Abstract
Purpose: To investigate whether altered functional activity, functional connectivity (FC), and structural connectivity (SC) following acute carbon monoxide (CO) poisoning contribute to delayed neurological sequelae (DNS) occurrence. Methods: Binary degree centrality (DC) and seed-based FC were investigated in 18 patients with DNS, 26 patients without DNS, and 30 healthy controls. Duration of CO exposure and coma severity indices-related fibers was detected by connectometry analysis and the identified fiber tracts were tracked and their SC alteration was quantify by fractional anisotropy (FA). Results: Acute CO exposure induced DC change in the prefrontal cortex (PFC), visual cortex, primary sensory cortex, and anterior cerebellum, and FC alteration between the right fusiform gyrus (seed) and bilateral PFC and left inferior occipital gyrus (Gaussian random field corrected, P < 0.05). Poisoning severity indices-related WM fibers consisted of corpus callosum and some association and projection fibers (false discovery rate corrected, P < 0.05). Only altered DC in the right fusiform gyrus and right postcentral gyrus and reduced FC of the PFC could identify DNS occurrence (P < 0.05). Conclusions: The functional abnormalities in the visual- and sensory- cortex and PFC subsequent to acute CO poisoning represent one of the potential neural mechanisms underlying the occurrence of DNS.
Similar content being viewed by others
Introduction
Delayed neurological sequelae (DNS), characterized by the recurrence of neurologic deficits such as cognitive impairment, urinary incontinence, parkinsonism, and psychiatric symptoms, can occur following acute carbon monoxide (CO) poisoning1,2. These sequelae may emerge after a symptom-free lucid interval, persisting for up to 6 months during follow-up. However, previous research has shown that only 56.3% of patients achieve a favorable outcome after 1 year2,3. The resulting poor prognosis not only significantly impacts the patient’s quality of life but also imposes a substantial burden on caregivers. At present, the pathophysiological mechanism of DNS occurrence is uncertain, and early identify patients who might develop DNS is still a concerned issue.
Despite the controversies surrounding the prediction of DNS, certain factors such as an earlier onset, a longer duration of exposure, and a decreased Glasgow Coma Scale (GCS) score are widely recognized as indicators of severe poisoning with a high likelihood of resulting in neurological injury4,5,6. By contrast, the presence of acute brain lesions (ABLs) on diffusion-weighted imaging (DWI) has been identified as a reliable predictor of DNS2,7,8. These clinical and imaging indices hold the advantage of being easily applicable for clinical diagnosis. However, they provide limited insights into the underlying pathophysiological mechanisms associated with neurological disorders.
In recent years, there was a growing interest in using MRI techniques to investigate the brain damage features in patients with CO poisoning at different disease phases, specifically by examining those brain alterations of functional activity and functional connectivity (FC) using resting-state functional magnetic resonance imaging (rs-fMRI) and that change of structural connectivity (SC) using diffusion tensor imaging (DTI). Previous studies have reported abnormal neural activity in regions such as the prefrontal-temporal cortices, right insula, and cerebellum, as well as altered FC intensity in networks associated with cognition and sensorimotor functions in patients with acute CO exposure9,10. Another study, using rs-fMRI degree centrality (DC) analysis, which quantifies altered FC between voxels to determine the relative importance of a node in a network, detected functional abnormalities in multiple brain hub regions, including the cerebral cortex, subcortical nuclei, and cerebellum in patients during the period of DNS11. Beyond these functional alterations, several studies have discovered a significant reduction in fractional anisotropy (FA), a metric for assessing white matter (WM) microstructural integrity. Specifically, this reduction was observed in the centrum semiovale and periventricular WM of patients who had experienced CO poisoning, two weeks after exposure12,13,14. Furthermore, a study employing graph theory analysis of structural networks reported reduced global efficiency and regional node efficiency in patients during DNS, indicating the potential impact of disrupted WM connectivity15. However, there is currently a lack of research using multi-modal MRI approaches to investigate the causal relationship between alterations in brain function and structure and the DNS development.
Connectome analysis, a novel tractography modality that tracks the WM fibers related to the research variables at the voxel-wise level16, has been used in clinical studies of major depression17, early Parkinson’s disease18, restless withdrawal syndrome19, and drug / alcohol addiction20. Whether this analysis can be applied to identify WM fiber injury trajectories associated with indicators of intoxication severity is unclear in patients with acute CO poisoning.
In current study, by integrating rs-fMRI and DTI sequences, we sought to examine the effects of acute CO poisoning on both functional and structural changes throughout the entire brain and further investigate the potential of these alterations in the early identification of patients who will develop DNS. Here, we hypothesized that the brain functional activity and connectivity alterations and WM structural disconnection induced by acute CO exposure might be the potential neuropathological basis for subsequent DNS.
Materials and methods
Ethical approval
This study was approved by the Research Ethics Committee of the first hospital of Lanzhou University (ID: LDYYLL2018-114). All methods were performed in the this were performed in accordance with relevant guidelines and regulations. Written informed consent was obtained from all participant before the study.
Study population
The clinical and MRI image data in patients with carbon monoxide (CO) poisoning from charcoal-burning incidents between May 2018 and February 2023 were collected prospectively. Screening by the inclusion and excluded criteria, a total of 54 patients were enrolled in this study. Among them, 24 patients experienced DNS, while the remaining 30 patients did not. Additionally, 32 healthy controls (HCs) were enrolled during the same time period, with matched age, sex, and educational level.
The diagnostic criteria of acute CO included a history of acute CO exposure and an increased carboxyhemoglobin (COHb) > 10% at acute admission21.
The diagnostic criteria of DNS are as follows: (1) had a clear history of acute CO exposure; (2) had a definite“false recovery period” in the course of the disease; (3) who appeared some new neurological symptoms, including cognitive impairment, dyskinesia, psychomotor abnormalities and so on.
All patients conform to the following inclusion criteria: (1) who underwent prospective multi-modal MRI scan within one week after acute CO poisoning, including three-dimensional T1-weighted imaging (3D T1WI), rs-fMRI, and DTI; (2) the follow-up result of whether had novel neurologic deficits within two months after CO poisoning was successfully obtained.
Patients were excluded if they had any of the following criteria: (1) under the age of 18; (2) had experienced symptoms of DNS before the MRI scan; (3) had a history of neuropsychological disorders, systemic diseases (such as infection, tumor, metabolic disease, or other chronic poisoning), and alcohol or substance abuse; (4) had unexpected brain lesions on MRI images; (5) patients with aging-related WM hyperintensity, with total Fazekas scores of 2 and 3, as this could potentially affect the measurement of SC parameters22. WM hyperintensity was defined as a visually hyperintense signal on fluid-attenuated inversion recovery (FLAIR) sequences, corresponding to isointense regions on DWI images with a b value of 1000 s/mm2, or as hyperintense regions on DWI due to T2 shine-through effects from chronic lesions (6) left-handed individuals; (7) had contraindications for MRI scan.
DNS evaluation process: (1) performing DNS-related knowledge propaganda and education to patients and their families during the hospital, and informing them of the contact information of the doctor; (2) each patient who had a routine neurological examination before discharge; (3) patients were followed up regularly in the outpatient department of Neurology after discharge; (4) followed up patients regularly by telephone.
Clinical materials collection
All subjects’ demographic characteristics including age, sex, and education level, and all patients’ clinical materials including duration of CO exposure, time interval from CO poisoning to MRI scan, Glasgow Coma Scale (GCS) score on admission, frequency of hyperbaric medicine, and ABLs on DWI images were collected. The ABLs were defined as unambiguous bright signal intensities on DWI (b = 1000 s/mm2) after excluding T2 shine-through effects. All patients received 100% oxygen therapy after a mask or endotracheal intubation, and other treatments included capacity expansion, anti-infection, symptomatic treatment, and hyperbaric oxygen therapy.
MRI data acquisition and analyses
MRI data acquisition
All subjects underwent scanning using a 3.0 Tesla MR scanner (Magneton Skyra; Siemens, Erlangen, Germany) equipped with a 32-channel phased-array head coil. To minimize head motion, foam padding was used, and earplugs were provided to reduce scanner noise. Participants were instructed to keep their eyes closed, not to think of anything specific, avoid falling asleep, and refrain from any head movement during the resting-state functional magnetic resonance imaging (rs-fMRI) scan. The scanning parameters for the 3D T1WI were consistent with our previous study23. It was a magnetization-prepared rapid acquisition gradient echo sequence with the following parameters: repetition (TR), echo (TE), and inversion (TI) times, (TR/TE/TI) = 2300/2.98/900 ms, respectively; flip angle = 9º, matrix = 512 × 512, field of view = 256 × 256 mm, slice thickness = 1 mm, and 176 single-shot interleaved slices with no gap with isotropic voxel size 1 × 1 × 1 mm. rs-fMRI data were acquired using a single-shot, gradient-recalled echo-planar imaging sequence with the following parameters: repetition time (TR) = 3200 ms, echo time (TE) = 30 ms, flip angle = 90º, acquisition matrix size = 64 × 64, slice thickness = 3 mm, number of slices = 40, and 200 volumes in total. DTI images were obtained using a spin echo-based echo-planar imaging sequence with TR = 4800 ms, TE = 95 ms, matrix size = 128 × 128, and slice thickness = 3.9 mm. Two sets of images were acquired with different diffusion sensitivities: one with 60-direction encoding and a b-value of 1000 s/mm2 for each direction and the other with no diffusion encoding (b = 0 s/mm2). Each volume consisted of 72 contiguous axial slices covering the entire brain.
rs-fMRI data preprocessing
The preprocessing of rs-fMRI data was performed in MATLAB R2018a using the Data Processing and Analysis for Brain Imaging (DPABI 6.2v) toolbox (http://rfmri.org/dpabi), following the standard procedure. The first 10 volumes were discarded, and slice-timing and head-motion correction were applied to the remaining 190 images. Subjects were excluded if the translational or rotational displacement between successive volumes exceeded 3.0 mm or 3.0°. The individual images were then normalized to the Montreal Neurological Institute (MNI) space using the normalization parameters derived from the 3D-T1 image and resampled to 3-mm isotropic voxels for inter-subject comparison. Linear regression was performed to remove nuisance variables, such as white matter and cerebrospinal fluid signals, as well as the Friston-24 head motion parameters. Finally, a temporal band-pass filter was applied to the images, retaining frequencies between 0.01 and 0.10 Hz.
Voxel-wise binary DC and seed-based FC analyses
After preprocessing, we calculated the binary DC in a voxel-wise manner using DPARSF. The time course of each voxel was correlated with all other voxels within the GM mask, yielding a voxel-wise Pearson’s correlation matrix and the binary DC map of the whole brain. Then, to eliminate the low time correlation interference caused by signal noise, we set the threshold of Pearson’s correlation coefficient to r > 0.25 according to the suggestion24. The FC analysis was then conducted using the seed-based approach, focusing on brain regions that showed significant changes in DC measures when comparing the DNS and non-DNS groups. For the seed-based FC analysis, we defined spherical seed regions with a radius of 3 mm centered on specific voxels. The reference time series for each seed region was obtained by averaging the time series of all voxels within the region. Pearson’s correlation analysis was then performed between the averaged time series from each seed region and the rest of the brain. This allowed us to calculate correlation maps for each subject. To enhance normality effectively, the obtained correlation maps were transformed into Fisher z-values, resulting in z-FC maps. A Gaussian kernel with a full width at half maximum (FWHM) of 4 mm was applied for enhancing the smoothness of the individual binary DC and FC maps (the data preprocessing was without smoothing) .
DTI data processing
The diffusion data and WM tractography were processed using the DSI studio software (http://dsi-studio.labsolver.org/) following standard procedures. First, individual diffusion images were corrected for motion and eddy current distortion using the FSL toolbox (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl/). The images were then resampled to 1 mm isotropic resolution. The diffusion data were reconstructed in MNI space using q-space diffeomorphic reconstruction, with a sampling length ratio of 1.25. To ensure data accuracy, the b-table was checked using an automatic quality control routine25. After reconstruction, streamlined tractography was performed using a random seeding strategy. The default parameters were used, including a Hausdorff distance tolerance of 16 mm, an angular threshold randomly selected from 15° to 90°, and a step size ranging from 0.5 voxels to 1.5 voxel distance. A FA tracking threshold of 0.2 was applied and tracks with lengths shorter than 30 mm or longer than 300 mm were discarded. To further refine the tractography, topology-informed pruning was applied with 16 iterations to remove false connections. In conclusion, the diffusion data and WM tractography were processed using the DSI studio software. The data underwent motion and eddy current distortion correction were resampled to 1 mm isotropic resolution, and reconstructed in MNI space using q-space diffeomorphic reconstruction. Tractography was performed using a random seeding strategy and parameters as mentioned above. Finally, topology-informed pruning was applied to refine the resulting tractography.
Connectometry analysis
Connectometry analysis was employed to establish correlational tractography, which revealed the correlation between FA value (between adjacent voxels within fiber tracts) and the clinical indices (duration of CO exposure and GCS score). These localized connectomes are traced along the core pathway of the fiber bundle using a fiber tracking algorithm and then compared to the null distribution of coherent associations through nonparametric permutation statistics16. Therefore, the Spearman partial correlation, a nonparametric test, was used to determine this correlation while accounting for the effects of age, sex, and education level using a multiple regression model. To obtain the correlational tractography, a deterministic fiber tracking algorithm was employed, with a T-score threshold of 2.5, and tracking was performed throughout the analysis. The resulting tracks were then refined using topology-informed pruning with four iterations. Furthermore, a false discovery rate (FDR) threshold of 0.05 was used to select tracks to conduct multiple corrections. A total of 4000 randomized permutations17,19 were applied to the group labels to obtain a null distribution of the track length .
SC analysis of fiber tracts
The fiber bundles identified through connectometry analysis of individual subject were automatically tracked using a batch automatic fiber tracking method (AutoTrack)26. This method is based on the shortest Hausdorff distance between streamlines and the HCP842 tractography atlas27. Which has been successfully applied in automatic fiber tracking in the previous similar studies28,29. Based on the previous study26, the Hausdorff distance is defined as the maximum of closest distance between a pair of streamlines X and Y as.
dH (X, Y) = max{maxx ∈ X miny ∈ Y d(x, y), maxy ∈ Y minx ∈ X d (x, y)}
X is a set of coordinates, i.e. X={x}, whereas Y is another set of coordinates, i.e. Y={y}. d(x, y) calculates the Euclidean distance between two coordinates x and y, and the dH(X, Y) calculates the Hausdorff distance between set X and Y26.
Similarly, the fiber bundles connecting the altered seed-based FC regions were also tracked. In this case, the seed region served as the starting point, while the significant regions in the FC analysis served as endpoints. The mean FA value of both the fiber bundles with abnormal connectivity identified by connectometry analysis and between the brain regions with altered FC was computed by automatically, which was used to define the strength of the SC.
After above procedures, a total of 10 patients were excluded from the study. The exclusions were based on the presence of an arachnoid cyst, excessive head motion, and age-related white matter hyperintensities. Additionally, 2 HCs were excluded due to excessive head motion. Finally, a total of 44 patients and 30 HCs obtained the follow statistical analyses.
Statistical analyses
Continuous variables were depicted using either mean ± standard deviation or median and interquartile range, depending on the normality assessment conducted using the Shapiro–Wilk test. Categorical variables were assessed using the chi-square test or Fisher test, while continuous variables were evaluated using either one-way analysis of variance (ANOVA), Kruskal–Wallis test, or Mann-Whitney test. All statistical analyses were performed using SPSS 22, and the threshold for statistical significance was set at P < 0.05, using a two-tailed approach.
We utilized the ANOVA to assess the presence of significant binary DC and seed-based FC differences across various groups while considering age, sex, education level, mean frame-wise displacement (FD), and GM volume as covariates. The DPABI software’s statistical analysis module facilitated this analysis. Afterward, a post hoc t-test was conducted within the mask that contained the significantly different results obtained from the ANOVA. All statistical significance complied with voxel-wise P < 0.001 (two-tailed) and a cluster-level P value < 0.05 after Gaussian random field (GRF) correction between groups.
Partial correlation analyses were conducted in SPSS 22 to examine the relationship between imaging indices and clinical indicators; The same covariates as mentioned earlier were used, except for GM volume. Independent t-tests were used to determine the differences in mean FA values of each WM fiber bundle between patient groups. The threshold for statistical significance was set at a FDR-corrected P < 0.05.
Last, receiver operating characteristic (ROC) curve analysis was utilized to assess whether the extracted brain imaging indicators could serve as reliable features for DNS identification. The uncorrected two-tailed P < 0.05 are considered to be significant.
Results
Subject characteristics
Significant differences were observed among patients who developed DNS in terms of the duration of CO exposure (P<0.001), GCS score (P = 0.002), and ABLs (P<0.001). However, no significant differences were found between the two patient groups in relation to the time interval from CO exposure to MRI scans (P = 0.528) and the number of hyperbaric oxygen therapy sessions (P = 0.185). Table 1 provides a detailed summary of the demographic and clinical data.
DC analysis
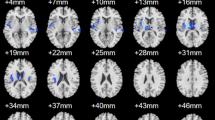
As depicted in Fig. 1; Table 2, significant differences in binary DC were observed between DNS patients and HCs in various brain regions, including the right anterior cerebellar lobe, left orbital frontal cortex (OFC.L), right postcentral gyrus (postCG.R), and right inferior temporal gyrus (ITG.R) (Fig. 1A). Similarly, Non-DNS patients also displayed significant alterations in binary DC compared to HCs, particularly in the left anterior cerebellar lobe, left lateral orbital frontal cortex (latOFC.L), and right inferior occipital gyrus (IOG.R) (Fig. 1B). Furthermore, inter-patient comparisons revealed significant changes in DC in the right fusiform gyrus (FG.R) and postCG.R (Fig. 1C) in individuals who eventually developed DNS. Notably, when discriminating between DNS patients and Non-DNS patients, the FG.R exhibited an area under the curve (AUC) of 0.815 (P < 0.001), whereas the postCG.R had an AUC of 0.811 (P = 0.001) (Fig. 1D). Similarly, distinguishing DNS patients from HCs yielded an AUC of 0.844 (P < 0.001) for the FG.R and an AUC of 0.744 (P = 0.005) for the postCG.R (Fig. 1E).
Significantly binary DC differences among groups (A-C) and the receiver operating characteristic curves of altered DC regions for identifying DNS from Non-DNS (D) and HCs (E). Abbreviations: DC, degree centrality; DNS, delayed neurological sequelae; HCs, healthy controls; postOFC.L, left posterior orbital frontal cortex; antOFC.L, left anterior orbital frontal cortex; CB.4.5.R, right cerebellar lobules 4 and 5; postCG.R, right postcentral gyrus; ITG.R, right inferior temporal gyrus; IOG.R, right inferior occipital gyrus; latOFC.L, left lateral orbital frontal cortex; CB.4.5.L, left cerebellar lobules 4 and 5; FG.R, right fusiform gyrus. Gaussian random field corrected with two-tailed P < 0.05.
Seed-based FC and correlation analysis
As depicted in Table 2; Fig. 2, we observed a decrease in FC between the FG.R (seed) and the right middle frontal gyrus (MFG.R) and left medial superior frontal gyrus (medSFG.L) in patients with DNS (Fig. 2A). Conversely, patients with Non-DNS displayed an increased FC with the left inferior occipital gyrus (IOG.L) (Fig. 2B). Furthermore, it is revealed that DNS patients exhibited reduced FC in the left anterior cingulum cortex (ACC.L), medSFG.L, and right dorsolateral superior frontal gyrus (dlSFG.L) compared to those Non-DNS patients (Fig. 2C). These altered FC patterns were found to effectively differentiate DNS patients from Non-DNS patients, as evidenced by AUC values of 0.811 (P = 0.001), 0.750 (P = 0.005), and 0.887 (P < 0.001) for the ACC.L, medSFG.L, and dlSFG.L, respectively (Fig. 2D). Additionally, except for the ACC.L, the altered FC in the medSFG.L and dlSFG.L proved effective in identifying DNS occurrence patients from HCs, with AUC values of 0.743 (P = 0.005) and 0.858 (P < 0.001), respectively (Fig. 2E). Supplementary Table 1 lists the detailed diagnostic efficacy of the DC and FC values.
There was no significant FC intensity difference between the postCG.R (seed) with other brain regions (GFR-corrected with two-tailed P > 0.05).
Significantly altered FC among groups (Figures A - C were generated using DPABINet, which is installed within the DPABI 6.2v toolbox). A, DNS vs. HCs; B, Non-DNS vs. HCs; C, DNS vs. Non-DNS. The receiver operating characteristic curves of altered FC for identifying DNS from Non-DNS (D) and HCs (E). Edge width denotes the significance lever between-group differences (thicker for more significant). Abbreviations: DNS, delayed neurological sequelae; HCs, healthy controls; medSFG.L, left medial superior frontal gyrus; MFG.R, right middle frontal gyrus; FG.R, right fusiform gyrus; IOG.L, left inferior occipital gyrus; ACC.L, left anterior cingulum cortex; dlSFG.R, right dorsolateral superior frontal gyrus; FC, functional connectivity. Gaussian random field corrected with two-tailed P < 0.05.
Correlation analysis
As showed in Table 3, in addition to FC value of the medSFG.L, we observed that altered binary DC in the FG.R and postCG.R and altered FC in the ACC.L and dlSFG.L have a significantly correlation with GCS score and duration of CO exposure (FDR-corrected P < 0.05).
Connectometry analysis
The connectometry analysis identified several brain regions that showed FA had a negative correlation with the duration of CO exposure but a positive correlation with the GCS score (Supplementary Fig. 1). The duration of CO exposure related WM fiber tracts include the minor forceps, body, and major forceps of the corpus callosum (minfCC, bCC, and majfCC), left arcuate fasciculus (AF.L), left and right corticostriatal anterior tract (CSAT.L and CSAT.R), left inferior frontal occipital fasciculus (IFOF.L), and right thalamic anterior radiation (TAR.R); The Glasgow Coma Scale score related WM fiber tracts include minfCC, IFOF.L and right inferior frontal occipital fasciculus (IFOF.R), AF.L, left corticospinal tract (CST.L), left corticostriatal superior tract (CSST.L), and right frontal and parietal tract of cingulum gyrus (FPCG.R) (Fig. 3). The two-dimensional images of fiber tracks was showed in Supplementary Fig. 1.
Three-dimensional reconstruction of white matter tracks with diffusion tensor imaging_fractional anisotropy correlated with duration of carbon monoxide exposure (A) and with Glasgow Coma Scale score (B) (false discovery rate-corrected P ≤ 0.05). The figures were generated with DSI studio software (versions 2022 August). Colors reflect fiber orientation. Blue: superior–inferior; green: anterior–posterior; red: medial–lateral. The duration of CO exposure and GCS score related WM fiber tracts include the minor forceps, body, and major forceps of the CC (minfCC, bCC, and majfCC), right frontal and parietal tract of cingulum gyrus (FPCG.R), left arcuate fasciculus (AF.L), left and right inferior frontal occipital fasciculus (IFOF.L and IFOF.R), left and right corticostriatal anterior tract (CSAT.L and CSAT.R), left corticostriatal superior tract (CSST.L), left corticospinal tract (CST.L) and right thalamic anterior radiation (TAR.R) (FDR corrected P ≤ 0.05).
Fiber tracts SC
Apart from the minfCC, FPCG.R, CSAT.R, CSST.R and TRA.R successfully tracked in all of the patients, the bCC and majfCC were not detected in 2 patients, the AF.L and IFOF.L were not detected in 3 patients, CSAT.L was not detected in1 patients. The fiber tract connecting the FG.R to the dlSFG.R was successfully identified in 15 DNS occurrence patients and 24 Non-DNS occurrence patients, while a few fiber bundles were detected between the FG.R and ACC.L (patients number: DNS/ Non-DNS = 14/14) and between FG.R and medSFG.L (patients number: DNS/ Non-DNS = 8/10).
There was a trend of decrease in mean FA values across all identified fiber bundles in the DNS occurrence group. Specifically, the CC minor forceps displayed a significantly reduced mean FA value (uncorrected P = 0.10). However, after FDR correction, all P values were rendered insignificant. These findings are summarized in Supplementary Table 2.
Discussion
In this study, by combining rs-fMRI and DTI techniques, we investigated the whole-brain functional and structural alteration patterns in acute CO poisoning patients with different clinical outcomes and further explored the feasibility of those alterations in early identifying the patients with DNS occurrence. The main findings were as follows: (1) acute CO exposure results in functional abnormalities in the bilateral PFC, bilateral visual cortex, right primary sensory cortex, and bilateral anterior cerebellum; (2) the duration of CO poisoning and GCS score are related to some brain WM fiber microstructural alteration, which included the CC and some association and projection fibers; (3) altered functional activity in the hub regions of the right visual cortex and right primary sensory cortex, altered FC between the subregion of right visual cortex and the subregions of bilateral PFC contribute to DNS occurrence. The reduced FA values in the minor forceps of CC has the potential in diagnosing the DNS occurrence.
We observed functional abnormalities in key brain regions following acute CO exposure. These regions include the OFC.L, postCG.R, right medial occipitotemporal cortices, and bilateral anterior cerebellum lobes, involving cognitive, sensory, and motor processing. Among these regions, a significantly decreased binary DC was exhibited in the FG.R while an increased binary DC was shown in the postCG.R in DNS patients compared to Non-DNS patients. It is worth noting that a lower DC value suggests a decline in node functional activity, indicating impaired connectivity. On the other hand, a higher binary DC value does not necessarily reflect better functioning, as excessively high DC values may indicate incorrect or invalid connectivity. Moreover, only the FG.R showed significant FC alterations with the other brain regions. The fusiform gyrus, which is also known as the occipitotemporal gyrus, is the largest part of the human ventral temporal cortex and plays a key role in high-level visual cognitive tasks30. Our previous study reported structural atrophy in the FG.R during the DNS period23. Here, even after controlling for GM volume, the decreased binary DC in the FG.R remained significant, supporting its reliable contribution to the functional abnormalities observed in DNS. Our findings highlight the importance of the identified brain regions, particularly the FG.R, in understanding the functional consequences of acute CO exposure and its association with DNS.
At 1 week after acute CO poisoning, decreased GM volumes were observed in multiple subregions of the frontal, occipital, and temporal lobes31. When controlling for GM volume, we found significantly decreased FC in the medSFG.L and the MFG.R in patients with DNS occurrence. Conversely, increased FC in the IOG.L was observed in patients without DNS. The medSFG.L and the MFG.R belongs to the PFC and the IOG.L is part of visual cortex. Our findings highlight the FC reduction in the right visual cortex and bilateral PFC, as well as the compensatory increase in FC in the bilateral visual cortex, as distinct imaging pathology characteristics of acute CO poisoning patients with and without DNS. Previous research has also reported significant hypo-metabolism in the PFC of patients exposed to acute CO32. Furthermore, a significant FC decrease was observed in three subregions of the PFC, specifically, the ACC.L, medSFG.L, and dlSFG.R, which could effectively serve as an early diagnostic marker for DNS patients. These regions are functionally linked to cognitive control processes and are central nodes of the default mode network (ACC.L and medSFG.L) and the executive control network (dlSFG.R)33. Therefore, our findings suggest that functional abnormalities in those nodes of the right visual network, executive control network, and default mode network during acute CO exposure might underlie the subsequent visuospatial and cognitive control deficits observed in DNS patients.
At the voxel-wise level, connectometry analysis provided us with a trajectory of WM damage associated with the duration of CO exposure and coma severity. These fiber tracts included the CC, several association fibers (e.g. right FPCG, left AF, and bilateral IFOF), and some projection fibers (e.g. bilateral CSAT, left CSST, left CST, and right TAR). These findings suggest a vulnerability of the fiber bundles connecting the frontal-temporal-occipital cortices and the prefrontal cortex-striatum-thalamus in patients with more longer duration of CO poisoning and higher GCS score, and the latter are involved in dopaminergic pathways. Similar to the findings of this study, a previous study has reported the underlying damage of the CC, cingulate tract, and CST in 1 week in patients with acute CO poisoning34. As the main WM fiber bundle anatomically connecting the left and right hemispheres, the CC played an important role in integrating the functional activity between the hemispheres; the AF connects the frontal and temporal cortex and functionally plays a role in memory and cognition35; the IFOF passes frontal cortex to occipital cortex through the insular and outer capsule, its damage could lead to complex cognitive dysfunction36; the TAR is the fiber of the medial thalamic nucleus and the anterior nucleus to the frontal lobe, involved in the planning and execution of daily tasks functional and affective disorders37,38; the corticostriatal tract, involved in the regulation of multiple motor, sensory, cognitive, and affective functions39; the cingulate bundle connects the frontal, top and medial temporal lobes, forming radiation fibers in the frontal and top, and reduced FA values of the cingulate band have been reported in Alzheimer’s disease, mild cognitive dysfunction, obsessive-compulsive disorder, schizophrenia, and other patients40; and last, the CST, mainly originates from the primary motor area, with contributions from the primary sensory cortex and premotor areas41. It is primarily responsible for regulating voluntary body movements and processing part of the sensory information41. Based on the above functional feature, we inferred that these fiber bundle impairments induced by acute CO exposure could be associated with the delayed cognitive, parkinsonian, and psychiatric sequela symptoms that appeared in patients with DNS.
Furthermore, upon conducting a comparison of the FA values of fiber tracts between groups, we discovered that, in contrast to the patients without the occurrence of DNS, those with DNS occurrence exhibited a trend of reduced FA values in the minor forceps of CC (uncorrected P = 0.010; corrected P = 0.130). This finding suggests that it might be a promising indicator for the early diagnosis of patients with DNS occurrence after increasing the sample size to improve the statistical power in the future. Specifically, the fibers of the genu of the CC cross over and form the forceps minor, which connect regions of the frontal cortices42. Previous research has also reported altered WM microstructure in the frontal lobes as an early identifier of patients with DNS occurrence43. Additionally, selective damage in the prefrontal and premotor subregions of the CC has been observed in patients during the DNS period44. Here, we added the literature that the CC minor forceps as a potential MRI biomarker to aid the early diagnosis DNS.
When examining the SC change between reduced FC intensity regions, WM fiber between the FG.R and the ACC.L or between the FG.R and the medSFG.L were detected in only a few patients. Meanwhile, although WM fiber was successfully identified between the FG.R and the dlSFG.R in most of patients, no significantly reduced FA value of it showed in the DNS group. Similar to a previous study on cerebral small vessel disease showed only functional disconnectivity between the posterior cingulate and thalamus in patients with severe WM lesions, with no significant changes in the structural connectivity45. These findings suggest a complex and non-corresponding relationship between alterations in structural and functional connectivity. There is not necessarily structural disconnection between the brain regions with dysfunction in patients with acute CO poisoning.
Conclusion
The decrease in activity in the right visual cortex, the increase in activity in the right primary sensor cortex and the weakened functional connectivity between the right visual cortex and bilateral PFC together contribute to the occurrence of DNS. The disruption in the structural connectivity of the CC minor forceps has a potential to be a biomarker to aid in the early diagnosis of DNS.
Limitation
Although this study had strict exclusion criteria for enrolled patients, it was limited by the inability to obtain multi-modal MRI images before CO exposure in the patient group. Therefore, absolute causal inferences cannot be made between the brain imaging alterations and CO exposure. Furthermore, although no differences were found in hyperbaric oxygen therapy at the group level, it is important to consider that the drug therapeutic regimens administered to the included patients might still have confounding effects on brain function and structure. This limitation should be taken into account when interpreting the current results. Finally, the small sample sizes of each group of this study affected the efficacy of the statistical results, and future studies with large cohorts are needed to better explore the causal relationship between brain function and structural changes and patients’ neurological outcomes.
Data availability
All data generated or analysed during this study are included in this article.
References
Beppu, T. The role of MR imaging in assessment of brain damage from carbon monoxide poisoning: a review of the literature. AJNR Am. J. Neuroradiol. 35 (4), 625–631 (2014).
Wang, T. et al. Neurological sequelae in acute carbon monoxide poisoning: A prospective observational study with MRI data. Acta Neurol. Scand. 145 (5), 590–598 (2022).
Liu, J. et al. Clinical and imaging prognosis in patients with delayed encephalopathy after acute carbon monoxide poisoning. Behav. Neurol. 7, 1719360 (2020).
Pepe, G. et al. Delayed neuropsychological sequelae after carbon monoxide poisoning: predictive risk factors in the emergency department. A retrospective study. Scand. J. Trauma. Resusc. Emerg. Med. 19, 17 (2011).
Huang, C. C. et al. Exposure duration and history of hypertension predicted neurological sequelae in patients with carbon monoxide poisoning. Epidemiology 30, S76-S81 (2019).
Lee, H. et al. Initial creatine kinase level as predictor for delayed neuropsychiatric sequelae associated with acute carbon monoxide poisoning. Am. J. Emerg. Med. 43, 195–199 (2021).
Jeon, S. B. et al. Acute brain lesions on magnetic resonance imaging and delayed neurological sequelae in carbon monoxide poisoning. JAMA Neurol. 75 (4), 436–443 (2018).
Nah, S. et al. Cerebral white matter lesions on Diffusion-Weighted images and delayed neurological sequelae after carbon monoxide poisoning: A prospective observational study. Diagnostics (Basel). 10 (9), 10:698 (2020).
Dinghua, L., Dongbo, L., Jianyu, Z. & Lan, P. A resting-state functional magnetic resonance imaging study of acute carbon monoxide poisoning in humans. Cell. Biochem. Biophys. 67 (3), 1029–1032 (2013).
Zheng, H. et al. Abnormal brain functional network dynamics in acute CO poisoning. Front. Neurosci. 15, 749887 (2021).
Wu, K., Liu, M., He, L. & Tan, Y. Abnormal degree centrality in delayed encephalopathy after carbon monoxide poisoning: a resting-state fMRI study. Neuroradiology 62 (5), 609–616 (2020).
Beppu, T. et al. Assessment of damage to cerebral white matter fiber in the subacute phase after carbon monoxide poisoning using fractional anisotropy in diffusion tensor imaging. Neuroradiology 52 (8), 735–743 (2010).
Kuroda, H., Fujihara, K., Takahashi, S., Shinozawa, Y. & Itoyama, Y. A case of delayed encephalopathy after carbon monoxide poisoning longitudinally monitored by diffusion tensor imaging. AJNR Am. J. Neuroradiol. 33 (4), E52–E54 (2012).
Beppu, T. et al. Fractional anisotropy in the centrum semiovale as a quantitative indicator of cerebral white matter damage in the subacute phase in patients with carbon monoxide poisoning: correlation with the concentration of Myelin basic protein in cerebrospinal fluid. J. Neurol. 259 (8), 1698–1705 (2012).
Jiang, W. et al. Study on brain structure network of patients with delayed encephalopathy after carbon monoxide poisoning: based on diffusion tensor imaging. Radiol. Med. 126 (1), 133–141 (2021).
Yeh, F. C., Badre, D. & Verstynen, T. Connectometry: A statistical approach Harnessing the analytical potential of the local connectome. Neuroimage 125, 162–171 (2016).
Zheng, H. et al. C-Reactive protein and the kynurenic acid to quinolinic acid ratio are independently associated with white matter integrity in major depressive disorder. Brain Behav. Immun. 105, 180–189 (2022).
Sanchez-Catasus, C. A. et al. Dopaminergic nigrostriatal connectivity in early Parkinson disease: in vivo neuroimaging study of 11 C-DTBZ PET combined with correlational tractography. J. Nucl. Med. 62 (4), 545–552 (2021).
Park, K. M. et al. Correlation of diffusion tensor tractography with restless legs syndrome severity. Brain Sci. 13(11), 1560 (2023).
Waters, A. B., Sawyer, K. S. & Gansler, D. A. White matter connectometry among individuals with self-reported family history of drug and alcohol use disorders. Drug Alcohol Depend. 206, 107710 (2020).
Rose, J. J. et al. Carbon monoxide poisoning: pathogenesis, management, and future directions of therapy. Am. J. Respir Crit. Care Med. 195, 596–606 (2017).
Zeng, W. et al. Severity of white matter hyperintensities: lesions patterns, cognition, and microstructural changes. J. Cereb. Blood Flow. Metab. 40 (12), 2454–2463 (2020).
Wang, T. et al. Surface-based morphometry study of brain in patients with carbon monoxide poisoning. Eur. J. Radiol. 160, 110711 (2023).
Buckner, R. L. et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. 29 (6), 1860–1873. https://doi.org/10.1523/JNEUROSCI.5062-08.2009 (2009).
Schilling, K. G. et al. A fiber coherence index for quality control of B-table orientation in diffusion MRI scans. Magn. Reson. Imaging. 58, 82–89. https://doi.org/10.1016/j.mri.2019.01.018 (2019).
Yeh, F. C. et al. Population-averaged atlas of the macroscale human structural connectome and its network topology. Neuroimage 178, 57–68. https://doi.org/10.1016/j.neuroimage.2018.05.027 (2018).
Yeh, F. C. Shape analysis of the human association pathways. Neuroimage 223, 117329. https://doi.org/10.1016/j.neuroimage.2020.117329 (2020).
Cui, Y. et al. Disturbed interhemispheric functional and structural connectivity in type 2 diabetes. J. Magn. Reson. Imaging. 55 (2), 424–434. https://doi.org/10.1002/jmri.27813 (2022).
Wang, H. et al. White matter BOLD signals at 7 Tesla reveal visual field maps in optic radiation and vertical occipital fasciculus. Neuroimage 269, 119916. https://doi.org/10.1016/j.neuroimage.2023.119916 (2023).
Sebastian, R. et al. The roles of occipitotemporal cortex in reading, spelling, and naming. Cogn. Neuropsychol. 31, 511–528 (2014).
Chou, M. C., Li, J. Y. & Lai, P. H. Longitudinal Gray matter changes of the pain matrix in patients with carbon monoxide intoxication: A voxel-based morphometry study. Eur. J. Radiol. 126, 108968 (2020).
Chen, N. C. et al. Cognitive severity-specific neuronal degenerative network in charcoal burning suicide-related carbon monoxide intoxication: a multimodality neuroimaging study in Taiwan. Med. (Baltim). 94, e783 (2015).
Friedman, N. P. & Robbins, T. W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 47 (1), 72–89 (2022).
Tsai, P., Chou, H. & Chiang, M. C. Early white matter injuries in patients with acute carbon monoxide intoxication A tract-specific diffusion kurtosis imaging study and STROBE compliant article. Med. (Baltim). 96 (5), e5982 (2017).
Vavassori, L. et al. The arcuate fasciculus: combining structure and function into surgical considerations. Brain Behav. 13 (8), e3107 (2023).
Chen, H., Huang, F. & Li, L. L. Microstructural disruption of the right inferior fronto-occipital and inferior longitudinal fasciculus contributes to WMH-related cognitive impairment. CNS Neurosci. Ther. 26 (5), 576–588 (2020).
Torso, M. et al. Strategic lesions in the anterior thalamic radiation and apathy in early Alzheimer’s disease. PLoS One. 10 (5), e0124998 (2015).
Niida, R. et al. Aberrant anterior thalamic radiation structure in bipolar disorder: A diffusion tensor tractography Study. Front. Psychiatry. 9, 522 (2018).
Gómez-Ocádiz, R. & Silberberg, G. Corticostriatal pathways for bilateral sensorimotor functions. Curr. Opin. Neurobiol. 83, 102781 (2023).
Bubb, E. J. et al. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 92, 104–127 (2018).
Welniarz, Q., Dusart, I. & Roze, E. The corticospinal tract: Evolution, development, and human disorders. Dev. Neurobiol. 77(7), 810–829 (2017).
Goldstein, A., Covington, B. P., Mahabadi, N. & Mesfin, F. B. Neuroanatomy corpus callosum. In StatPearls [Internet] (2023).
Chou, M. C., Li, J. Y. & Lai, P. H. Longitudinal white matter changes following carbon monoxide poisoning: A 9-Month Follow-Up Voxelwise diffusional kurtosis imaging Studys. AJNR Am. J. Neuroradiol. 40 (3), 478–482 (2019).
Chen, P. C. et al. Callosal damage and cognitive deficits in chronic carbon monoxide intoxication: A diffusion tensor imaging studys. J. Neurol. Sci. 355 (1–2), 101–107 (2015).
Chen, X. et al. Disrupted functional and structural connectivity within default mode network contribute to WMH-related cognitive impairments. Neuroimage Clin. 24, 102088 (2019).
Acknowledgements
The authors wish to thank the patients and their caregivers for their time and commitment to this research. The authors would also like to thank Xiaoming Chen, Xin Zhuang, Jianlin Li, Dapeng Liang, Hang Guo, and Jiang Nan for their assistance in recruitment of participants and MRI scan.
Funding
This study has received funding by research grants from the National Natural Science Foundation of China (NO. 82160930), Natural Science Foundation of Gansu province (NO. 22JR11RA026), Technology plan project of Lanzhou city (NO. 2020-ZD-71), and Research Project of Gansu provincial administration of traditional Chinese medicine (No. GZKZ-2021-8).
Author information
Authors and Affiliations
Contributions
Yan-Li Zhang wrote the original manuscript text. Tian-Hong Wang, Chao-ning Zhou, and Zhao-dong Liu contributed to the patients collections. Yan-Li Zhang and Shuai-Wen Wang analysed the data. All authors contributed to the design and interpretation of the study. Jun-Qiang Lei proposed the study and is the guarantor. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Wang, T., Zhou, C. et al. Brain functional and structural alteration following acute carbon monoxide poisoning contribute to delayed neurological sequelae. Sci Rep 15, 10417 (2025). https://doi.org/10.1038/s41598-025-94787-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94787-4