Abstract
Dimethylguanidino valeric acid (DMGV) is a group of endogenous metabolites derived from arginine-containing proteins and is associated with several metabolic disorders. Latest studies have identified the stereoisomers of DMGV: asymmetric dimethylguanidino valeric acid (ADGV) and symmetric dimethylguanidino valeric acid (SDGV), which are derived from asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA), respectively. However, the lack of commercial standards has hampered research into the molecular mechanisms of DMGV and its potential clinical application as biomarkers. Reported chemical synthesis methods have low yields and require specialized chemical synthesis skills and apparatus. Therefore, we aimed to develop a practical and efficient method to synthesize DMGV stereoisomers and determine their precise concentration profile in healthy subjects. A novel biocatalytic method for synthesizing DMGV using alanine-glyoxylate aminotransferase 2 (AGXT2) was developed. A metabolite panel including eight DMGV-related metabolites was also established for human plasma samples using HPLC-MS/MS, and its performance was comprehensively evaluated, especially in terms of quantitative sensitivity, precision and accuracy. Compared with the reported chemical synthesis methods, the biocatalytic approach demonstrates superior yield, conversion rate, and product purity, while being easy to implement in biological laboratories. The established DMGV quantification method has been well validated and successfully applied to measure the contents of these metabolites, especially the concentration profile of ADGV in the plasma of healthy individuals. To sum up, this study provides an efficient and practical biocatalytic approach for DMGV synthesis and determines the levels of DMGV in healthy subjects. These findings will undoubtedly promote future mechanistic studies of DMGV and its future clinical applications.
Similar content being viewed by others
Introduction
Dimethylguanidino valeric acid (DMGV) has been reported to be a potentially important biomarker associated with several metabolic disorders, such as obesity1, non-alcoholic fatty liver disease (NAFLD)2, type 2 diabetes2 and cardiovascular disease3. O’Sullivan et al. (2017) first discovered the importance of DMGV as a biochemical compound in metabolic diseases. In this work, DMGV levels were found to be an independent biomarker of CT-defined NAFLD in the Swedish cohort of the Framingham Heart Study, and the baseline levels of DMGV independently predicted future diabetes up to 12 years before disease onset in three distinct human cohorts2. Furthermore, elevated DMGV levels in plasma were associated with the incidence of coronary heart disease and cardiovascular mortality in three different Swedish cohorts involving 5768 participants4. A multicenter prospective cohort study of patients with suspected coronary artery disease also showed that DMGV was associated with the occurrence and severity of coronary artery disease3. Given that dietary habits and physical activity are the primary means of preventing metabolic disorders, studies have also shown that DMGV levels in the blood were highly associated with lifestyles5. Ottosson et al. (2019) revealed that individuals with higher plasma DMGV levels tended to consume more sweetened beverages while taking less vegetables4. It was further demonstrated that macronutrients affected DMGV-related metabolites in mouse models5,6. In addition, high levels of DMGV were associated with poor metabolic responses to long-term cardiovascular prognosis7, and both long-term exercise training intervention8 and acute exercise9 lowered plasma DMGV levels.
According to the latest published research10, the endogenous metabolites DMGV has two stereoisomers, including asymmetric dimethylguanidino valeric acid (ADGV) and symmetric dimethylguanidino valeric acid (SDGV). ADGV and SDGV are produced from asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA), respectively, by alanine-glyoxylate aminotransferase 2 (AGXT2)11,12, a mitochondrial enzyme mainly expressed in kidney and liver. The published results on DMGV mainly refer to ADGV, and only one article reported SDGV and showed that SDGV is also one of the determinants in NAFLD progression13.
Although endogenous DMGV, including ADGV and SDGV, are closely related to metabolic diseases, the lack of commercial standards has hindered their mechanistic research and potential clinical application. Strictly speaking, both external and internal standards are required for absolute quantitation for setting up a multiple reaction monitoring (MRM) method using HPLC-QQQ-MS/MS which is the most sensitive and quantitative MS-based approach. However, to the best of our knowledge, only some published articles on DMGV were measured using in-house synthesized DMGV standards1,10,14, and some were based on untargeted metabolomics without DMGV standards7,15,16,17,18. According to the literature, ADGV was first synthesized and detected using a derivatization procedure19. Besides, two other articles mentioned the process of chemical synthesis1,10. However, after numerous attempts in our own lab, we found that the chemical synthesis of DMGV, especially the process for ADMA synthesis, is very time-consuming and has relatively high technical requirements in chemical operation. The synthesized DMGV from ADMA had low yield (5.6%) and low purity (78.4%). Therefore, the establishment of alternative synthetic approaches is necessary.
Biological catalysis is currently a leading method for small-molecule synthesis, with the advantages of being environmentally friendly, efficient, easy to operate, and having low production costs20,21. Studies22,23 reported the biocatalytic production of keto-acid 6-guanidino-2-oxocaproic acid from homoarginine in HepG2 cells with the help of the AGXT2 enzyme overexpressed by an adenoviral vector, Therefore, it is promising to investigate the biocatalytic process of producing ADGV and SDGV from ADMA and SDMA, respectively, under the action of AGXT2 enzyme.
Thus, this study aimed to develop a method for the biosynthesis of DMGV with high yield, purity, and efficiency. Moreover, DMGV-related metabolites play a vital role in the occurrence and progression of metabolic diseases, especially ADMA and SDMA, which are important biomarkers for predicting cardiovascular diseases6,24. Consequently, an HPLC-MS/MS-based method was also established and comprehensively validated for the quantification of a panel of metabolites in the DMGV metabolic pathway, including ADGV, ADMA, β-aminoisobutyric acid (BAIBA), citrulline, homoarginine, NG-monomethyl-L-arginine (NMMA), SDGV, SDMA, and thymine with good analytical performance. The analytical method was further applied to determine the concentration profiles of these metabolites in human plasma samples (Fig. 1).
Materials and methods
DMGV synthesis
The chemical synthesis of ADGV mainly consists of two parts: (A) synthesis of ADMA from ornithine1; (B) synthesis of ADGV from ADMA, according to the references1,10,19. The detailed synthesis process is shown in the supplementary.
For biosynthesis, the gene of truncated human AGXT2Δ1-41 with a fused C-terminal His-tag was codon-optimized and constructed into the expression vector pET28b by Genewiz (Nanjing, China). The pET28b-AGXT2Δ1-41 plasmids were then transformed into E. Coli BL21 (DE3) competent cells by heat shock. A single colony of the AGXT2Δ1-41-His6 expression strain was inoculated into LB broth (100 mL) containing 50 µg/mL kanamycin and shaken at 37 °C overnight. When the culture reached an OD600 of 0.8, protein expression was stimulated by adding 0.4 mM isopropyl-β-D-1-thiogalactopyranoside and incubated at 20 °C, 200 g for 18 h.
Cells were harvested by centrifugation at 8000 g, 4 °C for 25 min and resuspended in phosphate buffer A (20 mM sodium phosphate, 300 mM NaCl, 10 mM imidazole, and 100 µM pyridoxal phosphate; pH = 7.4) supplemented with 1 mM phenylmethanesulfonyl fluoride. Then, cells were lysed under sonication (Misonix) in an ice bath for 10 min at 50% amplitude with a processing time pulse of 10s and an interval of 30s. The lysates were centrifugated at 18,000 g, 4 °C for 1 h. The soluble supernatants were filtered with a 0.45 µM filter and loaded onto a pre-equilibrated HisTrap FF column (Cytavia) with the same phosphate buffer. The enzyme activities were measured by an ultraviolet spectrophotometer (Agilent, USA). The enzyme product was aliquoted and stored at -80 °C before use.
The enzymatic catalysis of ADGV was conducted overnight at room temperature using the above-mentioned purified human AGXT2 enzyme (2.04 mg/mL) and a reaction solution containing 30 mM ADMA, 150 mM glyoxylate, and 10 mM potassium phosphate buffer (pH = 8). After lyophilization, compounds were extracted by protein precipitation using an organic solvent (ACN: MeOH, 9:1, v: v). After centrifugation at 18,000 g, 4 °C for 10 min, the supernatant was collected for further product purification and identification. Other DMGV standards, including d6-ADGV, SDGV, and d6-SDGV were successfully synthesized using the same protocol.
After chemical and biological synthesis, the synthesized standards were purified using preparative HPLC (1260 HPLC, Agilent) and qualified by HPLC-QToF MS/MS (1290II HPLC-6530 MS/MS, Agilent) and proton nuclear magnetic resonance 1H NMR; 400 MHz, Avance NEO, Bruker). The details are shown in the supplementary.
UPLC-QQQ MS/MS-based quantitative method for DMGV metabolite panel
A measurement method for a set of DMGV-related metabolites was established by using UPLC (Vanquish core, Thermo Scientific) coupled to a triple quadrupole (QQQ) MS/MS (TSQ Altis MS, Thermo Scientific), with d6-ADGV, d7-ADMA, d7-arginine, d3-BAIBA and d2-citrulline as internal standards. Moreover, method performance validation was carried out according to the “Guidance for Industry: M10 Bioanalytical Methods Validation and Study Sample Analysis”25 and published articles26,27. This section is described in detail in the supplementary materials.
Analysis of human plasma samples
Human plasma samples from the hospitals were prepared and analyzed using the same protocol as the standard samples in the AB Sciex 6500 QTrap MS/MS. In addition, to determine the levels of SDGV in plasma, another 10 human plasma samples (n = 5 for each gender) were applied. Wilcoxon-Mann–Whitney test was conducted using R (version 4.3.0) to determine the differences in metabolites between sexes. A p-value < 0.05 was considered statistically significant. Detailed information can be found in the supplementary materials.
The study involving human samples was ethically approved, and all experiments were performed in accordance with the Declaration of Helsinki.
Results and discussion
Synthesis of DMGV standards
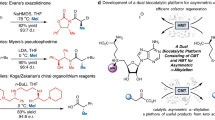
Generally, the chemical synthesis of ADMA went through a six-step reaction, starting from Boc-Orn(Z)-OH (Suppl. Fig. S1A). During this process, silica gel-based purification was required after each reaction step of reaction to improve the product purity. Thus, the first step of the chemical synthesis from Boc-Orn(Z)-OH to ADMA was very time-consuming and required relatively high chemical experimental operation skills.
The second part of the chemical synthesis was the synthesis of ADGV from ADMA, which was conducted via a two-step reaction (Suppl. Fig. S1B). In total, this two-step reaction took about 6 h, whereas based on the ratio of the amount of ADMA converted after the two-step reaction to the initial amount of ADMA, the conversion rate was about 98.8%. By calculating the ratio of the actual amount of ADGV obtained to the theoretical amount of ADGV, the chemical synthesis yield was about 5.6%. After purification using preparative HPLC, the product purity reached 78.4% (Fig. 2A).
In contrast, the biocatalytic synthesis of ADGV from ADMA with the help of AGXT2 enzyme took about 12 h. The AGXT2 enzyme was expressed and purified from engineered E. coli, which is highly efficient and easy to operate, making it particularly suitable for biological laboratories. According to the ADMA content before and after the reaction, the conversion rate of ADGV was about 99.6%. The product purity easily reached 97.6% after purification by preparative HPLC (Fig. 2B). Therefore, our experimental results showed that the established biocatalytic process of ADGV had a relatively high yield (~ 16.2%) and purity (~ 97.6%).
For compound structure confirmation, QToF MS/MS (m/z 71.1, 70.1, and 46.2 are the characteristic fragments of ADGV with [M + H]+ ion m/z 202.1; Fig. 2C) and 1H NMR (400 MHz, D2O; δ ppm 3.65 (2H, t, CH2CH2CH2), 2.19 (6H, s, N-CH3 × 2), 3.16 (2H, t, CH2CH2CH2), 1.56 (2H, t, CH2CH2CH2; Fig. 2D) were used. The other DMGV standards, d6-ADGV (conversion rate = 99.7%, purity = 76.2%), SDGV (conversion rate = 99.5%, purity = 94.6%), and d6-SDGV (conversion rate = 99.5%, purity = 17.6%), were also catalytically synthesized in the study using the same protocol (Suppl. Fig. S2). To our knowledge, this is the first study on the biosynthesis of these endogenous compounds.
UPLC-MS/MS-based quantitative method for DMGV metabolite panel
According to published articles5,10, a quantitative method for DMGV-related metabolites was established using UPLC-QQQ MS/MS in MRM acquisition mode, while a protein precipitation method using organic solvents, which was commonly used for sample preparation was adopted. For each analyte, the product ion with the highest response was usually chosen as the quantifier ion, and the next highest product ion as the qualifier ion. Representative extracted chromatograms (EIC) and MRM parameters of these DMGV metabolites from a human plasma sample are shown in Fig. 3.
The DMGV metabolite panel has three pairs of isomers: ADGV and SDGV, ADMA and SDMA, and homoarginine and NMMA. They have the same MRM transitions and the same highest product ions, such as ADGV and SDGV sharing 202.1→71.1 and 70.0, ADMA and SDMA sharing 203.1→70.1, and homoarginine and NMMA sharing 189.1→144.1. Therefore, unique ion transitions were selected for these isomers to ensure quantitative accuracy when creating the panel analysis. Still, the unique product ion intensities were sometimes relatively low, largely affecting their detection sensitivity, especially for SDGV. Moreover, arginine was removed from the panel due to severe carryover after sample runs (data not shown).
Furthermore, to mimic the matrix effects of plasma, different ratios of BSA (0.1%, 1%, 5%) were tested as matrix samples by comparing the intensity of the internal standards in these matrix samples with that in human plasma. Finally, 5% BSA was chosen as a surrogate matrix. These results are shown in Suppl. Table S1.
In addition, chromatographic columns commonly used for metabolite analysis were tested. The C18 column (100 × 2.1 mm, 2.2 μm, Acclaim RSLC column, Thermo Fisher) had limited ability to retain these water-soluble metabolites, which were washed out within the first two minutes with poor selectivity. Therefore, considering the analytical performance of RT, selectivity, peak intensity, and peak shape, the HILIC column (150 × 2.1 mm, 1.7 μm, Atlantis, Waters) was finally selected for analysis (Suppl. Fig. S3).
(A) Representative extracted ion chromatograms (EIC) of DMGV-related analytes in a plasma sample with spiked standards. The metabolites from left to right are: (a) thymine; (b) ADGV; (c) d6-ADGV; (d) BAIBA; (e) d3-BAIBA; (f) citrulline; (g) d2-citrulline; (h) d7-arginine; (i) homoarginine; (j) NMMA; (k) SDMA; (l) ADMA; (m) d7-ADMA, and (B) MRM parameters of DMGV analytes in detail.
Validation of HPLC-MS/MS detection method
Linearity, accuracy, precision, and sensitivity of the calibration curves
The calibration curves were established based on eight-point concentrations using three batches of standard samples dissolved in 5% BSA. The linear regression models of all analytes and the Limits of Detection (LOD) are shown in Table 1. Overall, the standard curves of all analytes satisfied the method requirements in accuracy (85 ‒ 115%) and precision (< 15%; Suppl. Table S2).
The linear ranges of ADGV and SDGV calibration curves were 0.006–0.8 µM (R2 > 0.99). The average accuracy of ADGV at all 8-point concentrations was 98.9% (94.8–107.2%), and their average precision was 2.9% (0.6–6.3%) (Suppl. Table S2a). The precision and accuracy results of other metabolites were listed in Suppl. Table S2. Regarding all analytes, most metabolites passed the acceptance criteria for precision and accuracy, except for NMMA at low levels of concentrations (0.003 and 0.006 µM).
Selectivity, specificity, and carryover of the analytes
The selectivity and specificity of the analytes and internal standards were carefully assessed by using 5% BSA as the matrix samples (n = 6) and human plasma samples (n = 6), respectively. There were no significant interference peaks at adjacent RTs of the analytes in the 5% BSA matrix samples (< 10.6% of the corresponding LLOQ level). The peak areas at the RTs adjacent to the internal standards were also negligible in the 5% BSA matrix samples (< 3.6% of the corresponding internal standards peak area; Suppl. Table S3) and in the human plasma samples (< 4.5% of the corresponding internal standards peak area; Suppl. Table S4).
Carryover of all analytes was evaluated by running a blank sample (50% ACN) after running the standard mixture with the highest concentration in the calibration curve. The carryover amounts in the blank samples were < 4.8% of the corresponding LLOQ for all analytes (Suppl. Table S5). Thus, the method was highly selective for the analytes, with no significant carryover observed at the highest concentration.
Accuracy and precision of the matrix samples with spiked standards
The standard mixtures at low, medium, and high concentrations were added into the 5% BSA matrix, denoted as Low-matrix, Medium-matrix, and High-matrix samples. Intra- and inter-assay precision and accuracy were calculated for these samples (n = 6) and shown in Suppl. Table S6. Taking ADGV as an example (Suppl. Table S6a), the average inter-assay accuracy of ADGV was 98.6% (94.6–102.7%), and the precision was 4.7% (3.6–6.1%). These results are within the acceptance criteria for accuracy (100 ± 15%) and precision (< 15%), demonstrating the reliability and reproducibility of the assay. For the other analytes, most metabolites had high accuracy and precision at all 3 spiking levels, except for low levels of NMMA (Suppl. Table S6f).
Recovery of standards during extraction
The extraction process may result in loss of analytes or internal standards, so recovery was evaluated by comparing the peak areas of the analytes between prior-to-extraction plasma samples spiked with standards and post-to-extraction plasma samples spiked with standards at different concentrations. They are denoted as Low-plasma, Medium-plasma, and High-plasma samples. The recovery results for these samples are shown in Table 2 and 3. The average recoveries of all analytes ranged from 92.7 to 128.6%, which means the extraction procedure had relatively small impacts on the analyte concentration.
Matrix effects of ADGV in plasma samples
The matrix effect was measured by calculating the ratio of the intensity of ADGV or internal standard d6-ADGV added in plasma samples to the intensity in 5% BSA matrix samples and the corresponding IS-normalized MF (Suppl. Table S7).
The average matrix correction factor (MF) was 42.4% for ADGV and 43.9% for d6-ADGV at low, medium, and high spiking concentrations. The internal standard normalized matrix correction factor (IS-normalized MF) which is defined as the ratio of MFanalyte to MFIS, was further used to evaluate the matrix effect. The IS-normalized MFs of ADGV was 96.6% with an RSD of 8.7%, which indicates the internal standard compensated for the matrix effect of the analyte and eliminated interference with the detection of ADGV. In summary, the matrix effect on ADGV was negligible, and potential effects on quantitation were eliminated by using a stable-isotope internal standard (Suppl. Table S7).
Reinjection reproducibility and Dilution integrity
The reinjection reproducibility of analytes at different concentrations is satisfactory (precision < 10%, 85%< accuracy < 115%) based on 5 replicates of standard samples, except for the low concentration of NMMA (0.006 µM; Suppl. Table S8).
Dilution integrity assesses the effect of sample dilution on the accuracy and precision of the measured analyte concentrations. According to our results, the recalculated analyte concentrations were close to the original levels, except for NMMA in the 128-time diluted sample (0.66µM; Suppl. Table S9).
The results of reinjection reproducibility and dilution integrity showed that the analytical performance of NMMA at a low concentration (0.006 µM) was not good enough.
Stability studies of processed samples
Because large sample preparation should be avoided for long sequence analysis to maintain batch-to-batch consistency, cohort samples are typically prepared in batches of 80–120 samples per batch. A single sample run time was approximately 17 min. Therefore, we evaluated the stability of the processed samples after 36 h of storage at the autosampler temperature of 4ºC, which is equivalent to the run time of one batch of samples. The stabilities of the analytes in the processed samples were good after storage, except for high concentrations of thymine (RSD > 15%; Suppl. Table S10).
Impact of internal standards and instruments on ADGV quantification
Using stable isotope-labeled internal standards is considered the most effective way to compensate for the matrix effect. However, considering the cost and availability of isotope-labeled internal standards, internal standards can sometimes be replaced by alternatives, but their impacts on ADGV quantification have not been evaluated. In this study, the impacts of different internal standards (d6-ADGV, d7-arginine, d7-ADMA, d3-BAIBA, and d2-citrulline) on the ADGV quantification were evaluated by using plasma samples spiked with different levels of ADGV (0.045 and 0.650 µM).
The concentrations of ADGV were calculated using the standard curves generated with different internal standards. The results showed that d6-ADGV had the highest quantitative accuracy, around 92.2%, compared with other isotope-labeled internal standards (Fig. 4). When isotopic ADGV is not available, we would recommend choosing d3-BAIBA as the internal standard.
In addition, there were no distinguished differences in ADGV quantification between analytical instruments (Suppl. Fig. S4).
Analysis of human plasma samples
The DMGV metabolites in human plasma samples were measured using the above-established quantitative method (Table 4). The levels of these analytes from our population were commensurate with previous literatures10,13,14. We determined the concentration distribution of ADGV in healthy plasma (n = 488; [2.9–16.1 nM]) which is within the reported range by measuring the plasma ADGV levels in 240 human subjects from outpatient clinics, preadmission clinics and the emergency department [1.8 to 74.2 nM]10. However, SDGV was reported to be less than 150 nM in recent research13, but it was not detected in our study. It is mainly due to the low intensity of its unique product ion for quantification (m/z 171.1) in the instrument and low content of SDGV in healthy human plasma. The levels of the other DMGV-related metabolites in our study agree with the reported results from others’ studies28,29. Thus, it is feasible to incorporate these DMGV-related metabolites in one analytical panel.
The content of these metabolites was further compared between males and females. Several metabolites (ADGV, citrulline, NMMA, homoarginine, SDMA, thymine) were significantly different between genders (Table 4, and Suppl. Fig. S5). For instance, ADGV in males (9.4 nM [4.1–16.1]) was much higher than in females (6.2 nM [2.9–11.2]; P < 0.001), and SDMA in males (269.2 nM [238.5-299.2]) is also higher than female (236.5 nM [209.1-266.3]; P < 0.001). However, obvious sexual dimorphism in the distribution of ADMA and BAIBA was not found.
Conclusion
The study successfully synthesized the endogenous metabolites DMGV, including ADGV, SDGV, d6-ADGV, and d6-SDGV, using a biocatalytic synthetic approach for the first time. Compared to the chemical synthetic method, the biocatalytic method is superior from the perspectives of product conversion rate, yield, product purity, and ease of operation. Furthermore, a targeted MS/MS-based method with high sensitivity, precision, accuracy, selectivity, and high throughput was established to quantify DMGV-related metabolites. The developed method has been applied to determine the concentration distribution of these metabolites in human plasma samples by measuring samples from 488 healthy participants, especially for DMGV. These findings provide an alternative way for DMGV synthesis and MS-based quantification, which will facilitate future mechanisms and biomarker studies on DMGV in different scenarios.
Data availability
Data that support the findings of this study have been attached as supplementary materials.
Abbreviations
- ACN:
-
Acetonitrile
- ADGV:
-
Asymmetric dimethylguanidino valeric acid
- ADMA:
-
Asymmetric dimethylarginine
- AGXT2:
-
Alanine glyoxylate aminotransferase 2
- BAIBA:
-
β-Aminoisobutyric acid
- BSA:
-
Bovine serum albumin
- DDAH1:
-
Dimethylarginine dimethylaminohydrolase
- DMGV:
-
Dimethylguanidino valeric acid
- HPLC-MS/MS:
-
High performance liquid chromatography-tandem mass spectrometry
- MeOH:
-
Methanol
- MMSA:
-
Methylmalonate semialdehyde
- MRM:
-
Multiple reaction monitoring
- NAFLD:
-
Non-alcoholic fatty liver disease
- NMMA:
-
NG-monomethyl-L-arginine
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- PBS:
-
Phosphate buffered saline
- PRMTs:
-
Protein-arginine methyltransferases
- SDGV:
-
Symmetric dimethylarginine
- SDMA:
-
Symmetric dimethylarginine
References
Poirier, J. et al. Bariatric surgery improves the atherogenic profile of circulating methylarginines in obese patients: Results from a pilot study. Metabolites 11, 759 (2021).
O’Sullivan, J. F. et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J. Clin. Invest. 127, 4394–4402 (2017).
Vernon, S. T. et al. Metabolic signatures in coronary artery disease: Results from the BioHEART-CT study. Cells 10, 980 (2021).
Ottosson, F. et al. Dimethylguanidino Valerate: A lifestyle-related metabolite associated with future coronary artery disease and cardiovascular mortality. JAHA 8, e012846 (2019).
Wali, J. A. et al. Impact of dietary carbohydrate type and protein-carbohydrate interaction on metabolic health. Nat. Metabolism 3, 810–828 (2021).
Wali, J. A. et al. Nutritional and metabolic regulation of the metabolite dimethylguanidino valeric acid: An early marker of cardiometabolic disease. Am. J. Physiol. Endocrinol. Metab. 319, E509–E518 (2020).
Robbins, J. M. et al. Association of dimethylguanidino valeric acid with partial resistance to metabolic health benefits of regular exercise. JAMA Cardiol. 4, 636–643 (2019).
Koay, Y. C. et al. Effect of chronic exercise in healthy young male adults: A metabolomic analysis. Cardiovascular. Res. 117, 613–622 (2021).
Nayor, M., Shah, R. V. & Lewis, G. D. Metabolic architecture of acute exercise response in middle-aged adults in the community. Circulation 142, 1905–1924 (2020).
Bonnitcha, P. et al. Design and validation of an LC-MS/MS method for simultaneous quantification of asymmetric dimethylguanidino valeric acid, asymmetric dimethylarginine and symmetric dimethylarginine in human plasma. Pathology 54, 591–598 (2022).
Martens-Lobenhoffer, J., Rodionovb, R. N. & Bode-Bögera, S. M. Probing AGXT2 enzyme activity in mouse tissue by applying stable isotope-labeled asymmetric dimethyl arginine as substrate. J. Mass Spectrom. 47, 1594–1600 (2012).
Wang, Z. et al. Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis. Commun. Biology. 3, 39 (2020).
Qadri, S. et al. The first human normative ranges and biomarker performance of dimethylguanidino valeric acid isoforms in fatty liver disease. Pathology 56, 391–397 (2024).
Martens-Lobenhoffer, J., Rodionov, R. N., Drust, A. & Bode-Böger, S. M. Detection and quantification of α-keto-δ-(NG,NG-dimethylguanidino)valeric acid: A metabolite of asymmetric dimethylarginine. Anal. Biochem. 419, 234–240 (2011).
Ottosson, F. et al. Metabolome-defined obesity and the risk of future type 2 diabetes and mortality. Diabetes Care. 45, 1260–1267 (2022).
Beckman, J. A. et al. Metabolomics reveals the impact of type 2 diabetes on local muscle and vascular responses to ischemic stress. Clin. Sci. 134, 2369–2379 (2020).
Zeleznik, O. A. et al. Circulating amino acids and amino acid-related metabolites and risk of breast cancer among predominantly premenopausal women. Breast cancer 54 (2021).
Ottosson, F., Smith, E., Fernandez, C. & Melander, O. Plasma metabolites associate with all-cause mortality in individuals with type 2 diabetes. Metabolites 10, 1–11 (2020).
Klein, C., Schulz, G. & Steglich, W. Umwandlung von o-guanidino- und o-ureido-a-aminosauren in a-ketosauren und deren heterocyclische folgeprodukte. Liebigs Ann. Chem., 1623–1637 (1983).
Sahu, S. et al. Algal carbohydrate polymers: Catalytic innovations for sustainable development. Carbohydr. Polym. 327 (2024).
Hu, H. et al. Strategies for the biological synthesis of D–glucuronic acid and its derivatives. World J. Microbiol. Biotechnol. 40 (2024).
Cellini, B., Bertoldi, M., Montioli, R. & Paiardini, A. Borri Voltattorni, C. Human wild-type alanine:glyoxylate aminotransferase and its naturally occurring G82E variant: Functional properties and physiological implications. Biochem. J. 408, 39–50 (2007).
Rodionov, R. N., Jarzebska, N., Weiss, N. & Lentz, S. R. AGXT2: A promiscuous aminotransferase. Trends Pharmacol. Sci. 35, 575–582 (2014).
Oliva-Damaso, E. et al. Asymmetric (ADMA) and symmetric (SDMA) dimethylarginines in chronic kidney disease: A clinical approach. Int. J. Mol. Sci. 20, 1–15 (2019).
U.S. Department of Health and Human Services. Food and Drug Administration, Center for Drug Evaluation and Research (CDER) & Center for Biologics Evaluation and Research (CBER) M10 bioanalytical method validation and study sample analysis: Guidance for industry. Int. Council Harmonisation May, 0–47 (2022).
Lai, X., Kline, J. A. & Wang, M. Development, validation, and comparison of four methods to simultaneously quantify l-arginine, citrulline, and ornithine in human plasma using hydrophilic interaction liquid chromatography and electrospray tandem mass spectrometry. J. Chromatogr. B. 1005, 47–55 (2015).
Chunduri, R. H. B. & Dannana, G. S. Development and validation of a high throughput UPLC–MS/MS method for simultaneous quantification of esomeprazole, rabeprazole and levosulpiride in human plasma. J. Pharm. Anal. 6, 190–198 (2016).
Molfino, A. et al. The metabolite beta-aminoisobutyric acid and physical inactivity among hemodialysis patients. Nutrition 34, 101–107 (2017).
Iritas, S. B. et al. Assessment of potential cardiovascular risk in trichloroethylene exposure by serum methylated arginine levels. Int. J. Environ. Health Res. 31, 63–74 (2019).
Funding
The research project was financially supported by the National Natural Science Foundation of China (Grant number: 82404254) and the Research Development Fund from Xi’an Jiaotong-Liverpool University (Grant number: RDF-21-02-069).
Author information
Authors and Affiliations
Contributions
Y.H. performed all LC-MS related experiments, data collection and analysis, and the first draft of the manuscript; Q.X. and Y.L. were mainly responsible for the construction of the clinical cohort, the collection of plasma samples and clinical data analysis; J.W. performed the biosynthesis and purification experiments; L.H. and Y.Q. mainly supported the method development of biosynthesis; D.J. and Y.L. mainly conducted the chemical synthesis; Z.Z., L.H. and M.W. supported the method establishment and validation of LC-MS; K.C. was mainly responsible for the study conception and design, guided the investigation, and revised the writing. All authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The research involving human samples complied with all relevant national regulations and institutional policies, including the ethics approvals from China Human Genetic Resources Management Office ([2023]GH1304), the Xi’an Jiaotong-Liverpool University Policy on Ethical Conduct in Research (ER-UEC-11000045920220817114316) and the Biomedical Research Ethics Committee of the School of Public Health, Sun Yat-sen University (SYSKY-2022-3336-03). All participants have signed an informed consent form before joining the cohort study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, Y., Wang, J., Xiong, Q. et al. A synthesis and quantification method for endogenous metabolites dimethylguanidino valeric acid. Sci Rep 15, 11100 (2025). https://doi.org/10.1038/s41598-025-94932-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94932-z