.L.: Data curation, Formal analysis, Methodology, Visualization; M.R.G.: review & editing, P.S.: review & editing, C.M.: review & editing , C.B.: review & editing, W.G.: Writing—review & editing, H.P.: Writing—review & editing, D.G.: Conceptualization, Investigation, Project administration, Writing—review & editing, M.I.: Conceptualization, Investigation, Project administration, Writing—review & editing;
Corresponding author
Ethics declarations
Competing interests
First insights and future research perspectives from the sarcoidosis registry at the Medical University of ViennaC Guttmann-Ducke, M Lutnik, MR Gysan, P Sarova, C Milacek, C Bal, W Graninger, H Prosch, D Gompelmann and M Idzko. The authors declare following conflicts of interest: Claudia Guttmann- Ducke: Speaker Fees Boehringer Ingelheim all outside the submitted work, Marco Idzko: lecture fees from AstraZeneca, Bayer, Berlin-Chemie, Boehringer Ingelheim, Chiesi, CSL-Behring, GSK, Menarini, MSD, Novartis, Roche, Sanofi, and Thermofischer; and advisory board fees from Alk-Pharma, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, CSL-Behring, GSK, Novartis, and Sanofi, all outside the submitted work; Daniela Gompelmann: lecture and travel fees from Olympus, Pulmonx, Astra Zeneca, Boehriger Ingelheim, Erbe, Berlin Chemie, Chiesi, MSD all outside the submitted work. Martin Lutnik: no conflict of interest; Christopher Milacek: no conflict of interest; Pavla Sarova: no conflict of interest; Maximilian Robert Gysan: Fees Boehringer Ingelheim all outside the submitted work. Wolfgang Graninger: no conflict of interest. Helmut Prosch: Fees from AstraZeneca, BMS, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novartis, Roche/Intermune, Sanofi, Siemens Healthcare, and Takeda; and research support from AstraZeneca, Boehringer, Siemens, and EU4Healthall outside the submitted work. Christina Bal: Fees Olympus all outside the submitted work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guttmann-Ducke, C., Lutnik, M., Gysan, M.R. et al. First insights and future research perspectives from the sarcoidosis registry at the Medical University of Vienna. Sci Rep 15, 8644 (2025). https://doi.org/10.1038/s41598-025-93708-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93708-9