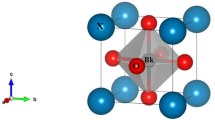
Abstract
High-pressure synthesized Pb-based perovskites exhibit diverse functional properties. PbCrO3, for instance, displays distinctive diffuse scattering and valence skipping, forming Pb2+ and Pb4+ ions. However, the spatial distribution of Pb ions in the crystal remains largely unexplored. Here, we elucidate the role of Pb ions with different valences through Sr substitution, using high-resolution transmission electron microscopy combined with elemental mapping. This approach allows us to accurately examine the distribution of Sr and Pb ions in the same atomic columns. The results reveal that in Pb0.8Sr0.2CrO3, Sr ions occupy a squared lattice, while Pb ions exhibit positional distortions. Simulations based on the experimentally determined Pb distribution reproduce the diffuse scattering observed in PbCrO3. These findings suggest that the lone pair electrons of s orbitals are responsible for the local lattice distortion. Our study provides atomistic insights into the local structures of materials exhibiting valence skipping.
Similar content being viewed by others
Introduction
Lead is a widely used metallic element known for its high workability and relatively simple refining process1. It remains essential in various applications, including crystal glass, pigments, soundproofing materials, and electronic components. Despite its toxicity and the urgent need for safer alternatives2, Pb’s high functional value makes replacement challenging, particularly in critical applications such as storage batteries and X-ray shielding. Additionally, perovskite Pb(Zr, Ti)O3 is a key Pb-based functional material3,4,5,6,7 with exceptional piezoelectric properties, for which no viable substitutes currently exist. Notably, it is exempt from the RoHS Directive (EU directive on the restriction of hazardous substances in electronic and electrical equipment). Understanding the mechanisms behind the unique properties of Pb compounds is crucial for developing effective alternatives and advancing toward a Pb-free society.
Pb-containing perovskite-type oxides exhibit various functional properties beyond ferroelectricity and piezoelectricity, including applications in solar cells, catalysis, and metal–insulator transitions8,9. The physical properties of perovskite oxides are often primarily attributed to the B-site ion in the ABO3 structure. However, when Pb occupies the A-site, its characteristics can play a crucial role in determining the crystal structure and overall properties. As a heavy element, Pb possesses 6s orbital electrons, which contribute to the unique physical properties of Pb-based perovskites. The electron configuration of Pb is [Xe]4f145d106s26p2, while Pb2+ is [Xe]4f145d106s2, and Pb4+ is [Xe]4f145d106s0. Hereafter, the electron configuration of Pb2+ is denoted as 6s2 and that of Pb4+ as 6s0. In Pb2+-containing crystals, the lone-pair electrons of 6s2 are believed to induce structural distortions that break inversion symmetry. Experimental studies have confirmed anisotropic covalent bonding between Pb and O ions in PbTiO310. Although some PbTiO3 derivatives can be synthesized at ambient pressure11, many perovskite oxides with distinctive properties, composed of Pb and 3d transition metals, have recently been reported using high-pressure synthesis methods12,13,14,15,16. In certain materials, Pb exhibits valence flexibility owing to the presence or absence of electrons in the 6s orbital, a phenomenon known as valence skipping17,18. Specifically, Pb2+ (6s2) and Pb4+ (6s0) states are stabilized, whereas the intermediate Pb3+ (6s1) configuration is inherently unstable.
Perovskite PbCrO3 is an interesting material with a unique structure and physical properties attributed to valence skipping. Recently, a “charge glass” state of Pb2+0.5Pb4+0.5Cr3+O3 was reported in PbCrO319. The study also revealed that under pressure, charge transfer occurs between Pb4+ and Cr3+, leading to the formation of Pb2+Cr4+O3. This charge redistribution results in a considerable change in the Cr–O bonding distance, causing a volume shrinkage of up to 10%, which is promising for developing giant negative thermal expansion materials based on PbCrO3. Additionally, its local structure exhibits distinctive features because high-angle annular dark-field scanning transmission electron microscopy (HAADF–STEM) images have shown an off-center displacement of Pb ions19,20. The observations revealed regions where Pb and Cr ions retained an undistorted cubic lattice, indicating that not all Pb ions were displaced20. This suggests that the presence or absence of off-centering may be influenced by the valence states of Pb2+ and Pb4+ in the charge glass state. R. Yu et al. constructed a structural model of diffuse scattering caused by Pb displacements, proposing a threefold structure based on pair-distribution function analysis19. However, discrepancies remain between experimental and simulation data because the diffuse scattering is not strictly confined to the threefold position but is instead located between the threefold and fourfold positions20. These unusual phenomena may stem from the valence flexibility of Pb ions, i.e., valence skipping. Furthermore, it is unclear whether Pb2+ and/or Pb4+ are responsible for off-centering. Therefore, further investigation is needed to clarify the origin of the characteristic diffuse scattering observed in PbCrO3.
In this study, we investigate Sr-substituted PbCrO3 to gain insight into diffuse scattering. When the solid solution Pb1–xSrxCrO3 is formed at x = 0.2, the coexistence of a Pb2+0.5Pb4+0.5Cr3+O3-like phase and a Pb2+Cr4+O3-like phase has been reported19. To maintain charge balance, the valence state of the former phase is considered to be Pb2+0.3Sr2+0.2Pb4+0.5Cr3+O3. Because Sr2+ ions substitute at Pb sites, they are expected to reduce the number of Pb2+ ions. Moreover, the role of Pb4+ in PbCrO3 can be inferred through Sr2+ substitution because Sr2+ lacks lone-pair electrons in its s orbitals. An additional advantage of Sr substitution is that it allows atomic displacements of both Pb and Sr ions to be observed in the same atomic columns using transmission electron microscopy. We investigated the local structure of Pb0.8Sr0.2CrO3, synthesized under high pressure, to clarify the origin of diffuse scattering. Using high-resolution scanning transmission electron microscopy (STEM) and atomic-resolution energy-dispersive X-ray spectroscopy (EDS), we analyzed structural details, including Pb off-centering. Our findings reveal that Pb off-centering is strongly related to the inactive lone-pair electrons of Pb2+. Furthermore, this study proposes a method for estimating the distribution of Pb2+ and Pb4+ ions in the charge glass state through Sr2+ substitution using atomic-resolution EDS mapping.
Results and discussion
Figure 1 shows the observation results of Pb0.8Sr0.2CrO3 along the [00\(\overline{1 }\)] axis. Figure 1a shows the electron diffraction pattern, which exhibits characteristic diffuse scattering (e.g., yellow arrow in the figure), suggesting that the structural features of PbCrO3 (Fig. 1b) are preserved in the matrix phase. The low-magnification HAADF–STEM image in Fig. 1c reveals straight contrast in the plane, indicating a mixed crystalline and amorphous microstructure. Previous studies on pure PbCrO3 have shown a combination of crystalline and amorphous regions in a single crystal grain20. This feature also remains consistent in Sr-substituted PbCrO3. Figure 1d shows the intensity profiles at the positions indicated by the red and blue arrows in Fig. 1a, b. When comparing PbCrO3 with Pb0.8Sr0.2CrO3, the diffuse scattering intensity in Pb0.8Sr0.2CrO3 is reduced at the locations marked by the black arrows. This decrease in intensity suggests that the reduction is linked to a decrease in the number of Pb2+ ions with inactive lone pair electrons resulting from Sr2+ substitution: Pb2+ ions may be responsible for the Pb off-center behavior.
Observation of diffuse scattering in Pb0.8Sr0.2CrO3. (a) Selected-area electron diffraction pattern of Pb0.8Sr0.2CrO3. (b) Selected-area electron diffraction pattern of PbCrO3 for comparison. (c) Low-magnification HAADF–STEM image of the (001) plane of Pb0.8Sr0.2CrO3. (d) Intensity profile of the diffuse scattering at the arrow positions in panels (a) and (b).
Atomic-resolution EDS observations were performed on Pb0.8Sr0.2CrO3, with the mapping results for each element presented in Fig. 2. Figure 2a shows a HAADF image where the bright spots correspond to the Pb positions at the A site. Figure 2b, c confirms that Cr occupies the B site and Pb the A site, as indicated by the crystal structure model (inset in Fig. 2a). When comparing Fig. 2c, d, it is evident that Sr is incorporated into the A site. It is important to note that the high and low intensities correspond to the number of atoms present in each column: columns with high Pb intensity exhibit low Sr intensity. These elemental maps reveal that Sr and Pb occupy different positions in the same A site, even though they were acquired simultaneously.
The dotted grid lines in the figure indicate that Sr is typically located at the intersection points. In contrast, the bright spots in the Pb map appear distorted or slightly displaced from the grid. Sr2+ (5s0) ions are found to occupy the lattice points, suggesting that Pb4+ (6s0) ions remain undisturbed owing to their similar electronic configurations, which lack lone pair electrons. Because Pb ions can exist in either divalent or tetravalent states and Pb4+ ions are assumed to be at the lattice points, the Pb ions slightly off-center from these lattice points are likely Pb2+ ions. Pb ions with a 6s2 configuration, possessing lone pair electrons, would distort the coordination environment in the crystal, causing off-centering similar to what is observed in ferroelectric perovskites.
To better understand the origin of the diffuse scattering, we simulated a diffraction pattern based on the positions of the Pb atoms. Figure 3a shows the diffuse scattering simulation using a model structure derived from the coordinates extracted from the Pb L map. The simulation was performed with a small number of 42 Pb atoms (2.5 nm2 range). Nonetheless, the simulation results qualitatively reproduce the characteristics of the diffuse scattering, which is consistent with the fast Fourier transform (FFT) pattern of a high-resolution TEM image shown in Fig. 3b. This confirms that the displacement of Pb atoms is responsible for this characteristic diffuse scattering. Notably, the simulated pattern shows intensity maxima at the threefold positions, while the experimental FFT pattern displays diffuse maxima. The charge glass state may blur the peaks of the diffuse scattering. The local off-centering of Pb2+ ions is attributed to the lone-pair effect of the 6s2 electrons, resulting from s–p orbital hybridization, as observed in other materials10,21,22,23. This hybridization interaction can be influenced by coordination. As a result, the charge glass state may locally change the amplitude of off-centering (i.e., the distance from the centrosymmetric position for each ion), as shown in Fig. 2c. This variation in off-centering amplitude could cause the threefold peak to disperse, as seen in the experimental diffraction pattern. This accounts for the earlier observation that the diffuse scattering occurs between the threefold and fourfold positions20. As a direction for future work, spectroscopic techniques such as extended X-ray absorption fine structure (EXAFS) could provide deeper insights into the local structure surrounding individual ions by selecting Pb, Cr, and Sr X-ray absorption edges. EXAFS is capable of detecting atomic displacements in the unit cell, offering information that could validate the microscopy images presented in this study.
We show that Pb ions undergo local displacement throughout the entire region of a grain. Figure 4 shows high-resolution EDS maps of the (001) planes of Pb0.8Sr0.2CrO3 from a grain different from that in Fig. 2. These maps are obtained using Pb L and Sr L absorption peaks. Square grid lines are included to aid visual interpretation. The green circles in Fig. 4a, c highlight regions where Pb ions are displaced from their lattice positions. These circles clearly indicate that the Pb ions are off the lattice. Thus, the results from Fig. 2 are confirmed in other areas. It is important to note that the grains shown in Figs. 2 and 4 likely exhibit the (Pb2+, Pb4+)Cr3+O3 phase because this phase induces local displacements of Pb ions, while the other phase, Pb2+Cr4+O3, is an ordered state without distortion. X-ray diffraction studies19 indicate that these two phases are mixed in the synthesized specimen. This observation confirmed that the two phases existed in different grains, with the grains of (Pb2+, Pb4+)Cr3+O3 exhibiting atomic displacement throughout the entire grain. Additionally, Fig. 4 shows a substantial displacement along the <100> direction, which corresponds to the high diffuse scattering intensity observed in this direction. Relatively weaker displacements are seen along the oblique < 110> direction, consistent with the finding that the intensity distribution of the diffuse scattering is nearly spherical20. These results further support the conclusion that this distortion pattern is the origin of the characteristic diffuse scattering in PbCrO3.
Observation of atomic-scale configuration in other regions. (a, b) Pb and Sr ion maps in the same field of view. (c, d) Observation of a different area in the same grain. These results were obtained from a grain different from that in Fig. 2. The energies used are (a, c) Pb L and (b, d) Sr L absorption peaks.
Summary
STEM analysis and atomic-resolution EDS mapping were used to examine the microstructural features and identify the origin of the observed diffuse scattering in the charge glass PbCrO3 upon partial substitution of Sr ions. The Pb0.8Sr0.2CrO3 specimen exhibits a phase coexistence of crystalline and amorphous regions, along with diffuse scattering around the fundamental reflections in the electron diffraction pattern, similar to that of PbCrO3. This indicates that Sr substitution does not disrupt the intrinsic composite structure and charge glass state of PbCrO3. The distribution of Pb2+ and Pb4+ can be inferred from the atomic-resolution EDS maps, which reveal Sr2+ positions at on-centered sites. Because Pb4+ lacks lone pair electrons, similar to Sr2+, Pb4+ ions are expected to occupy on-centered positions in PbCrO3. Consequently, Pb2+ ions, which possess lone pair electrons, are off-centered, contributing to the characteristic diffuse scattering. These behaviors resemble those of ferroelectric materials, such as relaxors, which exhibit small local distortions owing to the inactive lone pair electrons of Pb2+ ions24,25,26,27. We believe these atomistic insights could be valuable for designing Pb-free functional materials with high performance.
As a remark, elements such as Tl, Pb, Bi, Sn, and Sb cause valence skipping17, allowing them the flexibility to adopt either the s2 or s0 electron configuration in their 5s and 6s orbitals. Off-centering is frequently observed in materials containing these elements. Studies based on density functional calculations indicate that lone pair electrons contribute to the distortion28. However, establishing a structure, particularly for a randomly displaced configuration, has proven difficult because X-ray diffraction only provides information on average structures. The observation methods presented in this study will be useful for investigating local structures in materials exhibiting valence skipping.
Methods
High-pressure synthesis is an effective method for stabilizing the high-density structure of ABO3 (where A and B represent cations). The Pb0.8Sr0.2CrO3 polycrystalline specimen was synthesized following the same procedure as PbCrO319,20, using a cubic-anvil high-pressure synthesis technique. A powder mixture is enclosed in a platinum capsule, placed in a pyrophyllite cell, then pressurized and heated using a graphite heater.
Observations were performed using a JEM-ARM200F with aberration correctors (JEOL Co. Ltd.). The instrument is equipped with two 100 mm2 silicon-drift detectors, enabling atomic-resolution EDS observations29,30. The accelerating voltage was set to 200 kV, and all measurements were performed at room temperature. Because elemental maps were filtered to reduce noise, discussing absolute quantities based on intensity was challenging. Local structural analysis using STEM is particularly useful for specimens prepared by high-pressure synthesis owing to the ability to observe a small amount of sample and to distinguish grains of target materials from those of impurities.
For TEM thin foils, the powder specimen is embedded in epoxy resin, placed between Si wafers, and cut into approximately 3 mm2 pieces. After mechanical polishing to a thickness of approximately 10 μm, it was attached to a molybdenum single-hole mesh. The mesh is further processed by Ar-ion milling while rotating until a hole is formed. The cross section at the edge of the perforated sample has a wedge shape, and the area near the hole represents the thin film region that can be observed by TEM. This milling method offers the advantage of preserving the original structure without the damage typically caused by thin-film processing with focused ion beams. Additionally, the Ar-ion milling method allows for a 100 µm field-of-view, making it easier to search grains with target crystal orientations.
An electron diffraction pattern was simulated using xHREM (HREM Research Inc.). A multislice method based on a supercell was used to calculate the diffuse scattering intensity. The supercell for the simulation was constructed from EDS elemental maps. Ionic positions were determined from Sr and Pb elemental maps by automatically measuring the center position of each ion. Sr ion sites were assumed to be at the origin without distortion, and the displacement of Pb sites was calculated as the distance from the origin. In total, 42 Pb sites were extracted from the EDS maps.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Acharya, S. Lead between the lines. Nat. Chem. 5, 894 (2013).
Tsuchiya, K. & Harashima, S. Lead exposure and the derivation of maximum allowable concentrations and threshold limit values. Occup. Environ. Med. 22, 181–186 (1965).
Shirane, G., Sawaguchi, E. & Takagi, Y. Dielectric properties of lead zirconate. Phys. Rev. 84, 476 (1951).
Guo, R. et al. Origin of the high piezoelectric response in PbZr1−xTixO3. Phys. Rev. Lett. 84, 5423 (2000).
Noheda, B. et al. A monoclinic ferroelectric phase in the Pb(Zr1−xTix)O3 solid solution. Appl. Phys. Lett. 74, 2059–2061 (1999).
Zhang, N. et al. The missing boundary in the phase diagram of PbZr1−xTixO3. Nat. Commun. 5, 1–9 (2014).
Woodward, D. I., Knudsen, J. & Reaney, I. M. Review of crystal and domain structures in the PbZrxTi1−xO3 solid solution. Phys. Rev. B 72, 104110 (2005).
Peña, M. A. & Fierro, J. L. G. Chemical structures and performance of perovskite oxides. Chem. Rev. 101, 1981–2018 (2001).
Shai, X. et al. Efficient planar perovskite solar cells using halide Sr-substituted Pb perovskite. Nano Energy 36, 213–222 (2017).
Kuroiwa, Y. et al. Evidence for Pb-O covalency in tetragonal PbTiO3. Phys. Rev. Lett. 87, 217601 (2001).
Blum, J. B. & Gurkovich, S. R. Sol-gel-derived PbTiO3. J. Mater. Sci. 20, 4479–4483 (1985).
Belik, A. A., Azuma, M., Saito, T., Shimakawa, Y. & Takano, M. Crystallographic features and tetragonal phase stability of PbVO3, a new member of PbTiO3 family. Chem. Mater. 17, 269–273 (2005).
Arevalo-Lopez, A. M. & Alario-Franco, M. A. Structural Percolation in the PbM1−xMx′O3 (M, M′= Ti, Cr, and V) Perovskites. Inorg. Chem. 50, 7136–7141 (2011).
Oka, K. et al. Pressure-induced transformation of 6H hexagonal to 3C perovskite structure in PbMnO3. Inorg. Chem. 48, 2285–2288 (2009).
Inaguma, Y. et al. Synthesis, structural transformation, thermal stability, valence state, and magnetic and electronic properties of PbNiO3 with perovskite-and LiNbO3-type structures. J. Am. Chem. Soc. 133, 16920–16929 (2011).
Arévalo-López, Á. M. & Alario-Franco, M. Á. On the structure and microstructure of “PbCrO3”. J. Solid State Chem. 180, 3271–3279 (2007).
Matsuura, H., Mukuda, H. & Miyake, K. Valence skipping phenomena, charge Kondo effect, and superconductivity. AAPPS Bull. 32, 30 (2022).
Matsuura, H. & Miyake, K. Theory of charge Kondo effect on pair hopping mechanism. J. Phys. Soc. Jpn. 81, 113705 (2012).
Yu, R. et al. Melting of Pb charge glass and simultaneous Pb-Cr charge transfer in PbCrO3 as the origin of volume collapse. J. Am. Chem. Soc. 137, 12719–12728 (2015).
Kurushima, K. et al. Unusual inhomogeneous microstructures in charge glass state of PbCrO3. Jpn. J. Appl. Phys. 57, 50301 (2018).
Fu, Y., Jin, S. & Zhu, X.-Y. Stereochemical expression of ns2 electron pairs in metal halide perovskites. Nat. Rev. Chem. 5, 838–852 (2021).
Seshadri, R. & Hill, N. A. Visualizing the role of Bi 6s “lone pairs” in the off-center distortion in ferromagnetic BiMnO3. Chem. Mater. 13, 2892–2899 (2001).
Neaton, J. B., Ederer, C., Waghmare, U. V., Spaldin, N. A. & Rabe, K. M. First-principles study of spontaneous polarization in multiferroic BiFeO3. Phys. Rev. B 71, 14113 (2005).
Sato, Y. et al. Lamellar-like nanostructure in a relaxor ferroelectrics Pb(Mg1/3Nb2/3)O3. J. Mater. Sci. 56, 1231–1241 (2021).
Kumar, A. et al. Atomic-resolution electron microscopy of nanoscale local structure in lead-based relaxor ferroelectrics. Nat. Mater. 20, 62–67 (2021).
Nakajima, H. et al. Direct observation of monoclinic polar nanoregions in relaxor ferroelectric Pb(Yb1/2Nb1/2)O3-PbTiO3. Phys. Rev. Mater. 6, 74411 (2022).
Eremenko, M. et al. Local atomic order and hierarchical polar nanoregions in a classical relaxor ferroelectric. Nat. Commun. 10, 1–9 (2019).
Raulot, J.-M., Baldinozzi, G., Seshadri, R. & Cortona, P. An ab-initio study of the rôle of lone pairs in the structure and insulator-metal transition in SnO and PbO. Solid State Sci. 4, 467–474 (2002).
Nakajima, H. et al. Charged domain boundaries stabilized by translational symmetry breaking in the hybrid improper ferroelectric Ca3–xSrxTi2O7. Commun. Mater. 2, 109 (2021).
Kurushima, K. et al. Stabilization of layered perovskite structures via strontium substitution in Ca3Ti2O7 revealed via elemental mapping. J. Appl. Phys. 131, 24102 (2022).
Acknowledgements
This study was supported in part by JSPS KAKENHI (Grant Numbers JP19H05625, JP21K14538, and JP21H04625, JP24H00374), JST-CREST (JPMJCR22O1), and the Collaborative Research Projects of Materials and Structures Laboratory, Institute of Integrated Research, Institute of Science Tokyo.
Author information
Authors and Affiliations
Contributions
K. K., H. N., and S. M conceived the research. K. K. and H. N. performed the STEM observations and analyzed the data. T. O., Y. S., and M. A. synthesized the polycrystalline specimen via high-pressure synthesis. S. M initiated and supervised the research project. H. N. and K. K. wrote the manuscript. All authors discussed the results and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kurushima, K., Nakajima, H., Ogata, T. et al. Relationship between Pb ion off-centering and lone pair electrons. Sci Rep 15, 9314 (2025). https://doi.org/10.1038/s41598-025-93984-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93984-5