Abstract
Increasing salinity stress is a significant challenge in agriculture, affecting ~ 20% of irrigated areas worldwide. It can induce osmotic stress, oxidative stress, and nutrient imbalance in plants. Using rhizobacterial species and biochar can be an effective method to overcome this issue. Bacillus pumilus is a rhizobacteria that can enhance plant salt tolerance, facilitate nutrient solubilization in saline soils, and generate stress-alleviating metabolites. On the other hand, nitrilotriacetic acid mixed biochar (NAT-BC) can increase crop yields and mitigate salt stress by improving nutrients and water uptake. That’s why the present study was carried out to explore the combined effect of NAT-BC and Bacillus pumilus on sunflowers in both non-saline and salinity-stress on sunflowers. Four treatments, i.e., 0NAT-BC, Bacillus pumilus, 0.75NAT-BC, Bacillus pumilus + 0.75NAT-BC, were applied in four replications following a completely randomized design (CRD). Results showed that Bacillus pumilus + 0.75NAT-BC caused significant enhancement in sunflower plant height (103%), stem diameter (45%), head diameter (74%), stomatal conductance (60%), and protein content (11%) rate over control under salinity stress. A significant improvement in sunflower chlorophyll a (19%), chlorophyll b (35%), and total chlorophyll (54%) compared to the control confirm the efficacy of Bacillus pumilus + 0.75NAT-BC under salinity stress. It is concluded that applying treatment Bacillus pumilus + 0.75NAT-BC can alleviate salinity stress in sunflowers via improvement in total chlorophyll contents, which was the most representative attribute in the current study. Growers are recommended to apply Bacillus pumilus + 0.75NAT-BC to achieve better sunflower growth under salinity stress.
Similar content being viewed by others
Introduction
Globally, over 800 million hectares of land face salinity-related issues, primarily affecting arid and semi-arid regions with limited water resources and hot, dry climates1,2,3. Salt disrupts plant growth in varying concentrations and can even lead to plant death by disturbing cellular ion balance. This disruption results in various morphological, physiological, and metabolic issues, such as reduced seed germination, changes in cell membrane integrity, and inhibition of enzymatic activities4,5. High concentrations of sodium chloride, the predominant soil salt, induce osmotic and ionic alterations in plants, reducing water and mineral availability for root cells. This salinity stress leads to the overproduction of reactive oxygen species (ROS), causing oxidative damage to cellular membranes, lipids, DNA, proteins, and chlorophyll3,6,7. Excessive ROS accumulation can cause oxidative damage to proteins, lipids, and DNA, leading to cell death (Mansoor et al., 2022; Parab et al., 2023). Presence of ROS in various cellular compartments, i.e., chloroplasts, peroxisomes, and mitochondria8. In excess light energy electron leakage from photosystem I (PSI) and photosystem II (PSII), form superoxide radicals (O₂•⁻), hydrogen peroxide (H₂O₂), and singlet oxygen (¹O₂), which induced oxidative stress under ROS accumulation8.
Plant growth-promoting rhizobacteria (PGPR) improves plant tolerance under salinity stress. These rhizobacteria can improve plant growth by improving major physiological processes, i.e., nutrients and water uptake, source-sink relationships, and photosynthesis9. PGPR regulates salt stress via various mechanisms, i.e., osmotic balance, antioxidant activity, and ion homeostasis10. They also modulate gene expression, phytohormone status, and metabolite synthesis in plants9, increasing plant dry weight, chlorophyll content, root and shoot length under salt stress11.
Sunflower (Helianthus annuus L.) ranks third after soybean and peanut, among other oilseeds, including rapeseed and cotton, due to the quality of its oil12. Its seeds contain approximately 25–48% oil and 20–27% protein13. Furthermore, its wide adaptability to different soil and climatic conditions, short growth cycles, and significance in agricultural systems make it unique14. However, the global climate has increased temperatures, drought, and salinity, which are significant agricultural stress factors15. Salinity stress can decrease yields (5% yield decrease for each unit increase in salinity), plant height, water content, biomass, and physiological processes16,17,18,19.
A current study was conducted to mitigate the salt stress in sunflowers by using nitrilotriacetic acid mixed biochar (NAT-BC) and beneficial microorganisms, notably Bacillus pumilus. The NAT-BC, derived from the pyrolysis of organic waste, is renowned for its exceptional adsorption capacity and positive impact on critical soil properties such as bulk density, pH, cation exchange capacity, soil structure, and water retention capacity. Bacillus pumilus, known for its plant growth-promoting attributes, has shown potential in enhancing salt tolerance, nutrient solubilization, and the production of stress-alleviating metabolites.
The hypothesis driving this study suggests that the combined application of NTA-BC and Bacillus pumilus will significantly alleviate salt stress in sunflower plants. This is expected to occur through improvements in nutrient uptake, soil quality, reduction in soil salinity, and the mitigation of oxidative stress. The primary study objectives encompass evaluating the effectiveness of this innovative approach in salt stress mitigation, optimizing application methods, and advancing sustainable, eco-friendly solutions aligned with environmentally sensible agricultural practices.
Materials and methods
Experimental site
In 2022, a pot study was done at the research area of Anhui Science and Technology University. The climate of the area was semi-arid. A total of 6 samples were taken from different positions to characterize soil. A composite sample was then made for the analysis. The physiochemical attributes of the soil include pHs, 8.49 20, ECe = 2.98 dS/m EC, soil organic matter = 0.55% 21, available phosphorus = 4.67 mg/kg22, and extractable potassium = 89 mg/kg23.
Bacillus pumilus
Identification
The pure culture of isolate taken from cotton rhizosphere was prepared on LB agar plates and sent to Macrogen, South Korea, for 16 S rRNA sequencing using Sanger sequencing technology and identification. The primers 27 F (5’-AGA GTT TGA TCM TGG CTC AG-3’) and 1492R (5’-TAC GGY TAC CTT GTT ACG ACT T-3’) were used for PCR amplification with the addition of the universal primers 785 F (5’-GGA TTA GAT ACC CTG GTA-3’) and 907R (5’-CCG TCA ATT CMT TTR AGT TT-3’) as 16 S rRNA sequencing primers to ensure sequencing overlap. The crude sequences were trimmed and edited by using BioEdit software24. The National Center for Biotechnology Information (NCBI) server analyzed the final sequences using the Basic Local Alignment Search Tool (BLAST) program25 to compare a query sequence against a public sequence to find the closest related organism in the Genbank database. Evolutionary analyses were conducted using molecular evolutionary genetics analysis (MEGA X) software26.
Characterization
Spot inoculation of culture on petri plates containing LB agar medium was conducted and incubated at 28 ± 1 °C for 24 h. After incubation, individual colonies were observed under a magnifying glass following protocol as stated in Burgey’s Manual of Determinative Bacteriology27.
Characteristics
Rhizobacteria was tested for different morphological and biochemical characteristics. All tests were replicated 4 times with CRD arrangements. Skerman’s28 procedure was used to determine isolates’ cell morphology and gram-staining reactions. Oxidase activity was determined using freshly prepared culture following the method of Steel29. Catalase activity was tested following the procedure described by MacFaddin30. The protocol of Ashraf et al.31 was followed to determine exopolysaccharide production. The glucose utilization test and methyl red test were performed using methods reported by Holding and Collee32. Enzyme 1-aminocyclopropane-1- carboxylate (ACC) deaminase activity was tested using the method of Glick et al.33. As described by Sarwar et al.34, the protocol was followed to determine Indole-3-acetic acid (IAA) production. Isolates’ ability to solubilize zinc was detected following the procedure, as Kumar et al.35 reported. Siderophore production was tested following the method described by Schwyn and Neilands36. A qualitative test was performed to determine chitinase production following the technique of Dunne et al.37. HCN (cyanogen) production was tested using the procedure described by Bakker and Schippers38. The characteristics of Bacillus pumilus include cell shape = Rod; Gram staining = + ve, Catalase test = + ve, Oxidase test = + ve, Exopolysaccharide Production = -ve, Glucose utilization = + ve, Methyl red test = -ve, ACC-deaminase Activity = + ve, IAA production = -ve, Zn Solubilization = -ve, Siderophore production = + ve, Chitinase Production = + ve and HCN production = + ve.
Inoculation
Sunflower seeds were inoculated with Bacillus pumilus using a combination of peat and a 10% sugar solution as an adhesive. In this process, 10 g of peat were utilized with an addition of 10 milliliters of the sugar solution for every 50 g of seeds. Following the inoculation, the seeds underwent a drying period under controlled conditions to guarantee the effective binding of the inoculum to the seed surface.
NTA-BC preparation
Nitrilotriacetic acid
The nitrilotriacetic acid (NTA) used in the current study was identified as product number N9877 under the brand SIAL, and exhibits a physical appearance of white to off-white powder. Its formulation, registered under CAS Number 139-13-9 and with an MDL Number MFCD00004287, yields a molecular formula of C6H9NO6, weighing 191.14 g/mol.
Biochar preparation
Utilizing waste materials of spinach stems sourced from a local market, positioned at coordinates 30°11’29.8’’N and 71°28’48.8’’E, a biochar was prepared by semi-aerobic pyrolysis at a temperature of 305 ± 10 °C for 65 min. The physicochemical properties of the biochar includes pH = 7.66 20; EC = 3.11 dS/m39, ash content = 29%, volatile matter = 11%, fixed carbon = 60%, total nitrogen = 0.03%, total phosphorus = 0.52% and total potassium = 0.40% 40. For mixing NTA in biochar, a solution of 1% NTA was prepared in deionized water. In a mixing container, the 10 kg biochar was mixed with 1 L NTA solution (1%). Following this, the mixture underwent a sun-drying phase to eliminate excess moisture.
Treatment plan
The experiment involved manipulating three key factors: biochar type, salt stress level, and the introduction of Bacillus pumilus. Neither Bacillus pumilus nor NAT-BC (0NAT-BC) were introduced to the control group. Treatments involved Bacillus pumilus, 0.75NAT-BC, and the combination of Bacillus pumilus + 0.75NAT-BC were applied in a completely randomized design. Furthermore, in control (2.98 dS/m EC) soil no sat was added. A 1:1:1 mixture of NaCl, CaCl2, and MgSO4 was used to induce salt stress. The final EC of salinity stress soil was maintained at 6.44 dS/m EC after mixing and incubating the soil for 21 days.
Seed collection, sterilization, and sowing
The sunflower seeds S-278 were purchased from the local seed market. Before planting, the chosen seeds underwent a surface sterilization process involving treatment with a 5% sodium hypochlorite solution, followed by three consecutive rinses with 95% ethanol. To eliminate any residual traces of the sterilizing agents, the seeds were subsequently subjected to three washes using deionized water that had been sterilized41. Each pot containing 15 kg of soil was sown with 10 seeds. After germination, 2 seedlings per pot were maintained by thinning.
Fertilizer
To meet the nutritional needs of sunflowers, nitrogen (N), phosphorus (P), and potassium (K) were administered at rates of 60, 40, and 25 kg per acre, respectively. Urea was employed as the N source, while single superphosphate and potassium sulfate were used to supply P and K during sowing.
Irrigation
Each pot’s irrigation schedule was regulated with the help of a moisture gauge (ADVANCED™; 4 in 1 Soil Meter; China). Watering was adjusted daily to ensure the moisture level was kept at 70% of the soil’s field capacity, as indicated by the instrument.
Harvesting and data collection
Data collection occurred 60 days after planting when the plants were harvested. Subsequently, plant height (cm), stem diameter (cm), head diameter (cm), number of leaves/plants, number of achene/heads, 1000-achene weight, achene yield (kg ha− 1), and biological yield (kg ha− 1) were measured. We exposed the samples to a 72-hour oven-drying procedure at 65 °C to determine the dry outcomes until a consistent weight was attained.
Chlorophyll contents and carotenoids
To evaluate the concentrations of chlorophyll a, chlorophyll b, and total chlorophyll in freshly harvested wheat leaves, we employed a methodology inspired by Arnon’s approach42. The extraction process involved an 80% acetone solution, and absorbance measurements were recorded at distinct wavelengths: 663 nm, 645 nm, and 480 nm.
Gas exchange attributes
We conducted measurements of leaf gas exchange parameters, encompassing the transpiration rate and stomatal conductance. These measurements were performed utilizing an infrared gas Analyzer, specifically the CI-340 Photosynthesis system manufactured by CID, Inc. in the USA. The data collection took place on a sunny day during a one-hour window from 10:30 AM to 11:30 AM when the light intensity was optimal and saturating for photosynthetic processes, following the protocol outlined by43.
Antioxidants
To assess SOD activity, we measured the inhibition of nitro blue tetrazolium (NBT) reduction in the presence of riboflavin. The reaction mixture, which included enzyme extract, NBT, riboflavin, and phosphate buffer, was subjected to illumination, and changes in absorbance at 560 nm were continuously monitored44. POD activity was determined by monitoring the oxidation of a suitable substrate, such as guaiacol or o-dianisidine. We measured the increase in absorbance resulting from substrate oxidation at a specific wavelength, following the methodology outlined by45. CAT activity was assessed by monitoring the enzyme’s decomposition of hydrogen peroxide (H2O2). To evaluate APX activity, we monitored the oxidation of ascorbate in the presence of H2O2, observing the decrease in absorbance at a specific wavelength over time following the procedure developed by46. Finally, to determine the level of MDA, an indicator of lipid peroxidation, we reacted the sample extract with thiobarbituric acid (TBA) to form a colored complex. The absorbance of this complex was measured, and the MDA content was subsequently calculated.
Oil content
The oil content of intact seeds was assessed through nuclear magnetic resonance (NMR) utilizing the MQC23 instrument produced by Oxford Instruments, UK. A reference sample comprising 5 g of seeds from different sunflower hybrids with known oil content was employed for calibration. Near-infrared spectroscopy (NIRS) was utilized to analyze fatty acid profiles, encompassing stearic acid, palmitic acid, linolenic acid, and oleic acid47.
Statistical analysis
The analysis involved standard statistical methods to compare the data. A two-way ANOVA was employed to assess the significance of the treatments. Additionally, paired comparisons were made using the Tukey test at a significance level of p ≤ 0.05. For data visualization, cluster plots, convex hull plots, hierarchical cluster plots, and Pearson correlation were generated using OriginPro software provided by48.
Results
Plant height, stem diameter, and head diameter
Under 2.98 dS/m EC, the mean plant height for the 0NAT-BC treatment was 60.79 cm. When Bacillus pumilus was applied, the plant height increased to 29.01% over the 0NAT-BC, and treatment with 0.75NAT-BC showed a substantial 79.50% increase. The combination of Bacillus pumilus and 0.75NAT-BC led to a plant height of 123.69 cm, indicating an impressive increase over the 0NAT-BC treatment. In contrast, under SS (6.44 dS/m EC), the control (0NAT-BC) group had a mean plant height of 17.81 cm. When Bacillus pumilus was applied under the SS 6.44 dS/m EC, the plant height increased to 60.57%, and the 0.75NAT-BC treatment produced a mean plant height of 36.76 cm, showing a remarkable increase related to the 0NAT-BC treatment, while the combination of Bacillus pumilus and 0.75NAT-BC yielded a plant height of 41.14 cm (Table 1).
The 0NAT-BC treatment had an average stem diameter of 0.92 cm, while it increased by approximately 17.35% when treated with Bacillus pumilus in 2.98 dS/m EC. When subjected to 0.75NAT-BC under 2.98 dS/m EC, the stem diameter exhibited a significant increase of about 37.74%, and when Bacillus pumilus and 0.75NAT-BC were applied in combination, the stem diameter further increased by 44.03% over the 0NAT-BC treatment. Under SS (6.44 dS/m EC), the 0NAT-BC treatment had a mean stem diameter of 0.21 cm. However, when treated with Bacillus pumilus, the stem diameter increased substantially by 80.95% over the 0NAT-BC treatment under SS 6.44 dS/m EC. The 0.75NAT-BC treatment also resulted in an average stem diameter of 0.56 cm over the baseline treatment 0NAT-BC, and the combination treatment of Bacillus pumilus and 0.75NAT-BC yielded the highest stem diameter of 0.78 cm under salt stress (Table 1).
Head diameter was significantly influenced by different treatments under both 2.98 dS/m EC and high salinity stress (6.44 dS/m EC). Under 2.98 dS/m EC, Bacillus pumilus treatment resulted in a 31.34% increase in head diameter related to the control (0NAT-BC). Treatment 0.75NAT-BC showed a 60.69% rise over the 0NAT-BCtreatment, and the combination treatment of Bacillus pumilus and 0.75NAT-BC showed a 74.25% increase. Under high salinity stress (6.44 dS/m EC), the Bacillus pumilus treatment exhibited a remarkable 83.70% increase in head diameter parallel to the 0NAT-BC treatment. In contrast, the combination treatment of Bacillus pumilus and 0.75NAT-BC had a mean head diameter of 5.29 cm. The 0.75NAT-BC treatment also showed an average head diameter of 3.35 cm (Table 1).
Number of leaves/plants, number of achene/heads, and 1000-achene weight
Under 2.98 dS/m EC, the 0NAT-BC treatment had 8.35 leaves per plant. When Bacillus pumilus was applied, the number of leaves showed a 21.04% improvement, and the 0.75NAT-BC treatment led to a substantial 53.82% boost compared to the 0NAT-BC treatment. Combining Bacillus pumilus with 0.75NAT-BC resulted in an impressive 83.79% rise in 2.98 dS/m EC over the 0NAT-BC treatment. In contrast, the stress treatment with a higher salinity level (6.44 dS/m EC) 0NAT-BC treatment exhibits 1.60 leaves per plant. Applying Bacillus pumilus significantly 132.71% improved leaf number, and the 0.75NAT-BC treatment resulted in a significant 211.86% increase related to the 0NAT-BC treatment under salt stress. Combining Bacillus pumilus with 0.75NAT-BC under the stress condition yielded a remarkable 325.61% increase from 0NAT-BC in salt stress (Table 2).
Under 2.98 dS/m EC, the Bacillus pumilus treatment resulted in a 12.20% increase in achenes contrasted to 0NAT-BC. When 0.75NAT-BC was applied, there was a noTable 20.60% increase in the number of achenes over the 0NAT-BC treatment, and the combination of Bacillus pumilus and 0.75NAT-BC produced the most significant effect, leading to a 34.62% increase. Under saline stress (6.44 dS/m EC), the Bacillus pumilus treatment exhibited a substantial 62.48% increase in achenes related to 0NAT-BC treatment. When 0.75NAT-BC was applied under the salt stress, there was a remarkable 118.75% increase in the number of achenes, and the combination of Bacillus pumilus and 0.75NAT-BC produced the most significant effect, resulting in a substantial 158.41% increase in the number of achenes related to 0NAT-BC (Table 2).
Compared to the 0NAT-BC control, the Bacillus pumilus treatment increased 1000-achene weight by 17.12% under 2.98 dS/m EC. When 0.75NAT-BC was introduced, it showed a substantial 30.91% increase over the 0NAT-BC treatment, and the combination of Bacillus pumilus and 0.75NAT-BC produced the most significant 47.49% increase in 1000-achene weight. Under salt stress (6.44 dS/m EC), the Bacillus pumilus treatment demonstrated a remarkable 53.03% increase in 1000-achene weight evaluated to the 0NAT-BC control and 0.75NAT-BC treatment was applied under the salt stress, the 1000-achene weight exhibited a substantial 85.22% increase. The combination of Bacillus pumilus and 0.75NAT-BC showed a remarkable 150.98% increase in 1000-achene weight in salt stress over the 0NAT-BC (Table 2).
Achene yield and biological yield
In the case of 2.98 dS/m EC, the 0NAT-BC treatment had an average achene yield of 1251.3 kg h-1. Adding Bacillus pumilus increased the achene yield by 26.39% and the 0.75NAT-BC treatment, showing a 44.11% increase compared to the 0NAT-BC treatment under 2.98 dS/m EC. Under 2.98 dS/m EC, combining Bacillus pumilus and 0.75NAT-BC resulted in a significant 59.30% increase compared to the 0NAT-BC treatment. Under salinity stress (6.44 dS/m EC), the 0NAT-BC treatment exhibits an average achene yield of 436.6 kg h-1. The application of the Bacillus treatment represented a 27.49% increase over the 0NAT-BC treatment, and the 0.75NAT-BC treatment showed an impressive 63.73% increase related to the 0NAT-BC treatment. The highest achene yield under saline was achieved with the Bacillus pumilus + 0.75NAT-BC treatment, indicating a remarkable 121.73% increase related to the 0NAT-BC treatment (Table 3).
Under 2.98 dS/m EC, the control group (0NAT-BC) had a biological yield of 5178.8 kg h− 1. When treated with Bacillus pumilus, the yield increased by 18.80%, and a more substantial increase of 32.65% was observed in the 0.75NAT-BC treatment over the 0NAT-BC treatment under 2.98 dS/m EC, and the most significant improvement was noted in the Bacillus pumilus + 0.75NAT-BC treatment, with a 38.45% increase. In contrast, under a higher salinity level (6.44 dS/m EC), the 0NAT-BC treatment had an average biological yield of 1917.6 kg h− 1. Applying Bacillus pumilus substantially increased the biological yield of 56.92% and the 0.75NAT-BC treatment, showing a 101.47% increase over the 0NAT-BC treatment in salt stress. The combined treatment of Bacillus pumilus and 0.75NAT-BC resulted in a 137.79% increase (Table 3).
Leaf transpiration rate and stomatal conductance
The control group (0NAT-BC) had a 4.81 mmol H2O/m2/s transpiration rate under 2.98 dS/m EC. When Bacillus pumilus was introduced, there was an 11.69% increase, and the 0.75NAT-BC treatment led to a substantial 37.41% rise in transpiration rate over the 0NAT-BC treatment in 2.98 dS/m EC. The Bacillus pumilus + 0.75NAT-BC treatment showed a 59.76% increment in transpiration rate over the 0NAT-BC treatment. Under salt stress (6.44 dS/m EC), the control group (0NAT-BC) revealed a mean transpiration rate of 2.25 mmol H2O/m2/s. The introduction of Bacillus pumilus increased the transpiration rate by 28.83%, and when 0.75NAT-BC treatment was applied, there was a substantial 54.09% increase in transpiration rate under salt stress over the 0NAT-BC. The group treated with Bacillus pumilus and 0.75NAT-BC exhibited the highest increase of 80.46% in transpiration rate in salt stress over the 0NAT-BC treatment (Table 4).
The control group (0NAT-BC) showed a photosynthetic rate of 454.31 µmol CO2/m2/s in 2.98 dS/m EC. When Bacillus pumilus was introduced, there was a 10.93% increase, and the introduction of a combination treatment (Bacillus pumilus + 0.75NAT-BC) under 2.98 dS/m EC showed a noTable 59.90% increase in photosynthetic rate over the 0NAT-BC treatment. Under salinity stress (6.44 dS/m EC), the control group (0NAT-BC) exhibited a lower photosynthetic rate of 229.22 µmol CO2/m2/s. When Bacillus pumilus was applied, there was a substantial 44.29% increase, and the combination treatment (Bacillus pumilus + 0.75NAT-BC) under saline stress displayed a significant 68.66% increase in photosynthetic rate contrasted to the 0NAT-BC treatment (Table 4).
Palmitic acid, steric acid, oleic acid, and Linoleic acid
The palmitic acid content was 3.13% for the control (0NAT-BC) in 2.98 dS/m EC when Bacillus pumilus was applied, indicating a 3.70% increase compared to the 0NAT-BC treatment. When 0.75NAT-BC treatment was used, it showed an 8.81% rise in palmitic acid as opposed to the control. The most significant increase in palmitic acid was observed when both Bacillus pumilus and 0.75NAT-BC were combined, representing a 13.02% increase over the control under 2.98 dS/m EC. In the stress condition (6.44 dS/m EC), the control (0NAT-BC) had a mean palmitic acid of 2.80%. When Bacillus pumilus was introduced, it resulted 2.21% increase compared to the control in salinity stress. Adding 0.75NAT-BC treatment resulted in a 5.14% increase over the 0NAT-BC treatment, and the combination of Bacillus pumilus and 0.75NAT-BC led to a 9.74% increase under the stress condition (Table 5).
Under 2.98 dS/m EC, the steric acid content in 0NAT-BC was 4.29%. When Bacillus pumilus was added, steric acid increased to 3.73%, and the addition of 0.75NAT-BC further increased steric acid by 6.43% over the 0NAT-BC in 2.98 dS/m EC. The combination of Bacillus pumilus and 0.75NAT-BC shows a significant 10.30% increase in steric acid over the 0NAT-BC treatment. Under high salinity stress (6.44 dS/m EC), the steric acid content in 0NAT-BC was lower at 3.69%. When Bacillus pumilus was introduced, representing a 5.20% rise in steric acid, adding 0.75NAT-BC further boosted steric acid to 9.53% in contrast to the 0NAT-BC treatment under salinity stress. The combination of Bacillus pumilus and 0.75NAT-BC treatment showed a 13.62% increase related to the 0NAT-BC treatment under high salinity stress (Table 5).
Under 2.98 dS/m EC, applying Bacillus pumilus led to a 3.44% increase in oleic acid related to the control (0NAT-BC). When 0.75NAT-BC was applied, there was a 5.59% increase, and the combination of Bacillus pumilus and 0.75NAT-BC resulted in an 11.83% increase over the control under 2.98 dS/m EC. Under SS 6.44 dS/m EC, Bacillus pumilus application led to a 4.67% increase in oleic acid content evaluated to the 0NAT-BC, while 0.75NAT-BC resulted in an 8.68% increase. The combination of Bacillus pumilus and 0.75NAT-BC had the most significant impact, with a 13.61% increase in oleic acid content under salt stress over the 0NAT-BC treatment (Table 5).
When Bacillus pumilus was added to the 2.98 dS/m EC, there was a 2.43% increase in linolic acid, and with the combination of Bacillus pumilus and 0.75NAT-BC, the increase was even more pronounced at 7.77% over the 0NAT-BC treatment. Under salt stress, Bacillus pumilus alone led to a 4.30% increase in linolic acid, and the addition of 0.75NAT-BC resulted in a substantial 10.12% increase over the 0NAT-BC treatment. The combination of Bacillus pumilus and 0.75NAT-BC under stress exhibited the highest increase in linoleic acid content at 15.77% under salt stress (Table 5).
Oil and protein contents
In the case of 2.98 dS/m EC, the mean oil content was 35.34% in 0NAT-BC treatment. When Bacillus pumilus was introduced in the 2.98 dS/m EC treatment, there was a 2.85% increase in oil content, and adding 0.75NAT-BC to 2.98 dS/m EC further boosted the oil content by 5.75% in contrast to the 0NAT-BC. Combining Bacillus pumilus and 0.75NAT-BC with 2.98 dS/m EC had the most pronounced effect, raising the oil content to 10.56% over the 0NAT-BC treatment. Under SS 6.44 dS/m EC, the oil content for 0NAT-BC was 30.65%. Introducing Bacillus pumilus in salt stress treatment led to a 4.33% increase in salt stress. When 0.75NAT-BC was combined with the SS (6.44 dS/m EC) treatment, there was a notable 7.74% increase in oil content, and the most significant improvement was observed when Bacillus pumilus and 0.75NAT-BC were used in conjunction with the salt stress treatment, resulting in a substantial 11.62% increase in oil content over the 0NAT-BC (Table 6).
The use of Bacillus pumilus treatment under 2.98 dS/m EC resulted in a 3.55% increase in protein content compared to the control (0NAT-BC), and when 0.75NAT-BC treatment was applied, there was a 7.01% increase. In 2.98 dS/m EC, the combination of Bacillus pumilus and 0.75NAT-BC showed the highest increase at 10.78%, contrasted with the 0NAT-BC treatment. In the SS 6.44 dS/m EC, the Bacillus pumilus treatment led to a 6.68% increase in protein content evaluated to the control (0NAT-BC). Application of 0.75NAT-BC resulted in a substantial 12.52% increase in protein content, and the combination of Bacillus pumilus and 0.75NAT-BC showed a remarkable 19.79% rise in protein content over the 0NAT-BC treatment in salt stress (Table 6).
Chlorophyll (a, B, and total) and carotenoid contents
The chlorophyll a content was 1.08 mg/g for the control group (0NAT-BC) in 2.98 dS/m EC. When Bacillus pumilus was applied, there was an 18.55% increase in chlorophyll a content, and the 0.75NAT-BC treatment resulted in a 37.66% increase over the 0NAT-BC treatment. The most significant increase was observed in the group treated with Bacillus pumilus and 0.75NAT-BC, showing a 59.93% increase in chlorophyll a content in 2.98 dS/m EC. Under stress (6.44 dS/m EC), the chlorophyll a content for the control group (0NAT-BC) was 0.30 mg/g. When Bacillus pumilus was applied, there was a substantial 74.32% increase, and 0.75NAT-BC treatment led to a remarkable 172.97% increase in chlorophyll a content related to the 0NAT-BC treatment. The most significant improvement was observed in the group treated with Bacillus pumilus and 0.75NAT-BC under salt stress, where chlorophyll a content increased by 229.39% (Table 7).
Under 2.98 dS/m EC, chlorophyll b levels increased over the 0NAT-BC treatment by the addition of Bacillus pumilus treatment resulted in a 10.47% increase, 0.75NAT-BC treatment led to a 20.93% increase, and the combination of Bacillus pumilus and 0.75NAT-BC treatment showed the highest increase at 33.72%. Under SS 6.44 dS/m EC, the chlorophyll b content increase over the 0NAT-BC treatment with the application of Bacillus pumilus treatment resulted in a substantial 31.25% increase, 0.75NAT-BC treatment showed a 43.75% increase, and the combined treatment of Bacillus pumilus and 0.75NAT-BC exhibited a remarkable 79.17% increase in chlorophyll b levels (Table 7).
Under 2.98 dS/m EC, using Bacillus pumilus led to a 16.62% increase in chlorophyll related to the control group (0NAT-BC). When 0.75NAT-BC was introduced, there was a substantial 33.80% increase in total chlorophyll content over the 0NAT-BC treatment, and the combination of Bacillus pumilus and 0.75NAT-BC resulted in a remarkable 53.66% rise in total chlorophyll content related to the 0NAT-BC treatment. When plants were subjected to saline stress (6.44 dS/m EC), the effect of Bacillus pumilus became even more pronounced, with a substantial 59.91% increase in total chlorophyll compared to the control group (0NAT-BC), and the 0.75NAT-BC treatment exhibited an impressive 128.63% increase. The combination of Bacillus pumilus and 0.75NAT-BC also demonstrated a 177.90% increase in total chlorophyll content under salt stress (Table 7).
Under 2.98 dS/m EC, the control group’s carotenoids content was 0.41 mg/g (0NAT-BC). When Bacillus pumilus was introduced, there was a 26.21% increase in carotenoids content, and when 0.75NAT-BC treatment was applied, resulting in a 47.09% increase in contrast to the 0NAT-BC. The most substantial improvement occurred when Bacillus pumilus and 0.75NAT-BC were combined, leading to a remarkable 77.18% increase in carotenoids content in 2.98 dS/m EC. Under salt stress (6.44 dS/m EC), the control group (0NAT-BC) had a carotenoids content of 0.13 mg/g, notably lower than 2.98 dS/m EC. When Bacillus pumilus was introduced, there was a 38.46% increase in carotenoids content, and the application of 0.75NAT-BC resulted in a more significant 72.31% increase under salt stress. The combination of Bacillus pumilus and 0.75NAT-BC showed a remarkable 151.28% increase in carotenoids content over the 0NAT-BC treatment under SS 6.44 dS/m EC (Table 7).
Antioxidant activities
POD, SOD, CAT, and APX activity
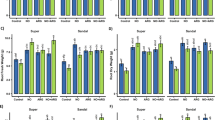
The peroxidase (POD) values for various treatments showed significant variations. Under 2.98 dS/m EC, the control (0NAT-BC) exhibited a POD value of 1.39 U/mg Protein, which served as the baseline treatment. When Bacillus pumilus was introduced, there was a decrease of 11.92% in POD activity, and the treatment with 0.75NAT-BC led to a 24.55% reduction in POD activity under 2.98 dS/m EC. The most substantial change was observed in the combination treatment of Bacillus pumilus and 0.75NAT-BC, where the POD activity dropped by 83.86% contrasted to the 0NAT-BC treatment in 2.98 dS/m EC. In the presence of higher salinity stress (6.44 dS/m EC), the control (0NAT-BC) exhibited a higher POD value of 2.12 U/mg Protein. The introduction of Bacillus pumilus decreased 8.28%, and the treatment with 0.75NAT-BC caused a 21.44% reduction in POD activity under salt stress. Furthermore, the combination treatment of Bacillus pumilus and 0.75NAT-BC reduced the POD activity by 33.35% compared to the 0NAT-BC treatment in salt stress (Fig. 1A).
Effect of treatments on peroxidase (POD) (A), superoxide dismutase (SOD) (B), catalase (CAT) (C), and ascorbate peroxidase (APX) (D) of sunflower growth under control condition (2.98 dS/m EC) and salinity stress (6.44 dS/m EC). Bars are means of 4 replicates ± SE. Difference letters on bars showed significant changes at p ≤ 0.05: Tukey test. Nitrilotriacetic acid mixed biochar (NAT-BC).
When plants were subjected to 2.98 dS/m EC, the 0NAT-BC treatment exhibited a superoxide dismutase (SOD) activity of 19.83 U/mg Protein. The addition of Bacillus pumilus resulted in a 9.91% decrease in SOD activity, and the 0.75NAT-BC treatment showed a significant 31.46% reduction in SOD activity in 2.98 dS/m EC over the 0NAT-BC treatment. In contrast to the 0NAT-BC treatment, when both Bacillus pumilus and 0.75NAT-BC were combined, there was a substantial 49.89% decrease in SOD activity. Under saline stress (6.44 dS/m EC), the 0NAT-BC treatment displayed a SOD activity of 28.56 U/mg Protein. The addition of Bacillus pumilus resulted in an 8.21% decrease in SOD activity, and the 0.75NAT-BC treatment exhibited a notable 19.21% reduction. The Bacillus pumilus + 0.75NAT-BC treatment led to a significant 34.32% decrease in SOD activity under saline stress (Fig. 1B).
Under 2.98 dS/m EC, Bacillus pumilus + 0.75NAT-BC showed a 49.89% decrease in catalase (CAT), Bacillus pumilus treatment resulted in a 9.91% drop, and 0.75NAT-BC treatment led to a 31.46% reduction over the 0NAT-BC treatment. In SS 6.44 dS/m EC, Bacillus pumilus + 0.75NAT-BC resulted in an 8.21% reduction, Bacillus pumilus treatment led to a 26.39% decrease, and 0.75NAT-BC treatment showed a 19.21% reduction over the 0NAT-BC treatment (Fig. 1C).
The 0NAT-BC treatment exhibited the mean APX activity of 1.10 U/mg protein. However, adding Bacillus pumilus resulted in a 25.69% decrease in APX activity, and 0.75NAT-BC treatment under 2.98 dS/m EC showed a substantial 57.93% reduction in APX activity parallel to the 0NAT-BC treatment. Combining Bacillus pumilus and 0.75NAT-BC led to a remarkable 115.75% decrease in APX activity under 2.98 dS/m EC. In the high salinity stress condition (SS 6.44 dS/m EC), the 0NAT-BC treatment demonstrated the mean APX activity at 1.79 U/mg protein. Bacillus pumilus treatment exhibited a modest 8.24% reduction in APX activity, and 0.75NAT-BC treatment demonstrated a more substantial 23.00% decrease in APX activity in salt stress. When Bacillus pumilus and 0.75NAT-BC treatment were applied, it showed a 40.82% reduction in APX activity in salt stress in contrast to the 0NAT-BC treatment (Fig. 1D).
MDA, H2O2, and GHS activity
The application of Bacillus pumilus under 2.98 dS/m EC led to a 43.32% decrease in MDA levels related to the control (0NAT-BC), while the combined treatment of Bacillus pumilus and 0.75NAT-BC resulted in a significant 215.08% decrease. The 0.75NAT-BC treatment exhibited the most significant reduction of 89.05% in 2.98 dS/m EC. Under SS 6.44 dS/m EC, the Bacillus pumilus treatment showed a 6.76% reduction in MDA levels evaluated to the control, and the combination of Bacillus pumilus and 0.75NAT-BC resulted in a more substantial 43.05% decrease in MDA levels, suggesting a potential synergistic effect of the two treatments under salt stress (Fig. 2A).
Effect of treatments on malondialdehyde (MDA) (A), hydrogen peroxide (H2O2) (B), and glutathione (GSH) (C) of sunflower under control condition (2.98 dS/m EC) and salinity stress (6.44 dS/m EC). Bars are means of 4 replicates ± SE. Difference letters on bars showed significant changes at p ≤ 0.05: Tukey test. Nitrilotriacetic acid mixed biochar (NAT-BC).
Under 2.98 dS/m EC, the 0NAT-BC treatment resulted in a mean H2O2 level of 1.13 nmol/g FW. When Bacillus pumilus was introduced, there was a 15.54% decrease in H2O2 levels, and the 0.75NAT-BC treatment led to a substantial 104.71% decrease in H2O2 levels in 2.98 dS/m EC over the 0NAT-BC treatment. The most dramatic effect was observed when Bacillus pumilus was combined with 0.75NAT-BC, resulting in a remarkable 292.36% decrease in 2.98 dS/m EC contrasted to the 0NAT-BC treatment. Under salt-stressed (6.44 dS/m EC), the 0NAT-BC treatment had a higher mean H2O2 level of 2.17 nmol/g FW. The introduction of Bacillus pumilus in salt stress led to a 19.45% decrease, and the 0.75NAT-BC treatment showed a notable 38.65% decrease in H2O2 levels parallel to the 0NAT-BC treatment. Combining Bacillus pumilus with 0.75NAT-BC under stress resulted in a substantial 74.15% decrease in H2O2 levels in salt stress (Fig. 2B).
The control (0NAT-BC) had a mean GHS of 0.49 U/mg protein while applying Bacillus pumilus resulted in a 35.71% decrease in GHS activity under 2.98 dS/m EC. A more significant reduction in GHS activity was observed with the 0.75NAT-BC treatment, representing a 60.39% decrease, and the combination of Bacillus pumilus and 0.75NAT-BC led to the most substantial 162.77% decrease in GHS activity over the 0NAT-BC treatment in 2.98 dS/m EC. Under stress (6.44 dS/m EC), the control (0NAT-BC) exhibited a GHS of 0.86 U/mg protein. Applying Bacillus pumilus slightly lowered the GHS activity, representing a 13.53% decrease under salt stress. The 0.75NAT-BC treatment further reduced the GHS activity, indicating a 20.56% decrease, and the most significant decrease in GHS under stress was observed with the Bacillus pumilus + 0.75NAT-BC treatment, which represented a substantial 32.71% decrease (Fig. 2C).
Lyc and scavenging
The application of Bacillus pumilus resulted in a 7.15% decrease, and in addition to 0.75NAT-BC treatment, the lycopene (Lyc) content decreased by 12.49% under 2.98 dS/m EC over the 0NAT-BC treatment. Combining Bacillus pumilus with 0.75NAT-BC led to a significant decrease of 16.93% in lycopene content in 2.98 dS/m EC. In contrast, under stressful (6.44 dS/m EC), the control (0NAT-BC) showed a higher mean lycopene content of 16.56 µg/g FW. The application of Bacillus pumilus resulted in a modest 2.97% decrease, the 0.75NAT-BC was introduced, and the lycopene content decreased by 10.26% under salt stress. Combining Bacillus pumilus with 0.75NAT-BC led to a notable decrease of 15.16% over the 0NAT-BC treatment in salt stress (Fig. 3A).
Effect of treatments on lycopene (Lyc) (A) and scavenging (B) of sunflower under control condition (2.98 dS/m EC) and salinity stress (6.44 dS/m EC). Bars are means of 4 replicates ± SE. Difference letters on bars showed significant changes at p ≤ 0.05: Tukey test. Nitrilotriacetic acid mixed biochar (NAT-BC).
Under 2.98 dS/m EC, adding Bacillus pumilus resulted in a 10.41% decrease in scavenging efficiency compared to 0NAT-BC. When 0.75NAT-BC was applied, there was a 25.30% decrease in scavenging efficiency related to 0NAT-BC. When Bacillus pumilus and 0.75NAT-BC were combined, there was a significant 48.04% decrease in scavenging efficiency as equated to using 0NAT-BC under 2.98 dS/m EC. In the case of saline soil (6.44 dS/m EC), the results showed that Bacillus pumilus led to a 6.51% decrease in scavenging efficiency over the 0NAT-BC. When 0.75NAT-BC was used, a 9.99% decrease in scavenging efficiency competed to 0NAT-BC. However, when Bacillus pumilus and 0.75NAT-BC were combined, there was a 24.09% decrease in scavenging efficiency as opposed to using 0NAT-BC under saline soil (Fig. 3B).
Convex hull and hierarchical cluster analysis
The results of the convex hull analysis reveal the treatment groupings and their corresponding percentages within the convex hull. In this analysis, treatment groups are represented by points in a multi-dimensional space, and the convex hull encapsulates these points, helping to identify the boundaries of these groups. The treatment 0NAT-BC exhibited a convex hull with a coverage of 97.60%, indicating that the data points associated with this treatment are closely grouped and form a relatively large convex hull. Similarly, Bacillus pumilus displayed a convex hull with a coverage of 2.08%, suggesting that its data points are more spread out and less tightly clustered compared to 0NAT-BC. The treatment 0.75NAT-BC had a convex hull with a coverage of 0.78%, indicating a smaller grouping of data points. Lastly, the combination treatment Bacillus pumilus + 0.75NAT-BC exhibited a convex hull with a coverage of 0.55%, indicating a relatively smaller grouping of data points (Fig. 4A).
The results of the convex hull analysis indicate the spatial distribution of data points in two-dimensional space, with PC1 and PC2 representing the coordinates. The percentage values represent the proportion of variance explained by each principal component. Under 2.98 dS/m EC, the data points exhibit a convex hull that encapsulates most of the data, suggesting a relatively compact and well-distributed set of scores. The stress values for this condition are 97.60% for PC1 and 0.81% for PC2, indicating the suitability of the convex hull representation for these data points. In contrast, under the SS (6.44 dS/m EC) soil condition, the data points form a convex hull that also encompasses the data, but the distribution appears to be slightly more spread out. The stress values for this condition are 0.46788% for PC1 and 0.76320% for PC2, suggesting a slightly higher stress level in the convex hull representation, potentially due to the greater dispersion of data points (Fig. 4B).
The results of the hierarchical cluster analysis illustrate the relationships and similarities between different variables. This analysis groups variables based on similarity, as the similarity scores indicate. Several distinct clusters emerge, such as the cluster including chlorophyll a (mg/g) and total chlorophyll (mg/g), which share a high degree of similarity with a score of 0.03764. In another cluster, steric acid (%) and palmitic acid (%) are closely related, reflecting a similarity score 0.2196. The variables “Number of leaves/plants, head diameter (cm), and achene yield (kg h-1) are grouped with a similarity score of 0.25456, suggesting common characteristics (Fig. 4C).
Pearson correlation analysis
The Pearson correlation analysis conducted on the dataset reveals significant associations and relationships between various variables. The results show that several variables are strongly positively correlated, indicating high interdependence. These groups include plant characteristics, such as plant height, stem diameter, head diameter, and others, all displaying strong positive correlations. Similarly, variables related to fatty acid composition, including oleic acid (%), linolic acid (%), palmitic acid (%), and steric acid (%), also exhibit strong positive associations. Additionally, chlorophyll-related variables, such as chlorophyll a (mg/g), chlorophyll b (mg/g), and total chlorophyll (mg/g), display positive correlations with each other, indicating their interrelatedness. On the other hand, the enzyme and stress-related variables exhibit strong negative correlations with most other variables, implying that they are inversely related (Fig. 5).
Discussion
High salinity in the soil, a major environmental stressor, continues to delay crop growth and yield, posing a significant threat to global food security49. Salt stress hampers plant physiological processes and affects various parameters, including plant height, stem diameter, head diameter, gas exchange attributes, fatty acid composition, oil and protein contents, photosynthetic pigments, and antioxidant activities50. Excess sodium (Na⁺) and chloride (Cl⁻) ions disrupt cellular homeostasis, inhibit enzyme activities, and interfere with the uptake of essential nutrients like potassium (K⁺) and calcium (Ca²⁺), resulting in nutrient deficiencies50,51,52,53. Osmotic stress induced by soil salinity reduces cell expansion and stomatal conductance, limiting CO₂ assimilation and decreasing biomass production54.
To overcome this issue B. pumilus has been well-documented to alleviate salinity stress in variable crops. These rhizobacteria can improve antioxidant enzyme activity and nutrient uptake under salinity stress55,56,57. Improvement in root growth by B. pumilus enhance the rhizosphere area which helps to maintain osmotic balance by metabolite synthesis58. These root extensions and metabolites secreted by B. pumilus regulate water and ion uptake within plant tissues9,59. Exopolysaccharide (EPS) synthesis by B. pumilus rhizobacteria can bind to soil cations, i.e., sodium ions, thus reducing their plant uptake and mitigating salt stress60,61. Furthermore, B. pumilus rhizobacteria also enhances the synthesis of antioxidants in plants. These antioxidants help to mitigate oxidative damage by minimizing ROS62,63. Similar results were also noted in a current study where inoculation of B. pumilus was imperative in improving chlorophyll contents, growth attributes and antioxidants in sunflower under salinity stress conditions.
In addition to the above, biochar application improves the soil structure and enhances the cation exchange capacity64,65. It enhanced the soil nutrients and water retention, thus improving their bioavailability in the plants. Better availability of nutrients, i.e., potassium and water, decreases the osmotic stress induced by the salinity problem66,67. Applying biochar in the soil also improves the soil organic matter, facilitating microbial proliferation in plants68,69. It also stimulates the antioxidant defense mechanism, which acts as a natural defense when plants are cultivated in salt-affected soils. Literature shows that biochar is a promising amendment in mitigating soil salinity due to its high surface area and cation exchange capacity. These attributes allow it to adsorb and immobilize Na+ ions, reducing their availability in soil solution70,71. In our experiment, we also experienced similar results where applying 0.75NAT-BC significantly improved the plant height, stem diameter, chlorophyll contents and antioxidants. Furthermore, improvement in soil and protein contents also validated the effectiveness of sole and combined application of 0.75NAT-BC against control in sunflower plants cultivated in salinity stress.
Conclusion
It is concluded that treatment Bacillus pumilus + 0.75NAT-BC treatment can potentially improve sunflower growth under salt stress by improving the total chlorophyll contents. Bacillus pumilus enhances plant salt tolerance due to metabolite production, which assists in antioxidants’ biosynthesis. The treatment Bacillus pumilus + 0.75NAT-BC also has the potential for regulating stomatal conductance, oil and protein contents in sunflower under salinity stress. Growers are recommended to apply Bacillus pumilus + 0.75NAT-BC as an amendment to improve sunflower growth under salinity stress. More investigations are recommended at the field level for the declaration of Bacillus pumilus + 0.75NAT-BC as the best treatment for mitigating salt stress in sunflowers and other crops.
Data availability
All data generated or analysed during this study are included in this published article.
References
Rafay, M. & Usman, M. Soil salinity hinders plant growth and development and its remediation-A review. J. Agric. Res. 61, 189–200 (2023).
Danish, S. et al. Effect of Methyl jasmonate and GA3 on Canola (Brassica Napus L.) growth, antioxidants activity, and nutrient concentration cultivated in salt-affected soils. BMC Plant. Biol. 24, 363 (2024).
Danish, S. et al. The role of Strigolactone in alleviating salinity stress in Chili pepper. BMC Plant. Biol. 24, 209 (2024).
Gupta, A. et al. Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int. J. Mol. Sci. 23, 3741 (2022).
Inayat, H. et al. Impact of Cobalt and proline foliar application for alleviation of salinity stress in radish. BMC Plant. Biol. 24, 287 (2024).
Sharma, K., Devi, P., Kumar, P., Dey, A. & Dwivedi, P. Hazardous Phytotoxic Nature of Reactive Oxygen Species in Agriculture. in Reactive Oxygen Species 135–146Springer, (2023). https://doi.org/10.1007/978-981-19-9794-5_8
Alotaibi, N. M. et al. Zn-quantum Dot Biochar regulates antioxidants and nutrient uptake to improve rapeseed growth and yield in drought stress. Plant. Stress. 11, 100286 (2024).
Tiwari, S., Tiwari, S., Singh, M., Singh, A. & Prasad, S. M. Generation mechanisms of reactive oxygen species in the plant cell: an overview. React. Oxyg Species Plants Boon Or. Bane-Revisiting Role ROS 1–22 (2017).
Ilangumaran, G. & Smith, D. L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant. Sci. 8, 1768 (2017).
Ha-Tran, D. M., Nguyen, T. T. M., Hung, S. H., Huang, E. & Huang, C. C. Roles of plant Growth-Promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 22, 3154 (2021).
Hmaeid, N., Metoui, O., Wali, M., Zorrig, W. & Abdelly, C. Comparative effects of rhizobacteria in promoting growth of hordeum maritimum L. plants under salt stress. J. Plant. Biol. Res. 3, 37–50 (2014).
Céccoli, G. et al. Salinity tolerance determination in four sunflower (Helianthus annuus L.) hybrids using yield parameters and principal components analysis model. Ann. Agric. Sci. 67, 211–219 (2022).
Kaur, R. & Ghoshal, G. Sunflower protein isolates-composition, extraction and functional properties. Adv. Colloid Interface Sci. 306, 102725 (2022).
Yu, T. et al. Benefits of crop rotation on climate resilience and its prospects in China. Agronomy 12, 436 (2022).
Santos, E. et al. Are nanomaterials leading to more efficient agriculture? Outputs from 2009 to 2022 research metadata analysis. Environ. Sci. Nano. 9, 3711–3724 (2022).
Khalifani, S., Darvishzadeh, R., Azad, N. & Rahmani, R. S. Prediction of sunflower grain yield under normal and salinity stress by RBF, MLP and, CNN models. Ind. Crops Prod. 189, 115762 (2022).
Francois, L. E. Salinity effects on four sunflower hybrids. Agron. J. 88, 215–219 (1996).
Taher, M., Beyaz, R., Javani, M., Gürsoy, M. & Yildiz, M. Morphological and biochemical changes in response to salinity in sunflower (Helianthus annus L.) cultivars. Ital. J. Agron. 13, 1096 (2018).
Ashraf, M., Shahzad, S. M., Akhtar, N., Imtiaz, M. & Ali, A. Salinization/sodification of soil and physiological dynamics of sunflower irrigated with saline–sodic water amending by potassium and farm yard manure. J. Water Reuse Desalin. 7, 476–487 (2017).
Mclean, E. O. Soil pH and Lime Requirement. in 199–224 (2015). https://doi.org/10.2134/agronmonogr9.2.2ed.c12
Nelson, D. W., Sommers, L. E., Total & Carbon Organic Carbon, and Organic Matter. in Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties (ed. Page, A. L.) vol. sssabookse 539–579American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, WI, USA, (1982).
Kuo, S. & Phosphorus in In Methods of Soil Analysis Part 3: Chemical Methods. 869–919 (eds Sparks, D. L.) (John Wiley & Sons, Ltd, SSSA, 2018). https://doi.org/10.2136/sssabookser5.3.c32Madison, Wisconsin.
Pratt, P. F. & Potassium in In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties. 1022–1030 (eds Norman, A. G.) (John Wiley & Sons, Ltd, 2016). https://doi.org/10.2134/agronmonogr9.2.c20
Hall, T., Biosciences, I. & Carlsbad, C. BioEdit: an important software for molecular biology. GERF Bull. Biosci. 2, 60–61 (2011).
The National Center. for Biotechnology Information-blastn program.
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Holt, J. G., Krieg, N. R., Sneath, P. H. A., Staley, J. T. & Williams, S. T. Bergey’s Manual of Determinative Bacteriology, nineth ed. Williams Wilkins Balt. MD (1994).
Skerman, V. B. A Guide To the Identification of the Genera Bacteria (The Williams and Wilkins, 1967).
Steel, K. J. The oxidase reaction as a taxonomic tool. Microbiology 25, 297–306 (1961).
MacFaddin, J. F. Biochemical tests for the identification of medical bacteria, Williams and Wilkins, Baltimore. Escherichia coli 25922, (1980).
Ashraf, M., Hasnain, S., Berge, O. & Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils. 40, 157–162 (2004).
Holding, A. J. & Collee, J. G. Chapter I routine biochemical tests. in Methods in Microbiology (eds And, J. R. N. & Ribbons, D. W.) vol. 6 1–32 (Elsevier, London and New York, (1971).
Glick, B. R., Karaturovíc, D. M. & Newell, P. C. A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can. J. Microbiol. 41, 533–536 (1995).
Sarwar, M., Arshad, M., Martens, D. A. & Frankenberger, W. T. Tryptophan-dependent biosynthesis of auxins in soil. Plant. Soil. 147, 207–215 (1992).
Kumar, P., Dubey, R. C. & Maheshwari, D. K. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 167, 493–499 (2012).
Schwyn, B. & Neilands, J. B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160, 47–56 (1987).
Dunne, C., Crowley, J. J., Moënne-Loccoz, Y., Dowling, D. N. & O’Gara, F. Biological control of pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiology 143, 3921–3931 (1997).
Bakker, A. W. & Schippers, B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant growth-stimulation. Soil. Biol. Biochem. 19, 451–457 (1987).
Rhoades, J. D. & Salinity Electrical conductivity and total dissolved solids. Methods Soil. Anal. Part. 3 Chem. Methods. 417–435. https://doi.org/10.2136/SSSABOOKSER5.3.C14 (2018).
Mills, H. A. & Jones, J. B. J. Plant Analysis Handbook II: A Practical Sampling, Preparation, Analysis, and Interpretation Guide. Plant Analysis Handbook. A Practical Sampling, Preparation, Analysis, and Interpretation Guide (Micro-Macro Publishing, Inc., 1991).
Ahmad, M. et al. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 99, 19–33 (2014).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant. Physiol. 24, 1–15 (1949).
Nazar, R., Khan, M. I. R., Iqbal, N., Masood, A. & Khan, N. A. Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulfur in mustard. Physiol. Plant. 152, 331–344 (2014).
Dhindsa, R. S., Plumb-Dhindsa, P. L. & Reid, D. M. Leaf senescence and lipid peroxidation: effects of some phytohormones, and scavengers of free radicals and singlet oxygen. Physiol. Plant. 56, 453–457 (1982).
Hori, M. et al. Changes in the hepatic glutathione peroxidase redox system produced by coplanar polychlorinated biphenyls in Ah-responsive and-less-responsive strains of mice: mechanism and implications for toxicity. Environ. Toxicol. Pharmacol. 3, 267–275 (1997).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by Ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22, 867–880 (1981).
CHEN, J. Y., MIAO, Y., SATO, S. & ZHANG, H. Near infrared spectroscopy for determination of the protein composition of rice flour. Food Sci. Technol. Res. 14, 132–138 (2008).
(OriginLab Corporation. OriginPro & OriginLab Northampton, MA, USA., (2021).
Al-Omran, A. M., Dhaouadi, L. & Besser, H. Irrigation and salinity management of date palm in arid regions. in Date Palm 241–265 (CABI GB, (2023).
Ma, Y. et al. γ-Aminobutyric acid (GABA) and Ectoine (ECT) impacts with and without AMF on antioxidants, gas exchange attributes and nutrients of cotton cultivated in salt affected soil. BMC Plant. Biol. 23, 476 (2023).
Zafar-ul-Hye, M. et al. Multi-strain inoculation with PGPR producing ACC deaminase is more effective than single-strain inoculation to improve wheat (Triticum aestivum) growth and yield. Phyton-International J. Exp. Bot. 89, 405–413 (2020).
Zafar-Ul-Hye, M. et al. Bacillus amyloliquefaciens and alcaligenes faecalis with biogas slurry improved maize growth and yield in saline-sodic field. Pakistan J. Bot. 52, 1839–1847 (2020).
Younis, U., Danish, S., Datta, R., Al Obaid, S. & Ansari, M. J. Synergistic effects of Boron and saponin in mitigating salinity stress to enhance sweet potato growth. Sci. Rep. 14, 12988 (2024).
Zhang, Y., Kaiser, E., Li, T. & Marcelis, L. F. M. NaCl affects photosynthetic and stomatal dynamics by osmotic effects and reduces photosynthetic capacity by ionic effects in tomato. J. Exp. Bot. 73, 3637–3650 (2022).
Kumar, A., Singh, S., Mukherjee, A., Rastogi, R. P. & Verma, J. P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 242, 126616 (2021).
Mahmoud, O. M. B., Hidri, R., Abdelly, C. & Debez, A. Bacillus pumilus isolated from Sabkha rhizosphere ameliorates the behavior of the facultative halophyte hordeum marinum when salt-challenged by improving nutrient uptake and soil health-related traits. Plant. Stress. 11, 100383 (2024).
Liu, Y. et al. Beneficial microorganisms: regulating growth and defense for plant welfare. Plant. Biotechnol. J. https://doi.org/10.1111/pbi.14554 (2024).
Han, Y. et al. Improving aerobic digestion of food waste by adding a personalized microbial inoculum. Curr. Microbiol. 81, 277 (2024).
Ozfidan-Konakci, C. et al. Halotolerant plant growth-promoting bacteria, Bacillus pumilus, modulates water status, chlorophyll fluorescence kinetics and antioxidant balance in salt and/or arsenic-exposed wheat. Environ. Res. 231, 116089 (2023).
Yasmin, H., Nosheen, A., Naz, R., Keyani, R. & Anjum, S. Regulatory role of rhizobacteria to induce drought and salt stress tolerance in plants. in Field Crops: Sustainable Management by PGPR (eds Maheshwari, D. K. & Dheeman, S.) 279–335 (Springer International Publishing, Cham, doi:https://doi.org/10.1007/978-3-030-30926-8_11. (2019).
Chowdhury, S. R., Basak, R. K., Sen, R. & Adhikari, B. Characterization and emulsifying property of a carbohydrate polymer produced by Bacillus pumilus UW-02 isolated from waste water irrigated agricultural soil. Int. J. Biol. Macromol. 48, 705–712 (2011).
Saberi Riseh, R., Ebrahimi-Zarandi, M., Tamanadar, E., Moradi Pour, M. & Thakur, V. K. Salinity stress: toward sustainable plant strategies and using plant Growth-Promoting rhizobacteria encapsulation for reducing it. Sustainability 13, 12758 (2021).
Upadhyay, S. K., Saxena, A. K., Singh, J. S. & Singh, D. P. Impact of native ST-PGPR (Bacillus pumilus; EU927414) on PGP traits, antioxidants activities, wheat plant growth and yield under salinity. Clim. Chang. Environ. Sustain. 7, 157 (2019).
Medyńska-Juraszek, A., Álvarez, M. L., Białowiec, A. & Jerzykiewicz, M. Characterization and sodium cations sorption capacity of chemically modified biochars produced from agricultural and forestry wastes. Mater. (Basel) 14, (2021).
Pan, X. R. et al. The influence of carbon dioxide on fermentation products, microbial community, and functional gene in food waste fermentation with uncontrol pH. Environ. Res. 267, 120645 (2025).
Hareem, M., Danish, S., Obaid, S., Al, Ansari, M. J. & Datta, R. Mitigation of drought stress in Chili plants (Capsicum annuum L.) using Mango fruit waste Biochar, fulvic acid and Cobalt. Sci. Rep. 14, 14270 (2024).
Sultan, H., Ahmed, N., Mubashir, M. & Danish, S. Chemical production of acidified activated carbon and its influences on soil fertility comparative to thermo-pyrolyzed Biochar. Sci. Rep. 10, 595 (2020).
Zafar-Ul-Hye, M., Danish, S., Abbas, M., Ahmad, M. & Munir, T. M. ACC deaminase producing PGPR Bacillus amyloliquefaciens and Agrobacterium fabrum along with Biochar improve wheat productivity under drought stress. Agronomy 9, 343 (2019).
Zafar-ul-Hye, M. et al. Compost mixed fruits and vegetable waste Biochar with ACC deaminase rhizobacteria can minimize lead stress in mint plants. Sci. Rep. 11, 6606 (2021).
Yang, K. et al. Structure–Activity mechanism of sodium ion adsorption and release behaviors in Biochar. Agriculture 14, 1246 (2024).
Awan, S. et al. Biochars reduce irrigation water sodium adsorption ratio. Biochar 3, 77–87 (2021).
Acknowledgements
Anhui Provincial Department of Education self-science key project—Study on aphid resistance and aphid control of Chuju under the concept of green control (2024AH051442). Anhui Province Quality Improvement Project - Construction of school-enterprise “Double qualified” teacher training base (Project number: 2022TZPY037-4);②Chuzhou Polytechnic 2022 University-level Scientific Research Project—Research on Green Control Technology of Spodoptera frugiperda (J.E.Smith) (ZKZ-2022-05);③Anhui Provincial Department of Education self-science key project— Research on intelligent system of Chuju Pest Control based on GIS (2023AH053092).
Funding
Anhui Provincial Department of Education self-science key project—Study on aphid resistance and aphid control of Chuju under the concept of green control (2024AH051442). Anhui Province Quality Improvement Project - Construction of school-enterprise “Double qualified” teacher training base (Project number: 2022TZPY037-4);②Chuzhou Polytechnic 2022 University-level Scientific Research Project—Research on Green Control Technology of Spodoptera frugiperda (J.E.Smith) (ZKZ-2022-05); ③Anhui Provincial Department of Education self-science key project— Research on intelligent system of Chuju Pest Control based on GIS (2023AH053092).
Author information
Authors and Affiliations
Contributions
Q.T.; S.T.; S.H.; contributed to the conceptualization and design of the study, as well as data collection, analysis, and interpretation. Q.T.; S.T.; S.H.; contributed to the statistical analysis; Q.T.; S.T.; S.H.; interpretation of the data. All authors have reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable.Study protocol must comply with relevant institutional, national, and international guidelines and legislation. Our experiment follows the with relevant institutional, national, and international guidelines and legislation
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tu, Q., Tang, S. & Huang, S. Mitigation of salinity stress via improving growth, chlorophyll contents and antioxidants defense in sunflower with Bacillus pumilis and biochar. Sci Rep 15, 9641 (2025). https://doi.org/10.1038/s41598-025-93959-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93959-6