Abstract
The aim of the study was to determine the incidence of chemobrain in breast cancer patients, define it, and review the various tools used to measure it. A comprehensive literature review was performed, and covered all publications up to and including September 30, 2024. Studies were eligible for inclusion if they reported data on chemobrain in adults with breast cancer receiving chemotherapy. The following data were extracted: study characteristics, information on chemobrain, and information on breast cancer treatment. A total of 287 records were identified by the literature search. After eliminating duplicates, irrelevant articles after title and abstract screening, or after full-text review, 11 records were included in the study. The incidence across studies ranged from 9.6 to 81.0%. The pooled incidence of chemobrain was estimated at 39.9% (95% CI 26.3%, 55.2%). Chemobrain appears to affect a significant proportion of breast cancer patients undergoing chemotherapy, with a pooled incidence estimate of approximately 40%. However, the wide range of reported incidences (9.6–81%) suggests that the true prevalence may vary depending on study design, definitions, and assessment tools used.
Similar content being viewed by others
Introduction
The use of chemotherapy has greatly improved survival in patients with breast cancer1. However, on top of their undeniable therapeutic benefits, the various chemotherapy agents may also have some undesirable short, medium and long-term effects, notably on the peripheral and central nervous system (CNS). Cognitive disorders occurring after chemotherapy, termed “chemobrain” or “chemofog”, are among the side effects that may occur when chemotherapy for breast cancer affects the CNS2,3. The impact of these post-chemotherapy cognitive disorders is increasing steadily, and with the improved longevity of breast cancer survivors, cognitive disorders may persist for up to several years2,4.
Chemobrain may affect millions of cancer survivors around the world3. Researchers are gaining a better understanding of the brain changes caused by chemotherapy, thanks to studies using lab experiments, brain imaging, and clinical trials3,5,6,7,8. Chemotherapy can affect the brain in many ways. It can make the blood-brain barrier more permeable, which allows harmful substances to enter and increase inflammation8. Chemotherapy can also affect the mitochondria, causing more oxidative stress6. It disrupts how brain cells move and grow3, and can lead to cell death while slowing down the growth of new brain cells3. These various impairments become clinically manifest in the form of cognitive difficulties, with variable clinical presentations depending on the chemotherapy molecule used. The cognitive disorders may occur during or after treatment. The cognitive domains that are most frequently affected are concentration, episodic memory, processing speed, verbal fluency, visuo-spatial ability and mental flexibility7.
The incidence of chemobrain in patients treated by chemotherapy for breast cancer is not clearly established, and varies from 10% to over 80% across reports9,10. Furthermore, although it is now well known that cognitive disorders may occur after chemotherapy, there is no consensus to date on the definition of chemobrain, or how it should be measured. Indeed, the lack of standardized definition is a key obstacle to the development of management strategies for this phenomenon.
In this context, the primary aim of this meta-analysis was to determine the incidence of chemobrain in patients who received chemotherapy for breast cancer. Secondary objectives were to describe the various definitions of chemobrain used in the literature, and to make an inventory of the different tools used to measure chemobrain.
Methods
The research question to be answered by this systematic review was to determine the incidence of chemobrain, the definitions of chemobrain, and the tools used to measure chemobrain in adults with breast cancer.
Search strategy
A comprehensive literature search was performed using Scopus© and PubMed©. The literature search covered all publications up to and including September 30, 2024, with no specific start date specified. The search algorithm was defined by two senior researchers (LG, MD) and included the following keywords in the title: chemobrain or chemotherapy-related cognitive impairment or chemo-fog. Filters were applied to select studies in English, French or Spanish, including human beings only. Additional studies were identified by manual review of the reference lists of retrieved studies. The authors of the identified studies were contacted to recover unpublished data when available. Study selection was performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. This review was registered with PROSPERO, under the number CRD42024590463.
Study selection criteria
Study eligibility criteria were defined before performing the literature search. Studies were eligible for inclusion if they reported data on chemobrain in adults with breast cancer receiving chemotherapy. Basic science articles, reviews, case reports, editorials, and correspondence were excluded. Case series of less than 10 subjects were also excluded.
Data extraction
Data analysis was performed using Covidence systematic review software© (Veritas Health Innovation, Melbourne, Australia), available at www.covidence.org (September 2024). After eliminating duplicates, two senior researchers independently anonymously reviewed the titles and abstracts of all articles. In case of disagreement about whether or not to include an article, the case was discussed until consensus was reached. The researchers then independently extracted the data using the same data extraction form. The following data were extracted: study characteristics (publication year, country, study design, sample size, mean and/or median age), information on chemobrain (definitions, tools used for assessment, number of events), and information on breast cancer treatment (chemotherapy regimen, time since treatment completion, and concurrent therapy). When several groups were compared in a publication, only the group receiving chemotherapy was included in the systematic review.
Quality assessment
The quality of included studies was independently assessed by two researchers (LG, MD) using the Newcastle-Ottawa scale (NOS)11. The NOS consists of three quality parameters: selection, comparability, and outcome assessment. It assigns a maximum of four points (five points for cross-sectional studies) for selection, two points for comparability, and three points for outcome. NOS scores of 7 or higher were considered as high quality studies, 5–6 as moderate quality, and scores below 5 as poor quality.
Meta-analysis
The pooled incidence of chemobrain was calculated using a DerSimonian-Laird random-effects meta-analysis12. When there is no heterogeneity between studies, the random-effects and the fixed-effect methods are identical. However, when heterogeneity was present, random-effects summary estimates were considered. To assess the heterogeneity across studies in the meta-analysis, the I2-statistic was calculated. Meta-analysis was performed using the “Metaprop” function in the R package “meta”, release 7.0–0 (2024-01-11) (R Foundation for Statistical Computing, Vienna, Austria) (14).
Results
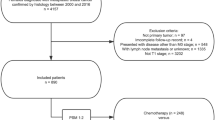
A total of 287 records were identified by the literature search (Fig. 1), including 61 duplicates. After examining the titles and abstracts of the remaining 226 records, 40 were retained for full-text assessment. After reading the full text of these 40 records, 29 were excluded because of wrong outcome, or wrong study population, or wrong language, or wrong study design, or overlapping data. Thus, 11 studies were included in the final review, totalling 1814 patients9,10,13,14,15,16,17,18,19,20,21.
As shown in Table 1, the majority of studies included in the systematic review were prospective cohorts (8 out of 11)9,10,13,14,15,16,17,20. The studies often involved populations with a mean age of 50 years or more (6 out of 10 studies9,14,15,16,18,19; age information was missing in one study21). Chemotherapy was combined with adjuvant treatment in 8 out of 11 studies13,14,15,16,17,18,19,21. Radiotherapy and hormone therapy were each used six times13,14,15,16,17,18; in four cases, they were both combined with chemotherapy13,15,17,18. Cognitive performances were tested before chemotherapy in 8 out of 11 studies9,10,13,14,15,16,17,20.
Table 2 presents the different definitions of chemobrain, as well as the tools used to assess it. In 6 out of 11 studies, objective assessment tools for cognitive decline were used9,10,16,17,18,21. Among the assessment tools for subjective cognitive impairment, the FACT-Cog was the most frequently used.
Figure 2 presents the overall pooled incidence of chemobrain. The incidence across studies ranged from 9.6 to 81.0%. Based on random-effects model analysis, due to high heterogeneity between studies (I2 = 97%; p < 0.01), the pooled incidence of chemobrain was estimated at 39.9% (95% CI 26.3%, 55.2%). As shown in the supplementary material, subgroup analyses of the incidence of chemobrain showed no statistically significant differences between age groups (age < 50 years: 49.8%; age > = 50 years:27.1%; p = 0.07) (Supplementary material S1), according to the presence or absence of adjuvant treatment (yes: 38.9%; no:42.2%; p = 0.92) (Supplementary material S2), according to the nature of the test used (objective test: 48.7%; subjective test: 27.0%; p = 0.11) (Supplementary material S3), or according to the time since completion of chemotherapy (< 6 months: 42.7%; ≥ 6 months: 34.4%; p = 0.69) (Supplementary material S4).
The quality of the studies (Table 3) was considered high for nine studies, and moderate for two.
Discussion
In this meta-analysis, the pooled incidence of chemobrain was estimated at 39.9% (95% CI 26.3%, 55.2%) in patients with breast cancer receiving chemotherapy. Time since completion of chemotherapy varied from 1 week to 2 years according to studies. Age (< 50 years or > = 50 years), presence or absence of adjuvant treatment, the nature of the test used to assess chemobrain (objective or subjective), and time since completion of chemotherapy had no influence on the pooled incidence of chemobrain.
For more than 40 years, cognitive impairment has been well known as a side-effect of chemotherapy7,22. Nevertheless, the incidence of chemobrain with the various chemotherapy regimens remains poorly documented, and there is no recommended, consensual strategy for its management. Several authors have reported that chemobrain has received insufficient attention in cancer survivors most likely because a lack of knowledge about its diagnosis and treatment. Millions of cancer survivors each year are affected by chemobrain, which can persist for the long-term2,23,24. The effects of chemotherapy on the CNS include, amongst others, a reduction in neurogenesis, an increase in apoptosis in the hippocampus and cortex, mitochondrial dysfunction with reduction production of ATP, disturbances in microtubule function that compromise neuronal migration, and vascular lesions associated with neuro-inflammation3,6. Taken together, these changes may translate into multi-domain cognitive dysfunction. Chemobrain is commonly defined as cognitive impairment including difficulties in verbal or visuo-spatial ability, information processing speed, and executive function25. Yet, there is no consensus on a definition or a recommended diagnostic test. In our systematic review about chemobrain in breast cancer, among the 11 articles studied, we found 9 different definitions for chemobrain (Table 2). Most often, chemobrain was defined by the scores achieved on cognitive assessment tools. This reflects the difficult of defining chemobrain accurately, in the absence of a consensual definition. The wide range of possible impairments7 compounds the difficulty of defining chemobrain precisely, and measuring its prevalence or incidence is difficult when different definitions are being used. The same is valid for the tools used to measure chemobrain. To determine whether or not chemobrain is present, in our systematic review, two different types of tools were mainly used, namely objective and subjective tools. Four studies used 2 or more tools10,16,17,18, all of which were objective. Seven studies used only one tool9,13,14,15,19,20,21, which was subjective in 5 of these (FACT-Cog or Squire memory Self-rating Questionnaire). In the 11 studies of our systematic review, more than 20 different objective tools and 2 subjective tools were used by the different authors. This reflects the difficulty for authors wishing to explore chemobrain of finding suitable objective tests for the purposes of screening or assessing chemobrain. Indeed, the methods used to develop assessment scales for cognitive function influence their capacity to detect or diagnose cognitive disorders in specific domains (language, executive function, working memory, etc.). The tests that are commonly used in neuropsychiatric evaluation were not specifically developed for the purpose of detecting cognitive disorders occurring post-chemotherapy, and thus, their performance may be suboptimal when used to this end. For this reason, some authors have questioned the capacity of common objective tools to detect chemobrain26. Despite the reserves expressed in the literature, our meta-analysis did not reveal any significant difference in the incidence of chemobrain reported with subjective versus objective tools. However, the total number of studies in our meta-analysis is relatively low, and this result warrants confirmation in larger-scale analyses.
Specific tests to screen for chemobrain have been developed, mainly in the form of self-report questionnaires. Von Ah et al. demonstrated that subjective tests developed specifically for detecting chemobrain (including the Functional Assessment of Cancer Therapy - Cognitive function, FACT-Cog) were correlated with objective tests, and could therefore be used to screen for chemobrain. Accordingly, the FACT-Cog has been widely studied and used, and has been adapted and validated in numerous languages27,28,29. It is a 37-item self-report questionnaire for use in cancer patients aged 18 years and older with chemotherapy-induced cognitive problems. There are 4 subscales, namely perceived cognitive impairments, impact of perceived cognitive impairments on quality of life, comments from others, and perceived cognitive abilities. They explore the individual’s perception of their own problems, but also the feedback from their entourage and the impact on their quality of life. Normal values have been defined based on age30, making it easy to use. Among the subjective tools used to measure the presence of chemobrain in this systematic review, the FACT-Cog was the most commonly used9,19,20. The use of subjective instruments, specifically the FACT-Cog, is easy for several reasons. First, it is a self-report questionnaire that the patient can complete on their own, so the completion is not time-consuming. Second, completion requires no specific learning phase and no complex instructions, contrary to certain objective tests. Third, the FACT-Cog has been adapted and validated in numerous languages, and finally, minimum scores have been identified to define improvement or deterioration in cognitive performance, which is useful for patient follow-up27. Taken together, all these features combine to make the FACT-Cog a user-friendly and attractive option for both clinical follow-up and for research purposes.
It would be beneficial if recommendations could be issued for the definition of chemobrain, and the instruments that are valid for its detection and follow-up. This said, detection and management should not be the only concern. Indeed, chemobrain has a negative impact on the quality of life of cancer survivors and their capacity to return to a normal life. There is a compelling need to identify strategies to limit post-chemotherapy cognitive disorders and promote cognitive recovery in patients receiving chemotherapy. A consensual definition of chemobrain, and a consensus on the most appropriate tools to detect and measure it could facilitate the implementation of studies on the management of chemobrain. Currently, there are no recommendations for the management of this entity, and various treatments have been proposed, such as Ginko Biloba31, prebiotics32, or cognitive rehabilitation33,34. However, the level of evidence remains low. Non-pharmaceutical therapies such as photobiomodulation, with proven efficacy in improving cognitive disorders of other aetiologies, warrant investigation in the setting of chemobrain.
This study has several strengths. It is the first meta-analysis to describe the incidence of chemobrain in breast cancer survivors. We included 11 studies, totalling over 1800 patients. The majority of studies included evaluated the cognitive performance of the study population prior to chemotherapy9,10,13,14,15,16,17,20, which strengthens the association between the chemotherapy and the cognitive disorders. Lastly, the quality of studies included was high overall. A major limitation of this meta-analysis is the lack of consensus on the definition and measurement of chemobrain across studies. The wide variety of assessment tools and definitions used may contribute to the high heterogeneity observed in the incidence estimates. Consequently, the measure of incidence may be imprecise. While a random-effects model was used to account for heterogeneity, the high I² statistic (97%) suggests substantial variation across studies. Future meta-analyses should consider exploring sources of heterogeneity, such as study design or patient population characteristics. Depending on the sensitivity of the tools used to determine the presence or absence of cognitive disorders, the incidence may have been over- or underestimated. In addition, most studies did not adjust for the possible presence of anxiety and/or depression, which could compound cognitive disorders and lead them to be incorrectly classed as a side effect of chemotherapy. Finally, the time since completion of chemotherapy and the determination of the presence of cognitive disorders varied across studies. Some patients may observe a spontaneous improvement in cognitive function at a distance from chemotherapy, notably due to the resolution of post-treatment asthenia. However, in our meta-analysis, analysis in subgroups according to the time since completion of chemotherapy (< 6 months; ≥ 6 months) did not reveal any significant difference in incidence between groups. The fact that we excluded trials because of language may have introduced a bias. However, we included other languages such as French and Spanish. Only one article in Korean was excluded for this reason.
In conclusion, chemobrain is frequent, affecting around 40% of patients receiving chemotherapy for breast cancer. There is currently no consensus on the definition of chemobrain, or the tools that could be used to identify it. This study underlines the multiplicity of definitions and assessment tools in use. Specific tools have been developed and validated, and expanding their use would help to promote the implementation of more standardized studies.
Data availability
The datasets used and/or analysed during the current study will be made available from the corresponding author on reasonable request.
References
Verrill, M. & S2-5. Chemotherapy for early-stage breast cancer: a brief history. Br. J. Cancer. 101 (Suppl 1). https://doi.org/10.1038/sj.bjc.6605268 (2009).
Alberti, P. et al. Neurological complications of conventional and novel anticancer treatments. Cancers (Basel). https://doi.org/10.3390/cancers14246088 (2022).
Nguyen, L. D. & Ehrlich, B. E. Cellular mechanisms and treatments for chemobrain: insight from aging and neurodegenerative diseases. EMBO Mol. Med. 12, e12075. https://doi.org/10.15252/emmm.202012075 (2020).
Cronin, K. A. et al. Annual report to the Nation on the status of cancer, part I: National cancer statistics. Cancer 124, 2785–2800. https://doi.org/10.1002/cncr.31551 (2018).
Chiaravalloti, A., Filippi, L., Pagani, M. & Schillaci, O. Functional imaging of chemobrain: usefulness of nuclear medicine in the fog coming after cancer. J. Nucl. Med. 64, 508–514. https://doi.org/10.2967/jnumed.121.263294 (2023).
Sahu, K., Langeh, U., Singh, C. & Singh, A. Crosstalk between anticancer drugs and mitochondrial functions. Curr. Res. Pharmacol. Drug Discov. 2, 100047. https://doi.org/10.1016/j.crphar.2021.100047 (2021).
Demos-Davies, K., Lawrence, J. & Seelig, D. Cancer related cognitive impairment: a downside of cancer treatment. Front. Oncol. 14, 1387251. https://doi.org/10.3389/fonc.2024.1387251 (2024).
Ren, X. et al. Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (chemobrain), a condition that significantly impairs the quality of life of many cancer survivors. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1088–1097. https://doi.org/10.1016/j.bbadis.2019.02.007 (2019).
Chae, J. W. et al. Association of mitochondrial DNA content in peripheral blood with cancer-related fatigue and chemotherapy-related cognitive impairment in early-stage breast cancer patients: a prospective cohort study. Breast Cancer Res. Treat. 168, 713–721. https://doi.org/10.1007/s10549-017-4640-7 (2018).
Juan, Z. et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: a randomised, double-blind, and placebo-controlled trial. Eur. J. Cancer. 161, 10–22. https://doi.org/10.1016/j.ejca.2021.11.006 (2022).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. (1995). https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin. Trials. 7, 177–188. https://doi.org/10.1016/0197-2456(86)90046-2 (1986).
Fan, H. G. et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J. Clin. Oncol. 23, 8025–8032. https://doi.org/10.1200/JCO.2005.01.6550 (2005).
Hurria, A. et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient’s perspective. Breast Cancer Res. Treat. 98, 343–348. https://doi.org/10.1007/s10549-006-9171-6 (2006).
Tager, F. A. et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res. Treat. 123, 25–34. https://doi.org/10.1007/s10549-009-0606-8 (2010).
Jansen, C. E., Cooper, B. A., Dodd, M. J. & Miaskowski, C. A. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 19, 1647–1656. https://doi.org/10.1007/s00520-010-0997-4 (2011).
Schrauwen, W. et al. Heterogeneous response of Chemotherapy-Related cognitive decline in patients with breast cancer: A prospective study. J. Int. Neuropsychol. Soc. 26, 806–814. https://doi.org/10.1017/S1355617720000296 (2020).
Syed Alwi, S. M., Narayanan, V., Mohd Taib, N. A. & Che Din, N. Chemotherapy-related cognitive impairment (CRCI) among early-stage breast cancer survivors in Malaysia. J. Clin. Exp. Neuropsychol. 43, 534–545. https://doi.org/10.1080/13803395.2021.1945539 (2021).
Liu, Y. et al. Construction and validation of a risk-prediction model for chemotherapy-related cognitive impairment in patients with breast cancer. J. Cancer Surviv.. https://doi.org/10.1007/s11764-024-01566-7 (2024).
Ma, Y. et al. Toward better understanding and management of chemobrain: The potential utilities of the memtrax memory test. BMC Womens Health. 24, 406. https://doi.org/10.1186/s12905-024-03251-4 (2024).
Wu, N. et al. Relationships between chemotherapy-related cognitive impairment, self-care ability, and quality of life in breast cancer survivors: A cross-sectional study. Semin Oncol. Nurs. 40, 151690. https://doi.org/10.1016/j.soncn.2024.151690 (2024).
Silberfarb, P. M., Philibert, D. & Levine, P. M. Psychosocial aspects of neoplastic disease: II. Affective and cognitive effects of chemotherapy in cancer patients. Am. J. Psychiatry. 137, 597–601. https://doi.org/10.1176/ajp.137.5.597 (1980).
Ahles, T. A. et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J. Clin. Oncol. 20, 485–493. https://doi.org/10.1200/JCO.2002.20.2.485 (2002).
Schagen, S. B. et al. Late effects of adjuvant chemotherapy on cognitive function: A follow-up study in breast cancer patients. Ann. Oncol. 13, 1387–1397. https://doi.org/10.1093/annonc/mdf241 (2002).
Jim, H. S. et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J. Clin. Oncol. 30, 3578–3587. https://doi.org/10.1200/JCO.2011.39.5640 (2012).
Vardy, J. et al. Assessing cognitive function in cancer patients. Support Care Cancer. 14, 1111–1118. https://doi.org/10.1007/s00520-006-0037-6 (2006).
Bell, M. L., Dhillon, H. M., Bray, V. J. & Vardy, J. L. Important differences and meaningful changes for the functional assessment of cancer Therapy-Cognitive function (FACT-Cog). J. Patient-Reported Outcomes. https://doi.org/10.1186/s41687-018-0071-4 (2018).
Joly, F. et al. French version of the functional assessment of cancer Therapy-Cognitive function (FACT-Cog) version 3. Support Care Cancer. 20, 3297–3305. https://doi.org/10.1007/s00520-012-1439-2 (2012).
Oliveira, A. F., Santos, I. M., Fernandes, S., Bem-Haja, P. & Torres, A. Validation study of the functional assessment of cancer Therapy-Cognitive Function-Version 3 for the Portuguese population. BMC Psychol. 10, 305. https://doi.org/10.1186/s40359-022-01018-w (2022).
Costa, D. S. J. et al. The structure of the FACT-Cog v3 in cancer patients, students, and older adults. J. Pain Symptom Manag. 55, 1173–1178. https://doi.org/10.1016/j.jpainsymman.2017.12.486 (2018).
Birks, J. & Grimley Evans, J. Ginkgo Biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003120.pub3 (2009).
Den, H., Dong, X., Chen, M. & Zou, Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment - a meta-analysis of randomized controlled trials. Aging (Albany NY). 12, 4010–4039. https://doi.org/10.18632/aging.102810 (2020).
Ercoli, L. M. et al. Assessment of the feasibility of a rehabilitation intervention program for breast cancer survivors with cognitive complaints. Brain Imaging Behav. 7, 543–553. https://doi.org/10.1007/s11682-013-9237-0 (2013).
Poppelreuter, M., Weis, J. & Bartsch, H. H. Effects of specific neuropsychological training programs for breast cancer patients after adjuvant chemotherapy. J. Psychosoc Oncol. 27, 274–296. https://doi.org/10.1080/07347330902776044 (2009).
Acknowledgements
Thanks to Fiona Ecarnot for editorial assistance.
Funding
None.
Author information
Authors and Affiliations
Contributions
L.G. and M.D. contributed to study conceptualization, data curation, formal analysis, methodology, manuscript writing, and validation of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Godaert, L., Dramé, M. The chemobrain in breast cancer patients: a systematic review and meta-analysis. Sci Rep 15, 35765 (2025). https://doi.org/10.1038/s41598-025-95380-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95380-5
Keywords
This article is cited by
-
Transcranial photobiomodulation for the treatment of chemobrain: new perspectives from a pilot study
Supportive Care in Cancer (2026)