Abstract
This study presents an appealing approach to sustainable catalysis using cellulose filter paper as a support for copper-catalyzed reactions. The paper was functionalized with thiol groups through a reaction with thioglycolic acid, which served a dual purpose: partially reducing Cu(II) to Cu(I) and stabilizing active copper species via Cu–S interactions. Spectroscopic analysis confirmed the formation of highly dispersed multi-valent Cu2O/CuO on the thiol-functionalized cellulose, resulting in a highly efficient copper catalyst. This catalyst demonstrated excellent performance in the oxidative coupling of various amines to imines, achieving yields of 39–99% within 10–30 min. A key advantage of this system is its reusability; the catalyst maintained remarkable stability and activity over ten reaction cycles with straightforward recovery. This paper-based catalyst offers a promising strategy for eco-friendly and cost-effective synthetic processes, with significant implications for green chemistry and industrial applications.
Similar content being viewed by others
Introduction
Copper is a versatile element for a wide range of applications because of its earth-abundant and unique properties such as high electrical and thermal conductivity, high stability, and low cost1. Moreover, copper can exist in multiple oxidation states, enhancing its performance in various important catalytic reactions, for example, oxidation2, coupling3, and click reactions4. Homogeneous copper catalysts typically offer excellent selectivity and high yield of desired products. Nevertheless, a challenge remains in the separation and recycling of these catalysts. To address these, numerous heterogeneous copper catalysts have been developed. In particular, copper catalysts immobilized on supporting materials can enhance activity, selectivity, and stability. High-surface-area and porous materials, such as metal oxides5, activated carbons6, and polymers have been exploited as supports. Among these, polymers offer notable advantages from the diverse fabrication processes to produce the desired structures and the simple modification strategies that can be employed to tune the surface chemistry. Polymers also possess a variety of functional groups, in addition to their mechanical robustness and flexibility, making them excellent for stabilizing metal active species for catalytic applications7,8,9.
Biopolymers have gained significant attention recently due to their renewability, biodegradability, non-toxicity, and cost-effectiveness. As a result, chitosan10, alginate11, and cellulose12 present an intriguing opportunity for the development of environmentally friendly heterogeneous catalysts. Cellulose stands out as one of the most interesting supporting materials due to its abundance and the presence of suitable functional groups for modification with catalysts13,14,15,16,17,18,19,20, amino acids21,22,23, or small biomolecules24,25,26,27,28,29,30. Copper-deposited cellulose-based heterogeneous catalysts have been proven to be highly efficient for a diverse range of reactions, including, hydroboration of alkynes31, click reactions32, and C–N coupling reactions33. In addition, the modification of the abundant hydroxyl groups in cellulose with functional groups like amino, carboxyl, and thiol groups can improve the coordination between cellulose and metal ions34. For example, in the application of cellulose as adsorbents, polyamine molecules have been used to improve the adsorption of copper ions onto cellulose35,36,37. Furthermore, the functionalization of cellulose with multi-carboxyl groups has demonstrated a high adsorption capacity via electrostatic interactions to form complexes with copper on the cellulose surface38,39. The modification of cellulose with sulfur-containing compounds is another effective approach to enhance copper adsorption. Thiosemicarbazone, for instance, has been utilized to modify cellulose, with nitrogen and sulfur atoms forming complexes with Cu(II) ions40. Similarly, the thiol group from the amino acid cysteine has been employed to coordinate with Cu(II) ions, with added ability to reduce Cu(II) to Cu(I)41. More interestingly, a paper-based colorimetric sensing device using thiol-modified cellulose to detect Cu(II) in water has also been developed42. Additionally, modified paper-like materials have been used to stabilize Pd nanoparticles for Suzuki–Miyaura reaction43 and Cr(IV) reduction44. Despite the ease of modifying commercial filter papers, their potential for copper-based catalysis remains largely unexplored.
Imines are valuable chemical intermediates commonly used in the production of pharmaceuticals and agrochemicals. The self-coupling reaction of amines is often preferred for imine synthesis due to its convenience compared to traditional condensation methods involving amines and carbonyl compounds catalyzed by acid catalysts under harsh conditions and long reaction times. Various metal catalysts such as V45,46, Au47,48,49, Pd50,51,52, Ce53, Co54,55,56, and Mn57,58 have been explored to promote the oxidative coupling of amines. Among these, copper-based catalysts stand out because of their remarkable efficiency and affordability, as copper is an earth-abundant and cost-effective alternative to precious metals. Copper-based catalysts are particularly attractive for this reaction, offering high catalytic performance while maintaining economic feasibility59,60,61,62.
However, the use of copper catalysts supported on porous materials, particularly in batch reactions, faces two main challenges. First, active copper species can be inherently unstable under high temperatures and pressures. Second, the separation of powder catalysts after the reaction is time-consuming, often requiring filtration or centrifugation, which can lead to catalyst loss and reduced process efficiency. To solve these problems, cellulose paper is proposed as a supporting material. Unlike powder catalysts, paper-based catalysts can be easily removed from the reaction mixture, simplifying the separation process. Moreover, heteroatoms like sulfur can be introduced onto the cellulose paper to stabilize active copper species, resulting in a higher copper loading and improved catalyst stability.
In this research, cellulose paper is modified with thiol groups via the reaction between thioglycolic acid and the hydroxyl groups of the paper. The thiol groups play a dual role by reducing Cu(II) to Cu(I) and stabilizing the active copper species. The resulting copper catalyst, supported on thiol-functionalized cellulose paper, shows excellent performance in the oxidative coupling reaction of various amines to imines. Moreover, the catalyst was easily recovered and reused for more than ten cycles, highlighting its stability and recyclability. This paper-based catalyst offers a promising strategy for the development of sustainable and eco-friendly synthetic processes.
Results and discussion
Morphology and structure
The color of the filter paper changed from white to light yellow after functionalization with thioglycolic acid (S-paper), and then to dark brown following the copper adsorption (Cu/S-paper), as shown in Supplementary Fig. S1 online. The darkening of Cu/S-paper intensified with longer adsorption times as a result of increasing copper deposition on S-paper. For this study, an adsorption time of 48 h was selected to achieve the maximum copper loading of 6.5% w/w in the prepared catalyst.
The morphology of the prepared materials was examined using field emission scanning electron microscopy (FE-SEM), as illustrated in Fig. 1. SEM images of untreated paper, S-paper, and Cu/S-paper disclosed similar cellulose fiber structures. A small blight flake appeared on the surface of the cellulose fibers in Cu/S-paper, potentially due to the fracture of fibers during the multistep chemical treatments. Despite this, the overall macroscopic integrity of Cu/S-paper remained intact. Elemental mapping by energy dispersive x-ray (EDX) analysis revealed homogeneous distribution of all expected elements. Additionally, SEM/EDX images of the Cu/S-paper cross-section showed a thickness of approximately 200 μm, with S and Cu present both on the surface and within the paper body. The atomic composition of S and Cu was found to be 6.97 and 6.16%, respectively, proposing an S/Cu atomic ratio close to one.
X-ray diffraction (XRD) patterns of the modified papers, shown in Fig. 2a, reveal the diffraction peaks at 2θ values of 15.5, 17.0, 23.0, and 34.5° attributed to (1 \(\overline{1 }\) 0), (1 1 0), (2 0 0), and (0 0 4) planes of the cellulose structure, respectively63. No other crystalline compounds were detected, suggesting good dispersion of copper species. Fourier transform infrared (FT-IR) spectroscopy was employed to validate the presence of thiol groups in the modified cellulose filter paper. In addition to the characteristic vibration signals from cellulose, the FT-IR spectra of S-paper and Cu/S-paper (Fig. 2b) exhibited a peak at 1727 cm−1, which corresponds to carbonyl group in the thioglycolate moiety42. Therefore, the thiol functionalization seemed successful.
(a) XRD patterns, (b) FT-IR spectra, (c) 13C CP MAS NMR spectra, (d) zoom in the spectral range between − 60 and 240 ppm, (e) ESR spectrum, and (f) images and Raman spectra of filter paper (black), S-paper (red), and Cu/S-paper (blue). Note: Spectra in (c) and (d) were measured at 8 kHz. Signals marked with asterisks (*) are spinning sidebands of the signal at 173 ppm. Signals marked with plus ( +) are spinning sidebands of the cellulose signals.
The functional groups present on the modified papers were further studied by 13C solid-state nuclear magnetic resonance (NMR) spectroscopy as shown in Fig. 2c. 13C cross-polarization magic angle spinning (CP MAS) NMR spectra of the filter and modified papers displayed the main characteristic peaks of cellulose at 105, 89, (72, 74), and 66 ppm for C1, C4, (C2, C3, C5), and C6, respectively. Detailed analysis (Fig. 2d) revealed small peaks in the range between 14 and 127 ppm at the noise level assigned to spinning sidebands and components in the paper substrate and a broad underlaying background signal from the NMR probe. Additionally, S-paper exhibited extra peaks at 173, 43, and 28 ppm (signals marked with 1–3) attributed to the ester linkage –COO–30, –CH2–SH, and –CH2–S– groups64,65, respectively. After copper deposition in Cu/S-paper, the intensity of the peak at 173 ppm decreased and the peak at 43 ppm disappeared giving a first hint for the coordination of the thiol group to paramagnetic Cu centers that may quench the signal. In good agreement, electron spin resonance (ESR) analysis of Cu/S-paper revealed a weak paramagnetic signal corresponding to Cu(II) species (Fig. 2e). Interestingly, given the copper content of 6.16 atom.% determined by EDX, a much stronger ESR signal would have been expected if copper were present solely as Cu(II). To further investigate this discrepancy, additional ESR studies were performed to examine the reduction of Cu(II) to Cu(I) during the reaction between thioglycolic acid and Cu(OAc)2. Notably, the Cu(II) signal disappeared entirely after thioglycolic acid was added to the Cu(OAc)2 solution (see Supplementary Fig. S2 online). These results suggested the co-existence of both Cu(II) and Cu(I) species in the Cu/S-paper catalyst.
Furthermore, the Raman spectrum of S-paper in Fig. 2f revealed peaks at 690 and 760 cm−1 corresponding to C–S vibration, and a peak at 941 cm−1 associated with S–H vibration. An additional peak at 502 cm−1 was attributed to S–S vibration perhaps from the oxidation of thiol groups in air. These results align well with the findings from FT-IR and solid-state NMR spectroscopic studies regarding the thiol group functionalization of the paper. Moreover, the interaction between the thiol group and copper was supported by the presence of Cu–S bond vibration at 265 cm−1 in the spectrum of Cu/S-paper. The vibrations related to copper oxides, typically observed at 299, 336, and 633 cm−1 for CuO or 413, 570, and 633 cm−1 for Cu2O66,67, were quite weak and difficult to clearly identify.
The oxidation state and chemical environment of copper in Cu/S-paper were further analyzed by using X-ray photoelectron spectroscopy (XPS). The atomic compositions of sulfur and copper were found to be 6.29 and 6.10%, respectively, closely matching the values obtained by the EDX technique. This resulted in a surface atomic S/Cu ratio of approximately one. The high-resolution XPS spectra of C 1s, O 1s, S 2p, and Cu 2p are depicted in Fig. 3. The C 1s spectrum (Fig. 3a) displayed peaks at 284.8 and 286.4 eV, corresponding to aliphatic carbon (C–C/C–H) and C–O–C/–C–OH groups, respectively, reflecting the cellulose structure of the paper. The peaks at 287.7 and 289.0 eV were attributed to C = O and O–C = O bonds68, respectively, indicating the presence of ester bond formed via the condensation of thioglycolic acid with the hydroxyl groups of cellulose. The O 1s spectrum (Fig. 3b) was deconvoluted into four peaks: 530.6 eV corresponding to O–Cu in copper oxides69,70, along with 531.7, 532.5, and 533.9 eV for O = C, O–C/O–H, and O–C = O, respectively68. Hence, the copper species was likely in the form of copper oxides. The S 2p XPS spectrum (Fig. 3c) revealed binding energies at 164.1 and 165.4 eV for unbound thiol groups, as well as at 162.4 and 163.2 eV for bound thiols, confirming the Cu–S interaction. The ratio of unbound to bound thiols was approximately 3.3, indicating the higher abundance of free thiol groups on Cu/S-paper. The Cu 2p spectrum (Fig. 3d) identified both Cu(I) and Cu(II) species. Cu(I) appeared at binding energies of 930.9 eV (Cu 2p3/2) and 950.9 eV (Cu 2p1/2), while Cu(II) was detected at 932.9 and 953.4 eV42, with a Cu(I)/Cu(II) ratio of approximately 1.9. Therefore, Cu(I) was the dominant species.
In conclusion, the spectroscopic studies confirmed the successful functionalization of the filter paper with thiol groups. These groups partially reduced the Cu(II) precursor to Cu(I) and stabilized the copper species through interactions between Cu and S. The copper species on the Cu/S-paper were primarily present as Cu2O/CuO, as indicated by XPS results, which revealed a Cu(I)/Cu(II) ratio of about 1.9. These multi-valent copper oxides were well dispersed on the modified paper with no aggregation observed by SEM/EDX and no detectable crystalline compounds identified by XRD analysis.
Catalytic activity
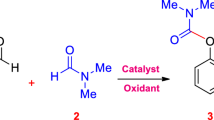
The catalytic performance of the prepared Cu/S-paper was investigated for the self-coupling reaction of benzylamine, using TBHP as the oxidant. The conversion of benzylamine and the yield of N-benzyl-1-phenylmethanimine under various reaction conditions are shown in Fig. 4. In the absence of the catalyst, as well as in the presence of either pristine filter paper or thiol-functionalized S-paper, benzylamine was barely converted to the imine product. However, under the same condition, Cu/S-paper achieved excellent conversion and yield. In addition, increasing the catalyst loading from 5 to 12.5 mg resulted in higher conversion and yield (Fig. 4a). Nonetheless, further increases in the catalyst loading led to a decrease in yield, and by-products such as benzaldehyde and benzonitrile appeared due to the decomposition of N-benzyl-1-phenylmethanimine. Higher reaction temperature also improved conversion and yield (Fig. 4b).
The effect of (a) Cu/S-paper catalyst loading, (b) temperature, and (c) oxidant on the conversion of benzylamine and yield of N-benzyl-1-phenylmethanimine. Reaction condition: 1.25 mmol benzylamine, 2.5 mmol TBHP, 5 mL CH3CN, 25 mg HMB, 12.5 mg Cu/S-paper, 80 °C, and 10 min, unless specified otherwise.
The effect of the amount and type of oxidant was also examined (Fig. 4c). Without any added oxidants, no reaction occurred, indicating that an oxidant is essential for the self-coupling of benzylamine with Cu/S-paper. Increasing the amount of TBHP resulted in higher conversion and yield. Although H2O2 could be employed as the oxidant, it produced lower conversion and yield compared to TBHP. Among the conditions studied, complete conversion of benzylamine and a 96% yield of imine could be achieved within 10 min of reaction in CH3CN at 80 °C, using 12.5 mg of the Cu/S-paper catalyst and 2 eq of TBHP as the oxidant.
To explore the versatility of Cu/S-paper as a catalyst in the coupling of amines, various amine substrates were tested. As shown in Fig. 5, benzylamine derivatives and heteroatom-containing amines produced excellent yields of imine products (2a-2g) within 10–15 min. Furthermore, the coupling of more sterically hindered amines provided high yields ranging from 86 to 99% (2h-2i). Aliphatic amines were also successfully converted to imines in the self-coupling reaction, though with moderate yields (2l-2n). These results demonstrate that Cu/S-paper is highly adaptable for the direct synthesis of imines via oxidative coupling reactions. To further validate the practicality of this catalytic system, a gram-scale reaction was investigated. Starting with 1.39 g of benzylamine, 1.22 g of imine product (2a) were obtained, corresponding to a 96% yield. This underlines the potential of the Cu/S-paper catalyst for scaling up reactions.
When the Cu/S-paper catalyst was placed directly in the reaction mixture, damage to the catalyst paper was observed as illustrated in Fig. 6a. Although the conversions and yields remained consistent over 10 consecutive cycles, wear and tear became increasingly evident, particularly around the edges, due to friction with the magnetic bar during the reaction. However, when the Cu/S-paper was suspended in the center of the reaction mixture using a sewing thread (see Supplementary Fig. S3 online), the integrity of the catalyst paper was preserved as depicted in Fig. 6b. The morphology of the Cu/S-paper after 1, 5, and 10 cycles remained unchanged, with no signs of copper species aggregation detected by SEM/EDX (Fig. 6c) or XRD (see Supplementary Fig. S4). Additionally, the Cu/S-paper catalyst demonstrated excellent reusability, maintaining activity for at least 13 reaction cycles with minimal copper leaching. The copper content found in the reaction medium ranged from 0.3 to 3 ppm, indicating insignificant loss of copper during the catalytic process.
Reusability tests for Cu/S-paper: (a) Cu/S-paper catalyst was placed directly in the reaction mixture, (b) Cu/S-paper catalyst was suspended in the center of the reaction mixture using a sewing thread, and (c) SEM/EDX images of Cu/S-paper after being used by suspending in the reaction mixture. Reaction condition: 1.25 mmol benzylamine, 2.5 mmol TBHP, 5 mL CH3CN, 25 mg HMB, 12.5 mg Cu/S-paper, 80 °C, and 10 min.
Generally, it is difficult to directly compare the catalytic performance of different systems due to variations in catalytic conditions. The performance of several heterogeneous catalysts for the oxidative self-coupling of benzylamine to N-benzyl-1-phenylmethanimine is summarized in Supplementary Table S1. The Cu/S-paper catalyst effectively catalyzed the reaction, achieving a 96% yield within 10 min. While many reported copper- and manganese-based catalysts delivered over 90% yields, they required significantly longer reaction times. More importantly, the Cu/S-paper catalysts demonstrated a higher number of reuse cycles compared to the reported catalysts, along with a much simpler separation process compared to powder catalysts.
Conclusion
In conclusion, the Cu/S-paper catalyst was successfully developed through the modification of cellulose filter paper with thiol groups, followed by the deposition of copper species. The paper catalyst was thoroughly characterized by SEM/EDX, XRD, FT-IR, ssNMR, ESR, Raman, and XPS spectroscopy. The thiol groups not only partially reduced Cu(II) in the precursor to Cu(I) in the Cu/S-paper but also stabilized the multi-valent Cu2O/CuO via Cu–S interactions. This resulted in well-dispersed copper oxides on the functionalized paper. The Cu/S-paper catalyst proved to be an effective and cost-efficient heterogeneous catalyst for the self-coupling reaction of amines. The reaction parameters were evaluated to achieve the best product yield under atmospheric pressure at 80 °C using 2 equivalents of TBHP as the oxidant. In addition to benzylamine, the catalyst successfully converted a variety of amines into their corresponding imines with moderate to excellent yields (39–99%) within 10–30 min. Furthermore, the active Cu2O/CuO remained well stabilized, allowing the Cu/S-paper catalyst to be reused for at least 13 cycles without significant loss of activity. Importantly, the Cu/S-paper catalyst could be easily recovered from the reaction solution, enhancing its practicality. The use of cellulose paper as a support provides a sustainable, cost-effective platform for catalytic applications, aligning with the principles of green chemistry. This work highlights the potential of paper-based catalysts as a versatile solution for industrial and environmental challenges in chemical synthesis.
Methods
Grade 5 qualitative filter paper was obtained from Whatman®. NaOH (≥ 98%, pellets), copper(II) acetate hydrate (Cu(OAc)2·H2O, 98%), tert-butyl hydroperoxide (TBHP, 5.0–6.0 M in decane), hexamethylbenzene (HMB, ≥ 99%), and all amine derivatives were purchased from Sigma-Aldrich. Thioglycolic acid (98%) and p-toluenesulfonic acid (PTSA, 97%) were received from Alfa Aesar. All organic solvents were analytical grade and purchased from RCI Labscan. De-ionized (DI) water was obtained from Nanopure® Analytical Deionization Water System with an electronic resistance ≥ 18.2 MΩ cm.
Preparation of copper catalyst supported on thiol-functionalized cellulose paper (Cu/S-paper)
First, the cellulose filter paper was pretreated as follows: 3 g of the filter paper, ca. 20 pieces measuring 4 × 3.7 cm2 each, were soaked in freshly prepared 10% NaOH solution (300 mL) for 24 h, washed with EtOH (3 × 50 mL), and kept in EtOH (100 mL). Next, the pretreated cellulose filter paper was functionalized with thiol groups using thioglycolic acid42. 600 mg of the pretreated paper, 1 mL of thioglycolic acid, 80 mL of dry toluene, and 90 mg of PTSA were mixed in a Schlenk flask. After that, the mixture was stirred at 100 rpm and refluxed at 110 °C under nitrogen atmosphere overnight. Then, the paper was separated and washed with 30 mL of MeOH, EtOH, acetone, and CH2Cl2, respectively. The thiol-functionalized cellulose paper, denoted S-paper, was dried under vacuum for 1 h and kept under nitrogen. Finally, copper species were deposited on the thiol-functionalized cellulose paper by reacting S-paper with Cu(OAc)2·H2O. 400 mg of dry S-paper were soaked in freshly prepared Cu(OAc)2·H2O solution (30 mM, 25 mL) for 48 h and subsequently washed with DI water (3 × 50 mL) and dried under vacuum at 100 °C for 1 h. This copper catalyst supported thiol-functionalized cellulose paper is denoted Cu/S-paper.
Characterization
Field emission scanning electron microscopy equipped with energy dispersive x-ray (FE-SEM–EDX, 10 kV, HITACHI, SU-8010) operated at 10 kV under high vacuum was used to observe the morphology and determine the elemental dispersion on surface. The samples were mounted on carbon tape attached to an aluminum stub and sputter coated with gold–palladium before SEM observation. Copper content was determined using flame atomic absorption spectroscopy (FAAS) on a PerkinElmer PinAAcle 900 T instrument. A Cu hollow cathode lamp at wavelength 324.5 nm was used with an acetylene flow rate of 2.5 L/min and oxidant (air) flow rate of 10.0 L/min. The structure of the prepared sample was studied by X-ray diffraction (XRD, Bruker D2 Phaser) technique with Cu Kα radiation (λ = 1.54184 Å) operated at 30 kV and 10 mA. Fourier transform infrared (FT-IR) spectra was recorded on Bruker Alpha with ATR technique. Solid-state nuclear magnetic resonance spectroscopy (ssNMR) experiments were performed at a Bruker Avance III 600 MHz operating at 14.1 T corresponding to frequencies of 600.11 MHz for 1H and 150.91 MHz for 13C. All measurements were recorded with a 4 mm H/X broadband probe at 8 kHz spinning using cross-polarization magic angle spinning (CP MAS). The contact time was set to 2.5 ms and a 50–100% ramp was applied on 1H during contact. Each spectrum was accumulated with 2048 scans with a repetition delay of 2 s. During data acquisition tppm1571 decoupling was applied. The spectra were referenced to tetramethylsilane (TMS) employing adamantane (38.5 ppm) as an external standard. Electron spin resonance (ESR) spectroscopy was conducted on a Bruker ELEYS 500 with frequency of 9.8 GHz, microwave power of 2 mW, magnetic field modulation of 100 kHz, and a sweep time of 40 s. Raman spectroscopy was carried out on HORIBA Scientific Xplora plus micro-Raman spectrometer using laser wavelength of 532 nm, laser slit of 100 µm, 1800 gr/mm diffraction grating and a 100 × (0.9 NA) objective. X-ray photoelectron spectroscopy (XPS, Shimadzu AXIS Ultra DLD) equipped with a monochromated Al Kα radiation source at 15 kV, 10 mA, and 150 W was used to study chemical species. Binding energy (BE) values were obtained by curve fitting and referenced to the C 1s band at 284.6 eV.
Catalytic activity
The catalytic activity of Cu/S-paper was evaluated using the self-coupling of benzylamine as a model reaction. Typically, benzylamine (1.25 mmol), TBHP (2.5 mmol), acetonitrile (5 mL), and HMB (25 mg) as the internal standard were mixed in a reaction tube under air. Then, a piece of catalyst paper was suspended in the center of the reaction mixture using a sewing thread as shown in Supplementary Fig. S3. The conversion and yield were monitored by gas chromatography-flame ionization detection spectrometry (GC‐FID with Auto-sampler & Headspace, Agilent Technology GC7890B and 7693A Autosampler). Isolated products were purified by silica column chromatography using ethyl acetate/hexane as eluent. The isolated products were determined by nuclear magnetic resonance spectroscopy (NMR, Bruker DPX-400 MHz Advance). In the substrate scope study, NMR yields were determined by integrating protons of imine products in the 1H NMR spectrum relative to HMB as an internal standard.
Data availability
The datasets generated or analyzed during the current study are included in this article and also available from the corresponding author on reasonable request.
References
Gawande, M. B. et al. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 116, 3722–3811. https://doi.org/10.1021/acs.chemrev.5b00482 (2016).
Li, L. et al. Amphiphilic ligands for Cu-catalyzed aerobic oxidation to synthesize 9-fluorenones in water. Catal. Commun. 127, 34–38. https://doi.org/10.1016/j.catcom.2019.04.022 (2019).
Sahoo, H., Mukherjee, S., Grandhi, G. S., Selvakumar, J. & Baidya, M. Copper catalyzed C-N cross-coupling reaction of aryl boronic acids at room temperature through chelation assistance. J. Org. Chem. 82, 2764–2771. https://doi.org/10.1021/acs.joc.7b00002 (2017).
Shin, J.-A., Oh, S.-J., Lee, H.-Y. & Lim, Y.-G. An efficient Cu-catalyzed azide–alkyne cycloaddition (CuAAC) reaction in aqueous medium with a zwitterionic ligand, betaine. Catal. Sci. Technol. 7, 2450–2456. https://doi.org/10.1039/c7cy00518k (2017).
Chen, M. et al. Re-utilization of spent Cu2+-immobilized MgMn-layered double hydroxide for efficient sulfamethoxazole degradation: Performance and metals synergy. Chem. Eng. J. 392, 123709. https://doi.org/10.1016/j.cej.2019.123709 (2020).
Wang, H. et al. Preparation of Cu-loaded biomass-derived activated carbon catalysts for catalytic wet air oxidation of phenol. Ind. Eng. Chem. Res. 59, 2908–2920. https://doi.org/10.1021/acs.iecr.9b05750 (2020).
Islam, R. U., Taher, A., Choudhary, M., Witcomb, M. J. & Mallick, K. A polymer supported Cu(I) catalyst for the ‘click reaction’ in aqueous media. Dalton Trans. 44, 1341–1349. https://doi.org/10.1039/C4DT02962C (2015).
Chetia, M., Konwar, M., Pegu, B., Konwer, S. & Sarma, D. Synthesis of copper containing polyaniline composites through interfacial polymerisation: An effective catalyst for Click reaction at room temperature. J. Mol. Struct. 1233, 130019. https://doi.org/10.1016/j.molstruc.2021.130019 (2021).
Wonoputri, V. et al. copper complex in poly(vinyl chloride) as a nitric oxide-generating catalyst for the control of nitrifying bacterial biofilms. ACS Appl. Mater. Interfaces 7, 22148–22156. https://doi.org/10.1021/acsami.5b07971 (2015).
Chutimasakul, T. et al. Uniform Cu/chitosan beads as a green and reusable catalyst for facile synthesis of imines via oxidative coupling reaction. RSC Adv. 10, 21009–21018. https://doi.org/10.1039/d0ra03884a (2020).
Rajender Reddy, K., Rajgopal, K. & Lakshmi Kantam, M. Copper-alginates: a biopolymer supported Cu(II) catalyst for 1,3-dipolar cycloaddition of alkynes with azides and oxidative coupling of 2-naphthols and phenols in water. Catal. Lett. 114, 36–40. https://doi.org/10.1007/s10562-007-9032-x (2007).
Yu, W., Jiang, L., Shen, C., Xu, W. & Zhang, P. A highly efficient synthesis of N-glycosyl-1,2,3-triazoles using a recyclable cellulose-copper(0) catalyst in water. Catal. Commun. 79, 11–16. https://doi.org/10.1016/j.catcom.2016.02.015 (2016).
Gutmann, T., Groszewicz, P. B. & Buntkowsky, G. in Annual reports on NMR spectroscopy Vol. 97 (ed Graham A. Webb) 1–82 (Academic Press, 2019). https://doi.org/10.1016/bs.arnmr.2018.12.001
Chutimasakul, T. et al. Size-controlled preparation of gold nanoparticles deposited on surface-fibrillated cellulose obtained by citric acid modification. ACS Omega 5, 33206–33213. https://doi.org/10.1021/acsomega.0c04894 (2020).
Quignard, F. & Choplin, A. Cellulose: A new bio-support for aqueous phase catalysts. Chem. Commun. https://doi.org/10.1039/B007776N (2001).
Zhou, Z., Lu, C., Wu, X. & Zhang, X. Cellulose nanocrystals as a novel support for CuO nanoparticles catalysts: Facile synthesis and their application to 4-nitrophenol reduction. RSC Adv. 3, 26066–26073. https://doi.org/10.1039/C3RA43006E (2013).
Koga, H. et al. Topochemical synthesis and catalysis of metal nanoparticles exposed on crystalline cellulose nanofibers. Chem. Commun. 46, 8567–8569. https://doi.org/10.1039/C0CC02754E (2010).
Shin, Y., Bae, I.-T., Arey, B. W. & Exarhos, G. J. Facile stabilization of gold-silver alloy nanoparticles on cellulose nanocrystal. J. Phys. Chem. C 112, 4844–4848. https://doi.org/10.1021/jp710767w (2008).
Liu, J. et al. Design of a heterogeneous catalyst based on cellulose nanocrystals for cyclopropanation: Synthesis and solid-state NMR characterization. Chem. Eur. J. 21, 12414–12420. https://doi.org/10.1002/chem.201501151 (2015).
Rösler, L. et al. Dirhodium complex immobilization on modified cellulose for highly selective heterogeneous cyclopropanation reactions. Cellulose 29, 6283–6299. https://doi.org/10.1007/s10570-022-04654-y (2022).
Kalaskar, D. M. et al. Characterisation of amino acid modified cellulose surfaces using TOF-SIMS and XPS. Cellulose 17, 747–756. https://doi.org/10.1007/s10570-010-9413-y (2010).
Cateto, C. A. & Ragauskas, A. Amino acid modified cellulose whiskers. RSC Adv. 1, 1695–1697. https://doi.org/10.1039/C1RA00647A (2011).
Höfler, M. V. et al. Fluorine-labeled N-Boc-L-proline as a marker for solid-state NMR characterization of biofunctionalizations on paper substrates. J. Phys. Chem. C 127, 3570–3578. https://doi.org/10.1021/acs.jpcc.2c08370 (2023).
Jirakittiwut, N., Panyain, N., Nuanyai, T., Vilaivan, T. & Praneenararat, T. Pyrrolidinyl peptide nucleic acids immobilised on cellulose paper as a DNA sensor. RSC Adv. 5, 24110–24114. https://doi.org/10.1039/C4RA15287E (2015).
Ohkawa, K., Nishibayashi, M., Devarayan, K., Hachisu, M. & Araki, J. Synthesis of peptide-cellulose conjugate mediated by a soluble cellulose derivative having β-ala esters. Int. J. Biol. Macromol. 53, 150–159. https://doi.org/10.1016/j.ijbiomac.2012.11.004 (2013).
Hilberg, V. et al. Light-controlled chemoenzymatic immobilization of proteins towards engineering of bioactive papers. Chem. Eur. J. 25, 1746–1751. https://doi.org/10.1002/chem.201804889 (2019).
Liebich, V. J. et al. Toward fabrication of bioactive papers: Covalent immobilization of peptides and proteins. Biomacromolecules 22, 2954–2962. https://doi.org/10.1021/acs.biomac.1c00354 (2021).
Khazanov, N., Iline-Vul, T., Noy, E., Goobes, G. & Senderowitz, H. Design of compact biomimetic cellulose binding peptides as carriers for cellulose catalytic degradation. J. Phys. Chem. B 120, 309–319. https://doi.org/10.1021/acs.jpcb.5b11050 (2016).
Tischer, T. et al. Modular ligation of thioamide functional peptides onto solid cellulose substrates. Adv. Funct. Mater. 22, 3853–3864. https://doi.org/10.1002/adfm.201200266 (2012).
Limprasart, W. et al. Peptides as model systems for biofunctionalizations of cellulose─synthesis and structural characterization by advanced solid-state nuclear magnetic resonance techniques. J. Phys. Chem. C 127, 22129–22138. https://doi.org/10.1021/acs.jpcc.3c05068 (2023).
Zhang, C. et al. Copper-loaded nanocellulose sponge as a sustainable catalyst for regioselective hydroboration of alkynes. Carbohydr. Polym. 191, 17–24. https://doi.org/10.1016/j.carbpol.2018.03.002 (2018).
Bahsis, L. et al. Cellulose-copper as bio-supported recyclable catalyst for the clickable azide-alkyne [3 + 2] cycloaddition reaction in water. Int. J. Biol. Macromol. 119, 849–856. https://doi.org/10.1016/j.ijbiomac.2018.07.200 (2018).
Goswami, M. & Das, A. M. Synthesis of cellulose impregnated copper nanoparticles as an efficient heterogeneous catalyst for CN coupling reactions under mild conditions. Carbohydr. Polym. 195, 189–198. https://doi.org/10.1016/j.carbpol.2018.04.033 (2018).
Gupta, A. D., Pandey, S., Jaiswal, V. K., Bhadauria, V. & Singh, H. Simultaneous oxidation and esterification of cellulose for use in treatment of water containing Cu(II) ions. Carbohydr. Polym. 222, 114964. https://doi.org/10.1016/j.carbpol.2019.06.003 (2019).
Manzoor, K., Ahmad, M., Ahmad, S. & Ikram, S. Synthesis, characterization, kinetics, and thermodynamics of edta-modified chitosan-carboxymethyl cellulose as Cu(II) ion adsorbent. ACS Omega 4, 17425–17437. https://doi.org/10.1021/acsomega.9b02214 (2019).
Nongbe, M. C. et al. Cellulose paper grafted with polyamines as powerful adsorbent for heavy metals. Cellulose 25, 4043–4055. https://doi.org/10.1007/s10570-018-1833-0 (2018).
Zhan, W. et al. Adsorption of Cu(II), Zn(II), and Pb(II) from aqueous single and binary metal solutions by regenerated cellulose and sodium alginate chemically modified with polyethyleneimine. RSC Adv. 8, 18723–18733. https://doi.org/10.1039/c8ra02055h (2018).
Liu, X., Lin, B., Zhang, Z., Lei, H. & Li, Y. Copper(II) carboxymethylcellulose (CMC-CuII) as an efficient catalyst for aldehyde–alkyne–amine coupling under solvent-free conditions. RSC Adv. 6, 94399–94407. https://doi.org/10.1039/c6ra18742k (2016).
Wang, J., Liu, M., Duan, C., Sun, J. & Xu, Y. Preparation and characterization of cellulose-based adsorbent and its application in heavy metal ions removal. Carbohydr. Polym. 206, 837–843. https://doi.org/10.1016/j.carbpol.2018.11.059 (2019).
Nguyen, T. A., Tran, D. B., Le, H. D. C., Nguyen, Q. L. & Pham, V. Thiosemicarbazone-modified cellulose: synthesis, characterization, and adsorption studies on Cu(II) removal. ACS Omega 5, 14481–14493. https://doi.org/10.1021/acsomega.0c01129 (2020).
Maiti, B. K. et al. Unusual reduction mechanism of copper in cysteine-rich environment. Inorg. Chem. 57, 8078–8088. https://doi.org/10.1021/acs.inorgchem.8b00121 (2018).
Rull-Barrull, J., d’Halluin, M., Le Grognec, E. & Felpin, F.-X. A paper-based biomimetic device for the reduction of Cu(II) to Cu(I) – application to the sensing of Cu(II). Chem. Commun. 52, 6569–6572. https://doi.org/10.1039/C6CC02305C (2016).
Xiang, Z., Chen, Y., Liu, Q. & Lu, F. A highly recyclable dip-catalyst produced from palladium nanoparticle-embedded bacterial cellulose and plant fibers. Green Chem. 20, 1085–1094. https://doi.org/10.1039/C7GC02835K (2018).
Zhao, Y., Liu, L., Shi, D., Shi, X. & Shen, M. Performing a catalysis reaction on filter paper: development of a metal palladium nanoparticle-based catalyst. Nanoscale Adv. 1, 342–346. https://doi.org/10.1039/c8na00095f (2019).
Chu, G. & Li, C. Convenient and clean synthesis of imines from primary benzylamines. Org. Biomol. Chem. 8, 4716–4719. https://doi.org/10.1039/C0OB00043D (2010).
Kodama, S. et al. Direct conversion of benzylamines to imines via atmospheric oxidation in the presence of VO(HHPIC)2 catalyst. Tetrahedron Lett. 51, 2450–2452. https://doi.org/10.1016/j.tetlet.2010.02.150 (2010).
Neeli, C. K. P., Ravi Kumar, M., Saidulu, G., Rama Rao, K. S. & Burri, D. R. Selective oxidation of benzylamine to n-benzyl benzaldimine over nanogold immobilized SBA-15 under solvent-free conditions. J. Chem. Technol. Biotechnol. 90, 1657–1664. https://doi.org/10.1002/jctb.4474 (2015).
Opriş, C. M. et al. New multicomponent catalysts for the selective aerobic oxidative condensation of benzylamine to N-benzylidenebenzylamine. Catal. Sci. Technol. 4, 4340–4355. https://doi.org/10.1039/C4CY00795F (2014).
Neeli, C. K. P., Ganji, S., Ganjala, V. S. P., Kamaraju, S. R. R. & Burri, D. R. Oxidative coupling of primary amines to imines under base-free and additive-free conditions over AuNPs/SBA-NH2 nanocatalyst. RSC Adv. 4, 14128–14135. https://doi.org/10.1039/C4RA00791C (2014).
Meilin, J. et al. Performance of supported Au-Pd alloy nano particles catalyst for base-free synthesis of imines by self-coupling of amine. Rare Metal Mater. Eng. 47, 442–446. https://doi.org/10.1016/S1875-5372(18)30085-7 (2018).
Rodríguez-Lugo, R. E. et al. Synthesis, characterization and Pd(II)-coordination chemistry of the ligand tris(quinolin-8-yl)phosphite. Application in the catalytic aerobic oxidation of amines. Dalton Trans. 47, 2061–2072. https://doi.org/10.1039/C7DT04000H (2018).
Xu, J., Li, J., Yang, K. & Li, H. Efficient gold–palladium nanoparticles stabilized by poly(amic acid) salt: Synthesis and application in catalytic oxidation of amines to imines. J. Inorg. Organomet. Polym. Mater. 30, 1384–1392. https://doi.org/10.1007/s10904-019-01317-7 (2020).
Sudarsanam, P., Rangaswamy, A. & Reddy, B. M. An efficient noble metal-free Ce–Sm/SiO2 nano-oxide catalyst for oxidation of benzylamines under ecofriendly conditions. RSC Adv. 4, 46378–46382. https://doi.org/10.1039/C4RA04397A (2014).
Ge, D. et al. Novel transition bimetal–organic frameworks: Recyclable catalyst for the oxidative coupling of primary amines to imines at mild conditions. New J. Chem. 40, 5531–5536. https://doi.org/10.1039/C5NJ03544A (2016).
Hazra, S., Pilania, P., Deb, M., Kushawaha, A. K. & Elias, A. J. Aerobic oxidation of primary amines to imines in water using a cobalt complex as recyclable catalyst under mild conditions. Chem. Eur. J. 24, 15766–15771. https://doi.org/10.1002/chem.201803251 (2018).
Zhang, C. et al. Co–N–C supported on SiO2: A facile, efficient catalyst for aerobic oxidation of amines to imines. RSC Adv. 7, 47366–47372. https://doi.org/10.1039/C7RA09516C (2017).
Zhang, Z. et al. tert-Butyl hydroperoxide (TBHP)-mediated oxidative self-coupling of amines to imines over a α-MnO2 catalyst. Green Chem. 16, 2523–2527. https://doi.org/10.1039/C3GC42312C (2014).
Jia, X. et al. Switching acidity on manganese oxide catalyst with acetylacetones for selectivity-tunable amines oxidation. Nat. Commun. 10, 2338. https://doi.org/10.1038/s41467-019-10315-9 (2019).
Patil, R. D. & Adimurthy, S. Catalytic methods for imine synthesis. Asian J. Org. Chem. 2, 726–744. https://doi.org/10.1002/ajoc.201300012 (2013).
Marui, K., Nomoto, A., Ueshima, M. & Ogawa, A. Eco-friendly copper sulfate-catalyzed oxidation of amines to imines by hydrogen peroxide in water. Tetrahedron Lett. 56, 1200–1202. https://doi.org/10.1016/j.tetlet.2015.01.090 (2015).
Hu, Z. & Kerton, F. M. Simple copper/TEMPO catalyzed aerobic dehydrogenation of benzylic amines and anilines. Org. Biomol. Chem. 10, 1618–1624. https://doi.org/10.1039/C2OB06670J (2012).
Liu, H., Chuah, G.-K. & Jaenicke, S. Self-coupling of benzylamines over a highly active and selective supported copper catalyst to produce N-substituted amines by the borrowing hydrogen method. J. Catal. 329, 262–268. https://doi.org/10.1016/j.jcat.2015.05.029 (2015).
Beyene, D. et al. Characterization of cellulase-treated fibers and resulting cellulose nanocrystals generated through acid hydrolysis. Materials (Basel) 11, 1272. https://doi.org/10.3390/ma11081272 (2018).
Arakaki, L. N. H. et al. Thioglycolic acid grafted onto silica gel and its properties in relation to extracting cations from ethanolic solution determined by calorimetric technique. J. Colloid Interface Sci. 273, 211–217. https://doi.org/10.1016/j.jcis.2004.01.006 (2004).
Silva Filho, E. C., Lima, L. C. B., Sousa, K. S., Fonseca, M. G. & Pereira, F. A. R. Calorimetry studies for interaction in solid/liquid interface between the modified cellulose and divalent cation. J. Therm. Anal. Calorim. 114, 57–66. https://doi.org/10.1007/s10973-012-2868-3 (2013).
Akgul, F. A., Akgul, G., Yildirim, N., Unalan, H. E. & Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 147, 987–995. https://doi.org/10.1016/j.matchemphys.2014.06.047 (2014).
Ravichandiran, C. et al. Influence of rare earth material (Sm3+) doping on the properties of electrodeposited Cu2O films for optoelectronics. J. Mater. Sci.:Mater Electron. 30, 2530–2537. https://doi.org/10.1007/s10854-018-0527-6 (2019).
Mansur, A. A. P. et al. Carboxymethylcellulose/ZnCdS fluorescent quantum dot nanoconjugates for cancer cell bioimaging. Int. J. Biol. Macromol. 96, 675–686. https://doi.org/10.1016/j.ijbiomac.2016.12.078 (2017).
Piñon-Espitia, M., Lardizabal-Gutiérrez, D., Camacho-Ríos, M. L., Herrera-Pérez, G. & Ochoa-Lara, M. T. Electronic structure comparison of Cu 2p and O 1s X-Ray photoelectron spectra for CuxO nanofibers (x = 1, 2, i). Mater. Chem. Phys. 272, 124981. https://doi.org/10.1016/j.matchemphys.2021.124981 (2021).
Wang, Y. et al. Synthesis of porous Cu2O/CuO cages using Cu-based metal–organic frameworks as templates and their gas-sensing properties. J. Mater. Chem. A 3, 12796–12803. https://doi.org/10.1039/C5TA01108F (2015).
Bennett, A., Rienstra, C., Auger, M., Lakshmi, K. & Griffin, R. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 103, 6951. https://doi.org/10.1063/1.470372 (1995).
Acknowledgements
This research project has been supported by Mahidol University (Fundamental Fund: fiscal year 2025 by National Science Research and Innovation Fund (NSRF)). J.S. and W.L. also thank the Development and Promotion of Science and Technology Talents (DPST) Project for their scholarship. T.G. and M.V.H. gratefully acknowledge the German Science Foundation (DFG) under contract GU-1650/3 − 2 project number 429632542 for financial support. We thank Prof. Buntkowsky (TU Darmstadt) for generous allocation of measurement time at his Bruker Avance III HD 600 MHz spectrometer .
Author information
Authors and Affiliations
Contributions
J.S. and W.L. contributed equally to the research work. J.S. was the primary writer of the manuscript and J.T. did the major edition. J.S. and W.L. designed and performed the preparation and characterization of the catalysts as well as the catalytic activity studies. M.H. and T.G. performed experiments and analysis for the ssNMR studies. S.P. did the ESR measurement and assisted with data interpretation. T.B. gave suggestions on the preparation of thiol functionalized paper. Overall data analysis was carried out by J.S. and W.L. with consultation with J.T., who conceptualized the research and received the funding. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sangkaworn, J., Limprasart, W., Höfler, M.V. et al. Copper-supported thiol-functionalized cellulose as a paper-based catalyst for imine synthesis. Sci Rep 15, 9893 (2025). https://doi.org/10.1038/s41598-025-95144-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95144-1