Abstract
Amid the ongoing trend of global warming, the distribution of habitable areas for Rhododendron is facing significant risks. To investigate the possible spatial distribution of Rhododendron on the Qinghai-Xizang Plateau in light of future global warming scenarios, we employed the Maximum entropy model (MaxEnt model) to map its suitable habitat using geographic distribution data and environmental factors projected for 2050s and 2070s, considering three representative concentration pathway (RCP) scenarios, while identifying the key factors influencing their distribution. The results show that: [1] The area under curve (AUC) values of the five Rhododendron were all greater than 0.98, indicated that the model prediction effect was excellent; [2] Isothermality is the most important environmental factor affecting the distribution of Rhododendron (excluding Rhododendron przewalskii). The most important environmental factor for Rhododendron przewalskii is altitude (alt: 51%), with an optimum range of 2700–3300 m, and Rhododendron trichostomum are affected by altitude (alt 18%), with an optimum range of 3200–3900 m. Rhododendron przewalskii (bio12: 21%) and Rhododendron trichostomum(bio12: 19%) are also affected by annual precipitation, and Rhododendron laudandum(bio12: 6%) is less affected by annual precipitation, The optimal amount of precipitation is 400–500 mm as well as 500–800 mm. Rhododendron przewalskii and Rhododendron trichostomum are suitable for survival in high altitude, semi-arid areas [3]. The suitable areas for survival for Rhododendron przewalskii, Rhododendron trichostomum, Rhododendron hypenanthum, and Rhododendron nyingchiense is expanding, while the suitable areas for survival for Rhododendron laudandum is shrinking [4]. The optimal zone for Rhododendron przewalskii is primarily found in the eastern section of the Qinghai-Xizang Plateau, while suitable areas for survival for the other four Rhododendron species are predominantly located in the southern region of the same plateau. Therefore, these regions will be designated as the primary conservation zones for in-situ preservation. The results of the study provide a basis for the in situ conservation of Rhododendron in response to global warming, relocation conservation, and the construction of nature reserve communities and ecological corridors.
Similar content being viewed by others
Introduction
It is a widely recognized fact that the 21st century presents a significant challenge to both humans and ecosystems in the form of global climate change1. According to a recent report released by the Intergovernmental Panel on Climate Change (IPCC), the earth’s average temperature has increased by approximately 1.5 °C compared to levels before industrialization, impacting the survival and geographical distribution of various species2,3. Numerous research studies have indicated that species are able to cope with the effects of climate change by adjusting their ecological niches and migrating towards higher altitudes or latitudes4,5. However, the ecological niches of species in different climates may evolve slowly compared to changes in climate. The gradual increase in temperature may lead to the extinction of certain species with limited ability to migrate. In a recent analysis of the suitable habitat for the coast redwood (Sequoia sempervirens D. Don), it was observed that its habitat has significantly reduced in size, putting the species at risk of extinction6. Most plants on the Qinghai-Xizang Plateau are at risk of extinction under future warming scenarios. Therefore, it is crucial to accurately forecast and understand the distribution of suitable habitats for endangered species in future scenarios, at both spatial and temporal scales, in order to conserve species diversity7,8.
The Qinghai-Xizang Plateau, also referred to as the “Third Pole of the Earth”9,10, can be found in the western region of China with an average elevation exceeding 4000 m11,12. Greenhouse gas emissions contribute to global warming13,14, and studies indicate that from 1961 to 2020, the annual average temperature on the Qinghai-Xizang Plateau is projected to increase by 0.35 °C per decade, which is more than double the global warming rate over the same timeframe. Additionally, the annual mean temperature on the Qinghai-Xizang Plateau is forecasted to rise by 1.6 °C to 2.0 °C by the year 2050. By the 2070s, the mean annual temperature on the Qinghai-Xizang Plateau is expected to increase by 1.2 °C to 3.0 °C. Climate models suggest that global warming could potentially exceed 6.5 °C by the end of the century9. Alpine plants are particularly susceptible to the impacts of global warming due to their evolutionary adaptation to cold environments15,16. Species on the Qinghai-Xizang Plateau exhibit high sensitivity to climate variations. Research indicates that six types of alpine plants in the Arctic exhibit varied responses to global warming, specifically affecting perennials and annuals, resulting in reduced suitable habitats. Consequently, a decline in the alpine plant population is anticipated in the future due to the effects of global warming17. Moreover, plants in alpine regions face greater challenges compared to those in lower altitudes and warmer climates18.
The genus Rhododendron belongs to the family Rhododendronaceae and there are eight species worldwide, divided into eight subgenera. These species are widely distributed in Europe, Asia and North America. Rhododendrons have great ornamental and medicinal value19, some of the plants in the genus Rhododendron can be used as a medicinal herbit, some plants of the genus Rhododendron are edible and can be used as a cash crop. Rhododendron, as one of the most prevalent woody plants in China, plays a crucial role in stabilizing slope topography and protecting river basins due to its significant biomass, flexible soil requirements, adaptability, and the formation of a well-developed lateral root system that intertwines effectively20. Additionally, Rhododendron exhibits a high capacity for enriching heavy metals such as lead, cadmium, and zinc, making it valuable for the remediation of heavy metal contaminated soil. Research on Rhododendron has focused more on morphological identification, plant metabolism, and molecular level21. Little research has been done on predicting the distribution of Rhododendrons and identifying suitable habitats22. In China, Rhododendrons are mainly distributed in the southwest, of which Yunnan, Sichuan and Xizang are the most abundant. It is important to understand the spatial distribution pattern of Rhododendron on the Qinghai-Xizang Plateau23.
The quantification of the correlation between species occurrence and environmental variables through ecological niche modeling helps describe a species ecological niche or habitat suitability, providing insights into changes in species distribution influenced by climate and other environmental factors12,24. Currently, popular ecological niche models include the Genetic Algorithm Rule Set Model (GARP), Bioclimatic Analysis and Prediction System Model (BIOCLIM), Random Forests Models (RFs), and the MaxEnt model. Among these, the MaxEnt model is widely utilized in various fields by scholars globally due to its user-friendly interface, high accuracy in predictions, simplicity in operation, and strong explanatory abilities25,26,27. In previous studies that the model is widely used on the Qinghai-Xizang Plateau. Some researchers utilized the MaxEnt model to predict the current and future potential distribution of six musk species on the Qinghai-Xizang Plateau28.
In this study, we selected five species of Rhododendron that are common on the Qinghai-Xizang Plateau, the species distribution of Rhododendron on the Qinghai-Xizang Plateau has not been previously studied. Geographic distribution data along with environmental factors were utilized to forecast both current and future distributions on the Qinghai-Xizang Plateau in light of climate change scenarios, employing the MaxEnt model, where eight soil factors were added to increase the comprehensiveness of the research. The objectives of this study were1 Determine the existing distribution of Rhododendron in the context of global warming;2 Analyze the primary environmental elements that influence Rhododendron distribution;3 Clarify changes in the area of suitable habitat under future climate change.
Materials and methods
Data and variable sources
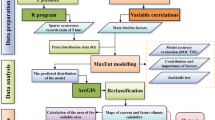
This research acquired distribution data of five Rhododendron species from various sources:1 CNKI database (http://www.cnki.net/ accessed on 7 October 2022);2 China Digital Herbarium (https://www.cvh.ac.cn/ accessed on 7 October 2022);3 NSII China National Herbarium Resource Leveling (http://www.nsii.org.cn/2017/home.php accessed on 7 October 2022);4 Global Biodiversity Information Facility (https://www.gbif.org/zh/);5 China Plant Image Library (http://ppbc.iplant.cn/ accessed on 7 October 2022). In cases where precise latitude and longitude coordinates were absent in the recorded data, we utilized Google Earth (http://ditu.google.cn/ accessed on 7 October 2022) to determine these coordinates based on described geographic positions. To mitigate spatial autocorrelation effects resulting from close sample point proximity, ENMTools were employed to eliminate redundant points. Data on the geographical distribution of the five species of Rhododendron are given in Table 1. The sample latitude and longitude coordinates were saved in an excel file and converted to CSV format for MaxEnd model construction (Fig. 1).
We obtained 19 bioclimatic factors and elevation variables from the global climate database WorldClim (version 1.4) (http://www.worldclim.org/ accessed on 7 October 2022) that have been most frequently used to study plants on the Qinghai-Xizang Plateau in recent years, with a spatial resolution of 30 arcseconds (approximately 1 km). Additionally, 8 soil variables were obtained from the National Tibetan Plateau Data Center ( https://data.tpdc.ac.cn/home accessed on 7 October 2022), resampled to a spatial resolution of 30 arcseconds (approximately 1 km). Future climate data were determined using the CCSM4 model with the most favorable climate effects in China for years 2050s and 2070s. Three different concentration pathways (RCP2.6, RCP6.0, and RCP8.5) were selected to predict the potential extent of climate change. These pathways correspond to low, medium, and high levels of greenhouse gas (GHG) concentrations in the future, respectively. Lastly, all bioclimatic variables were converted to ASCII format for MaxEnt analysis. (Table S1)
Environmental variable processing
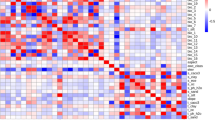
The issue of multicollinearity with climatic factors may impact our accuracy to assess the connection between species and their environment29. Past research has highlighted the seriousness of this problem with bioclimatic variables. To address this, We calculated the Pearson correlation coefficients between the environmental factors and set the correlation coefficients at a threshold of 0.830, retaining those with correlation coefficients less than 0.8, of which those with correlation coefficients greater than 0.8 were retained with the highest contribution, while removing those with a contribution of zero31.
MaxEnt parameter optimization
The latitude and longitude data of five Rhododendron species, along with the filtered environmental variables, were entered into the MaxEnt software. The model was configured with the following settings: 25% of the samples were designated as the test set, while the remaining 75% comprised the training set. The number of replicates was specified as 10, with the replicates run type set to Bootstrap. The “jackknife” method was employed to evaluate the impact of environmental factors. The maximum number of iterations was limited to 500032. Output format was selected as “Logistic”, and the file type was designated as “ASC”. All other parameters were kept at their default values33.
MaxEnt model evaluation
Researchers utilized the receiver-operating characteristic curve (AUC) to evaluate the precision of the species distribution model34. The AUC presents the extent of accuracy, with values ranging from 0 to 1. A higher AUC value signifies increased model accuracy35. An AUC value below 0.7 indicates poor prediction results, while 0.7 to 0.8 suggests an average outcome. The model is considered better with an AUC value between 0.8 and 0.9 and excellent with a value exceeding 0.927,36,37,38.
Classification of habitat suitability ranks
Species suitability values modeled by MaxEnt ranged between 0 and 139, with higher values indicating greater suitability in the specified area. After performing 10 repetitions of the MaxEnt model, a threshold value (10 percentile training presence Logistic threshold) was applied to the “maxent-results” to distinguish unsuitable from suitable regions. The five Rhododendron species were reassigned in the ArcGIS software, dividing suitable areas into three categories (lowly suitable region, moderately suitable region, and highly suitable region). The suitability indices were then classified into four ranks. (Table S3)
Results
Model selection and evaluation
According to the ROC curve analysis, the average AUC value for current and future predictions of five different Rhododendron species was determined through 10 repetitions, higher AUC values indicate high predictive accuracy of model results. The findings revealed that Rhododendron nyingchiense(AUC = 0.988), Rhododendron przewalskii(AUC = 0.985), Rhododendron laudandum(AUC = 0.989), Rhododendron hypenanthum(AUC = 0.995), and Rhododendron trichostomum(AUC = 0.985) had highly accurate potential distribution results as predicted by the model (Fig. 2).
Critical environmental factors
The MaxEnt jackknife test tool was used to assess the effects of various environmental variables on predicting the distribution of five species of Rhododendron (Fig. 3, Figure S1). We analyzed the environmental factors of five species of Rhododendron, the results showed that the distribution of Rhododendron was mainly influenced by isothermality (Bio3), altitude (Alt), annual precipitation (Bio12) and some soil factors. Figure 3 illustrates that isothermality significantly affect the distribution of Rhododendron, with the exception of Rhododendron przewalskii. With the combined contribution rate of isothermality reaching 54.5%. The existence probability of Rhododendron nyingchiense, Rhododendron hypenanthum, and Rhododendron trichostomum reaches the maximum when isothermality is 55%. The existence probability still unchange as the isothermality increases. The existence probability of Rhododendron laudandum reached the maximum when the isothermality reached 44%, The existence probability still decreases as the isothermality increases. The existence probability of Rhododendron przewalskii reached the maximum when the isothermality reached 34%. The existence probability still decreases as the isothermality increases (Fig. 4).
We further analyzed the effects of altitude, precipitation and soil factors on Rhododendron. The most important environmental factor of Rhododendron przewalskii was the altitude (alt:51%), with the optimum range of 2700–3300 m, and the optimum altitude range of the Rhododendron trichostomum (alt:18%) is 3200–3900 m. It is an important variable that affects most plants living on the Qinghai-Xizang Plateau. Rhododendron in the Qinghai-Xizang Plateau are particularly sensitive to altitude. Among the soil factors, Rhododendron laudandum was affected by soil organic matter (som: 18%) and effective phosphorus (ap: 4%), Rhododendron hypenanthum was affected by effective phosphorus (ap: 10%) and pH (pH: 3%), and Rhododendron trichostomum was affected by effective nitrogen (an: 6%) and pH (4%). Rhododendron nyingchiense was affected by soil organic matter (som: 2%), Rhododendron przewalskii (bio12: 21%) and Rhododendron trichostomum (bio12: 19%) are impacted by annual precipitation levels, The optimal amount of precipitation is 400–500 mm and 500–800 mm. In contrast, Rhododendron laudandum (bio12: 6%) is less influenced by annual precipitation, The optimal amount of precipitation is 400–500 mm (Fig. 4, Figure S2).
Current potential distribution of Rhododendron
Current distribution of MaxEnt models for five Rhododendron species in China, The color scale from white to red indicates the habitat suitability value from 0 to 1((a) Rhododendron ningchiense, (b) Rhododendron przewalskii, (c) Rhododendron laudandum, (d) Rhododendron hypenanthum, (e) Rhododendron trichostomum).
The results show that the distribution of Rhododendron nyingchiense, Rhododendron laudandum and Rhododendron hypenanthum is wider than that of the other two species (Fig. 5). The total habitat area of Rhododendron nyingchiense covers 26.83 × 104 km2, which represents 2.78% of the entire country’s area. It is divided into a highly suitable region spanning 2.14 × 104 km2 and a lowly suitable region covering 12.43 × 104 km2. This habitat is primarily found in the southern regions of the Qinghai-Xizang Plateau, encompassing the southwest and southeast of Xizang, the north of Yunnan, and the southwest of Sichuan. The suitable habitat area for Rhododendron laudandum is 19.98 × 104 km2, which represents 2.08% of the entire country’s area. It is divided into a highly suitable region spanning 0.85 × 104 km2 and a lowly suitable region covering 13.68 × 104 km2. This habitat is primarily found in the eastern part of Xizang, including the southern part of Xizang and the western part of Xizang, and part of Sichuan. The suitable habitat area of Rhododendron hypenanthum is 15.06 × 104 km2, which represents 1.57% of the entire country’s area. It is divided into a highly suitable region spanning 0.37 × 104 km2 and a lowly suitable region covering 12.49 × 104 km2. This habitat is primarily found in the southern part of Xizang, the southwestern part of Sichuan, and the northern part of Yunnan. The suitable habitat area of Rhododendron przewalskii is 21. 18 × 104 km2, which represents 2.20% of the entire country’s area. It is divided into a highly suitable region spanning 0.50 × 104 km2 and a lowly suitable region covering 14.74 × 104 km2. This habitat is primarily found in the eastern part of the Qinghai-Xizang Plateau, including the eastern part of Qinghai, the south-central part of Gansu, and the north-central part of Sichuan. The suitable habitat area of Rhododendron trichostomum is 28. 17 × 104 km2, which represents 2.93% of the entire country’s area. It is divided into a highly suitable region spanning 1.09 × 104 km2 and a lowly suitable region covering 18.85 × 104 km2. This habitat is primarily found in the eastern part of Xizang, most parts of Sichuan, and the northern part of Yunnan (Table 2).
Future distribution prediction and fluctuation analysis of suitable habitats
Four different levels of fluctuation in China’s future climate scenarios: Green, orange, red and blue represent the proportion of low, medium and high suitability areas and unsuitable habitats, respectively. A positive value indicates an increase in area and a negative value indicates a decrease in area.
In the future, considering the impacts of global warming, the habitat suitable for Rhododendron trichostomum and Rhododendron przewalskii will expand as CO2 concentrations rise (Fig. 6). In comparison to the current suitable habitat area, the regions appropriate for these two species are projected to grow by 118% and 134.5%, respectively. The areas classified as highly suitable for Rhododendron nyingchiense are expected to increase by 42%, whereas the medium-suitable zones will experience a reduction of 20.5%. This species is primarily located in the southern and southwestern regions of the Qinghai-Xizang Plateau. For Rhododendron laudandum, both the medium and high suitable areas are anticipated to decrease by 12.5% and 16%, respectively, predominantly in the southern portion of the Qinghai-Xizang Plateau. Furthermore, the zones classified as medium and high suitability for Rhododendron hypenanthum will see increases of 20% and 9% in the future, respectively, with this species primarily found in southern Xizang, southwestern Sichuan, and northern Yunnan (Figs. 7 and 8). Comprehensive data can be found in Table S1.
Discussion
The MaxEnt model is widely used to estimate the habitat suitability of species and to predict the effects of global climate on the geographical distribution of specific species40,41, and the model is widely used in disciplines such as conservation biology27. Environmental factors are categorized into biotic and abiotic factors. Abiotic factors mainly include climatic factors42, and large-scale hydrothermal conditions (including temperature and precipitation) are important factors of species distributions38. While biotic factors include factors that are directly related to species distributions and usually act on species distributions at small spatial scales. Many studies have shown that the effects of biotic factors are negligible at larger scales12,43. Therefore, in this study, we modeled the distribution ranges and patterns of five Rhododendron species on the Qinghai-Xizang Plateau using common large-scale bioclimatic and soil factors.
Analysis of key environmental variables
Environmental variables consist of abiotic factors and biological factors. Abiotic factors primarily include climatic variables, which mimic the spatial distribution pattern and species range on a large scale. We used ArcGIS software and MaxEnt model to analyze the relationship between key environmental factors and the existence probability of Rhododendron. The results showed that: in addition to Rhododendron przewalskii, isothermality is the most important environmental variable affecting the remaining four Rhododendron. From the current distribution of the five Rhododendron species. The Rhododendron przewalskii is mainly distributed in the eastern part of the Qinghai-Xizang Plateau, while the other four species are distributed in the southern part of t the Qinghai-Xizang Plateau. Species specificity and differences in distribution areas can affect the contribution of environmental factors40. This explains the different contributing factors affecting species distribution. From the studies that have been done: Isothermality = (Mean Diurnal Range / Temperature Annual Range)×100. Some researchers investigated the potential geographical distribution of two peony species under climate change. The results show that temperature seasonality and isothermality made the greatest contributions to the distribution model for P. delavayi relative to other variables. Greater isothermality indicates a greater diurnal temperature difference in plants. Increased isothermality guarantees that plants can benefit from higher daytime temperatures for photosynthesis. While lower nighttime temperatures minimize energy usage for respiration, ultimately aiding in nutrient absorption by the plant’s root system, promoting plant growth44.
Rhododendron przewalskii and Rhododendron trichostomum thrive in high-altitude region. Many studies have shown that altitude is the most influential factor in plant distribution on the Qinghai-Xizang Plateau, a previous study conducted a quantitative synthesis of various environmental factors using 400 variables and 1900 species. The study revealed that temperature, precipitation, and altitude were the most commonly studied factors45. For terrestrial species, precipitation, temperature, and proximity to water were the most influential factors. Our results also suggest that altitude is a key factor influencing species distribution. With the importance of each variable changing depending on how often they were tested and the specific species46. Altitude usually interacts with temperature and light and has a significant effect on the accumulation of metabolites and nutrients in alpine plants. Some researchers investigated the relationship between niche breadth and phylogenetic characteristics of eight species of rhubarb on the Qinghai-Xizang Plateau47. The results show that altitude is the most important environmental variable affecting six plant species.
Rhododendron przewalskii and Rhododendron trichostomum thrive in semi-arid regions. From previous studies that plant respiration and transpiration are directly influenced by temperature and precipitation, impacting plant growth and development. The hydrothermal conditions, including temperature and precipitation plays a significant role in determining the distribution of species across large areas. Therefore, the distribution of the five Rhododendron plants is primarily impacted by climate change46.
Additionally, prior studies suggest that abiotic factors have a more significant impact on Rhododendron species than soil factors. This study investigated the influence of various soil conditions, particularly those on the Qinghai-Xizang Plateau. Soil composition variations directly affect their growth. Rhododendron laudandum was impacted by soil organic matter (SOM: 18%) and available phosphorus (AP: 4%), Rhododendron hypenanthum by available phosphorus (AP: 10%), and Rhododendron trichostomum by available nitrogen (AN: 6%). Soil organic matter provides soil fertility retention and buffering qualities, available phosphorus supplies the soil with phosphorus and enhances plant growth, while available nitrogen is a key indicator of soil nitrogen fertility and is closely linked to crop growth. The findings suggest that Rhododendrons in this area exhibit specific soil preferences. Consequently, this research serves as a significant demonstration of how environmental factors can influence the distribution of Rhododendrons species in the future48 (Fig. 8).
Changes in suitable habitat for 5 rhododendrons on the Qinghai-Xizang plateau
Over the next few decades, significant warming will be felt across the entire Qinghai-Xizang Plateau9,28. Plant growth and distribution in alpine ecosystems will be especially affected by temperature, as it is a major limiting factor49,50. Most of the available studies show that the area of suitable habitat for most alpine plants is contracting, but there are also cases where suitable habitat is expanding. Habitat expansion and contraction is related to the ability of target species to adapt to environmental stresses. Yang and colleagues investigated the distribution pattern of 12 endangered medicinal plants on the Qinghai-Xizang Plateau. Their findings revealed that a quarter of these plants experienced a reduction in suitable habitat across various future scenarios. Conversely, approximately a third of the endangered medicinal plants expanded their suitable habitat due to their heightened adaptability to environmental pressures. This has resulted in a tendency for some plants living on the Qinghai-Xizang Plateau to have an elevated area of suitable habitat under future warming scenarios. Our results show that under the future warming scenario, the highly suitable area of Rhododendron przewalskii, Rhododendron trichostomum, Rhododendron hypenanthum as well as Rhododendron nyingchiense shows increasing. The area of the suitable area of Rhododendron laudandum shows decreasing. This shift is likely due to increasing temperatures, which have created more favorable conditions for Rhododendrons34. It is evident that the Rhododendron habitat has become more temperate. This study suggests that, in the context of future global warming, plants living on the Qinghai-Xizang Plateau will not always show a decrease in the size of their habitats, and that plants that are more responsive to environmental stresses may show an increase in the size of their habitats in the future51.
Response measures and issues
The genus Rhododendron is the largest genus in the Rhododendron family and one of the large genera in the Chinese and Himalayan flora. There are about 960 species in the world, which are widely distributed in Europe, Asia and North America. The plants of this genus occupy an important position in horticulture and have a high ornamental value52, the Qinghai-Xizang Plateau has already been established with a lot of different grades of national parks and nature reserves. Most of which are for the purpose of protecting the endangered wildlife. The conservation of alpine plants should also be emphasized53. The five Rhododendron species selected for this study all grow on the Qinghai-Xizang Plateau. Because alpine plants are distributed in a limited range, they are more sensitive to climate change. Many alpine plants have smaller migratory ranges and are more vulnerable to extinction in the face of global warming than their temperate counterparts54. This study may provide insights into the conservation of Rhododendron growing on the Qinghai-Xizang Plateau.
Conclusion
The spatial distribution of five species of Rhododendron growing on the Qinghai-Xizang Plateau was modeled under future climate change scenarios (three RCP scenarios). The key ecological niche indicators affecting their distributions were examined. Besides, the distribution of suitable habitats for Rhododendron was simulated for the 2050s and 2070s. Our findings suggest that:
1 Currently, the suitable habitat of Rhododendron nyingchiense is primarily found in the southern region of the Qinghai-Xizang Plateau. Rhododendron laudandum thrives in the eastern part of Xizang, while Rhododendron hypenanthum is predominantly located in the southern areas of Xizang. Rhododendron przewalskii is mainly distributed in the eastern section of the Qinghai-Xizang Plateau, covering parts of Qinghai, Gansu, and Sichuan provinces. Rhododendron trichostomum is predominantly found in eastern Xizang, large portions of Sichuan, and the northern area of Yunnan province.
2 Isothermality plays a crucial role in influencing the distribution of Rhododendron species (excluding Rhododendron przewalskii). Altitude emerges as the key environmental determinant for Rhododendron przewalskii, representing 51% importance, within the preferred range of 2700–3300 m. Altitude holds significance for Rhododendron trichostomum, constituting 18% weightage, within the optimal range of 3200–3900 m. This adaptation allows both Rhododendron przewalskii and Rhododendron trichostomum to thrive in high-altitude, semi-arid regions.
3 The suitable habitat for Rhododendron przewalskii, Rhododendron trichostomum, Rhododendron hypenanthum, and Rhododendron nyingchiense is expanding, while the suitable habitat for Rhododendron laudandum is shrinking.
Data availability
We used open- access data from the CNKI database (http://www.cnki.net/ accessed on 7 October 2022), China Digital Herbarium (https://www.cvh.ac.cn/ accessed on 7 October 2022), NSII China National Herbarium Resource Leveling (http://www.nsii.org.cn/ 2017/home.php accessed on 7 October 2022), Global Biodiversity Information Facility (https://www.gbif.org/zh/ ), China Plant Image Library (http://ppbc.iplant.cn/ accessed on 7 October 2022).
References
Bertrand, R. et al. Changes in plant community composition lag behind climate warming in lowland forests. Nature 479, 517–520. https://doi.org/10.1038/nature10548 (2011).
Butler, C. J., Stanila, B. D., Iverson, J. B., Stone, P. A. & Bryson, M. Projected changes in Climatic suitability for kinosternon turtles by 2050 and 2070. Ecol. Evol. 6, 7690–7705. https://doi.org/10.1002/ece3.2492 (2016).
Chen, I. C., Hill, J. K., Ohlemuller, R., Roy, D. B. & Thomas, C. D. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. https://doi.org/10.1126/science.1206432 (2011).
He, X. et al. Upward elevation and Northwest range shifts for alpine Mec-onopsis species in the Himalaya-Hengduan mountains region. Ecol. Evol. 9, 4055–4064. https://doi.org/10.1002/ece3.5034 (2019).
Quintero, I. & Wiens, J. J. Rates of projected climate change dramatically exceed past rates of Climatic niche evolution among vertebrate species. Ecol. Lett. 16, 1095–1103. https://doi.org/10.1111/ele.12144 (2013).
Fernandez, M., Hamilton, H. H. & Kueppers, L. M. Back to the future: using historical climate variation to project near-term shifts in habitat suitable for Coast Redwood. Glob Chang. Biol. 21, 4141–4152. https://doi.org/10.1111/gcb.13027 (2015).
Sony, R. K., Sandeep, S., Sunil, K., Monalisa, S. & Jayahari, K. M. Niche models inform the effects of climate change on the endangered Nilgiri Tahr (Nilgiritragus hylocrius) populations in the Southern Western Ghats, India. Ecol. Eng. 120, 355–363. https://doi.org/10.1016/j.ecoleng.2018.06.017 (2018).
Remya, K., Ramachandran, A. & ,Jayakumar, S. Predicting the current and future suitable habitat distribution of myristica dactyloides Gaertn. Using maxent model in the Eastern Ghats. India [J] Ecol. Eng. 82, 184–188. https://doi.org/10.1016/j.ecoleng.2015.04.053 (2015).
Yw, A. et al. Chinese caterpillar fungus (Ophiocordyceps sinensis) in China: currentdistribution, trading, and futures under climate change and overexploitation. Sci. Total Environ. 755, 142548. https://doi.org/10.1016/j.scitotenv.2020.142548 (2020).
Li, W. et al. Influence of Tibetan plateau snow cover on East Asian atmospheric circulation at medium-range time scales[J]. Nat. Commun. 9(1), 4243. https://doi.org/10.1038/s41467-018-06762-5 (2018).
Wang, B. et al. Temperature thresholds drive the biogeographic pattern of root endophytic fungal diversity in the Qinghai-Tibet plateau. Sci. Total Environ. 889, 164270. https://doi.org/10.1016/j.scitotenv.2023.164270 (2023).
Ma, B. & Sun, J. Predicting the distribution of stipa purpurea across the Tibetan plateau via the maxent model[J]. BMC Ecol. 18, 1–12. https://doi.org/10.1186/s12898-018-0165-0 (2018).
Wan, D. S. et al. The quaternary evolutionary history, potential distribution dynamics, and conservation implicationsfor a Qinghai-Tibet plateau endemic herbaceous perennial, anisodus tanguticus(Solanaceae). Ecol. Evol. 6, 1977–1995. https://doi.org/10.1002/ece3.2019 (2016).
Prieto-Torres, D. A., Navarro-Siguenza, A. G., Santiago-Alarcon, D. & Rojas-Soto, O. R. Response of the endangered tropical dry forests to climate change and therole of Mexican protected areas for their conservation. Glob Chang. Biol. 22, 364–379. https://doi.org/10.1111/gcb.13090 (2016).
Pauli, H., Gottfried, M., Reiter, K., Klettner, C. & Grabherr, G. Signals of range expansions and contractions of vascular plants in the high Alps: observations (1994–2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Glob Change Biol. 13, 147–156. https://doi.org/10.1111/j.1365-2486.2006.01282.x (2010).
RumpfS.B. et al. Range dynamics of mountain plants decrease with elevation. Proc. Natl. Acad. Sci. USA. 115, 1848–1853. https://doi.org/10.1073/pnas.1713936115 (2018).
Lesica, P. & Steele, B. M. A method for monitoring Long-Term population trends: an example using rare Arctic-Alpine plants. Ecol. Appl. 6, 879–887. https://doi.org/10.2307/2269491 (1996).
Thuiller, W., Lavorel, S., Araujo, M. B., Sykes, M. T. & Prentice, I. C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA. 102, 8245–8250. https://doi.org/10.1073/pnas.0409902102 (2005).
Wang, D. et al. Integrated metabolomics and transcriptomics reveal molecular mechanisms of corolla coloration in rhododendron dauricum L[J]. Plant Physiol. Biochem. 207, 108438 (2024).
Liang, J. et al. Comprehensive evaluation of appreciation of rhododendron based on analytic hierarchy Process[J]. Plants 13(4), 558. https://doi.org/10.3390/plants13040558 (2024).
Syfert, M. M., Smith, M. J. & Coomes, D. A. The effects of sampling bias and model complexity on the predictive performance of maxent species distribution models[J]. PloS One. 8(2), e55158. https://doi.org/10.1371/journal.pone.0055158 (2013).
Fitzpatrick, M. C., Gotelli, N. J. & Ellison, A. M. MaxEnt versus MaxLike: empirical comparisons with ant species distributions. Ecosphere 4 https://doi.org/10.1890/ES13-00066.1 (2013). art55[J].
Gogol-Prokurat Melanie. Predicting habitat suitability for rare plants at local Spatial scales using a species distribution model.[J]. Ecol. Applications: Publication Ecol. Soc. Am. 21(1), 33–47 (2011).
Li, Y. et al. Optimized maxent model predictions of climate change impacts on the suitable distribution of Cunninghamia lanceolata in China[J]. Forests 11(3), 302. https://doi.org/10.3390/f11030302 (2020).
Renner, I. W. & Warton, D. I. Equivalence of MAXENT and Poisson point process models for species distribution modeling in ecology[J]. Biometrics 69(1), 274–281. https://doi.org/10.1111/j.1541-0420.2012.01824.x (2013).
Phillips, S. J., Anderson, R. P. & Schapire, R. E. Maximum entropy modeling of species geographic distributions[J]. Ecol. Model. 190(3–4), 231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026 (2006).
Warren, D. L. & Seifert, S. N. Ecological niche modeling in maxent: the importance of model complexity and the performance of model selection criteria[J]. Ecol. Appl. 21(2), 335–342. https://doi.org/10.1890/10-1171.1 (2011).
Chen, Y. R., Xie, H. M. & Luo, H. L. Impacts of climate change on the distribution of cymbidium Kanran and the simulation of distribution pattern. J. Appl. Ecol. 30, 3419–3425. https://doi.org/10.13287/j.1001-9332.201910.011 (2019).
Jiang, F. et al. Musk deer (Moschus spp.) face redistribution to higher elevations and latitudes under climate change in China[J]. Sci. Total Environ. 704, 135335. https://doi.org/10.1016/j.scitotenv.2019.135335 (2020).
Wei, J. et al. Modeling the potential global distribution of phenacoccus madeirensis green under various climate change scenarios[J]. Forests 10(9), 773. https://doi.org/10.3390/f10090773 (2019).
Hill, M. P. et al. Distribution of cryptic blue oat mite species in Australia: current and future climate conditions[J]. Agric. For. Entomol. 14(2), 127–137 (2012).
Chen, K. et al. MaxEnt modeling to predict the current and future distribution of Pomatosace filicula under climate change scenarios on the Qinghai–Tibet plateau[J]. Plants 11(5), 670. https://doi.org/10.3390/plants11050670 (2022).
Heikkinen, R. K. et al. Methods and uncertainties in bioclimatic envelope modelling under climate change[J]. Prog. Phys. Geogr. 30(6), 751–777. https://doi.org/10.1177/0309133306071957 (2006).
Moreno-Amat, E. et al. Impact of model complexity on cross-temporal transferability in maxent species distribution models: an assessment using paleobotanical data[J]. Ecol. Model. 312, 308–317. https://doi.org/10.1016/j.ecolmodel.2015.05.035 (2015).
Qin, X. & Li, M. Predicting the potential distribution of oxalis debilis Kunth, an invasive species in China with a maximum entropy model[J]. Plants 12(23), 3999. https://doi.org/10.3390/plants12233999 (2023).
Guisan, A., Thuiller, W. & Zimmermann, N. E. Habitat Suitability and Distribution Models: with Applications in R[M] (Cambridge University Press, 2017). https://doi.org/10.1017/9781139028271
Ab Lah, N. Z. et al. Predicting the habitat suitability of melaleuca cajuputi based on the maxent species distribution model[J]. Forests 12(11), 1449. https://doi.org/10.3390/f12111449 (2021).
Phillips, S. J., Dudík, M. & Schapire, R. E. A maximum entropy approach to species distribution modeling[C] Proceedings of the twenty-first international conference on Machine learning. 83. (2004). https://doi.org/10.1145/1015330.1015412
Gong, L. et al. Prediction of potential distribution of soybean in the frigid region in China with maxent modeling[J]. Ecol. Inf. 72, 101834. https://doi.org/10.1016/j.ecoinf.2022.101834 (2022).
Duan, R. Y. et al. The predictive performance and stability of six species distribution models[J]. PloS One. 9(11), e112764. https://doi.org/10.1371/journal.pone.0112764 (2014).
Anderson, R. P. & Gonzalez, I. Jr Species-specific tuning increases robustness to sampling bias in models of species distributions: an implementation with Maxent[J]. Ecol. Model. 222(15), 2796–2811 (2011).
Fourcade, Y. et al. Map** species distributions with MAXENT using a geographically biased sample of presence data: a performance assessment of methods for correcting sampling bias[J]. PloS One. 9(5), e97122. https://doi.org/10.1371/journal.pone.0097122 (2014).
Byeon, D., Jung, S. & Lee, W. H. Review of CLIMEX and maxent for studying species distribution in South Korea[J]. J. Asia-Pacific Biodivers. 11(3), 325–333 (2018).
Zhang, K. et al. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change[J]. Sci. Total Environ. 634, 1326–1334. https://doi.org/10.1016/j.scitotenv.2018.04.112 (2018).
Bradie, J. & Leung, B. A quantitative synthesis of the importance of variables used in maxent species distribution models[J]. J. Biogeogr. 44(6), 1344–1361. https://doi.org/10.1111/jbi.12894 (2017).
Baker, B. B., Moseley, R. K., Yunnan, P. R. & China Advancing treeline and retreating glaciers: Implications for conservation in Arct. Antarct. Alp. Res. 39, 200–209. https://doi.org/10.1657/1523. (2007).
Chen, K. et al. The relationship between niche breadth and phylogenetic characteristics of eight species of rhubarb on the Qinghai-Tibet plateau, Asia[J]. Ecol. Evol. 14(3), e11040. https://doi.org/10.1002/ece3.11040 (2024).
Wang, M. et al. Fungal, but not bacterial, diversity and network complexity promote network stability during roadside slope restoration[J]. Sci. Total Environ. 171007. https://doi.org/10.1016/j.scitotenv.2024.17 (2024).
Yang, X. Q., Kushwaha, S., Saran, S., Xu, J. & Roy, P. S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia Adhatoda L. in lesser Himalayan foothills. Ecol. Eng. 51, 83–87. https://doi.org/10.1016/j.ecoleng.2012.12.004 (2013).
Muhlfeld, C. C. et al. Climate change links fate of glaciers and an endemic alpine invertebrate. Clim. Chang. 106, 337–345. https://doi.org/10.1007/s10584-011-0057-1 (2011).
Yang, L. et al. Predicting the potential distribution of 12 threatened medicinal plants on the Qinghai-Tibet plateau, with a maximum entropy model[J]. Ecol. Evol. 14(2), e11042. https://doi.org/10.1002/ece3.11042 (2024).
Liu, N. et al. The rhododendron plant genome database (RPGD): a comprehensive online omics database for rhododendron. BMC Genom. 22(1), 376. https://doi.org/10.1186/s12864-021-07704-0 (2021).
Yan, L. et al. Climatic warming over the Tibetan plateau paused or continued in recent years? Earth Ocean. Atmos. Sci. 1, 13–28 (2014).
Lenoir, J., Gegout, J. C., Marquet, P. A., de Ruffray, P. & Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 320, 1768–1771. https://doi.org/10.1126/science.115683 (2008).
Acknowledgements
We thank the Qinghai Province key research and development and transformation project (2023-SF-126)and the Qinghai Natural Science Foundation Team Program (2023-ZJ-902T)for financial support.
Author information
Authors and Affiliations
Contributions
C.S.X, M.L.P, M.Z.W, L.Y.Tand Y.Y.Q conducted the data collection, M.L.P, M.Z.W, L.Y.T, Y.Y.Q, W.B, X.Y.Mand Y.Y provided the methodology, W.B, X.Y.M and Z.G.Y provided the concept and the software needed, C.S.X wrote the first draft, the data analysis as well as the visualization, and Z.G.Y provided the acknowledgement and the supervision of the paper. All authors contributed equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no known competing financial, interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chai, SX., Ma, LP., Ma, ZW. et al. Predicting the impact of climate change on the distribution of rhododendron on the qinghai-xizang plateau using maxent model. Sci Rep 15, 10055 (2025). https://doi.org/10.1038/s41598-025-95016-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95016-8