Abstract
Enteroaggregative Escherichia coli (EAEC) is a diarrheagenic pathotype associated with traveler’s diarrhea, foodborne outbreaks, and sporadic diarrhea in industrialized and developing countries. Regulation of virulence factors in EAEC is mediated by the master regulator AggR, an AraC/XylS family member controlling the expression of more than 44 genes associated with metabolism and virulence. Although the AggR regulon is well-characterized, the mechanism and upstream signaling cascades that regulate its activation are poorly understood. This study demonstrates that Lpp (Braun’s lipoprotein) and L, D-transpeptidases are required for AggR activation. We found that deletion lpp in EAEC resulted in the downregulation of more than 100 genes involved in transport, metabolism, and virulence. Among the genes, fourteen transcriptional factors, including AggR, were differentially expressed in 042Δlpp. Our findings also showed that Lpp anchoring to the peptidoglycan is a requisite for AggR-activation. Hence, chemical inhibition or genetic deletion of L, D-transpeptidases encoding genes involved in the crosslink of Lpp to the peptidoglycan abolished AggR activation. Moreover, the 042Δlpp mutant exhibited reduced biofilm formation on abiotic surfaces and reduced colonization of human intestinal colonoids. This is the first study to demonstrate the tight regulation of the AraC/XylS transcriptional regulator AggR, essential in EAEC virulence and intestinal colonization by components of the bacterial cell envelope.
Similar content being viewed by others
Introduction
Enteroaggregative Escherichia coli (EAEC) is a diarrheagenic pathotype associated with traveler’s diarrhea, foodborne outbreaks, and sporadic diarrhea in industrialized and developing countries. It is also linked to linear growth faltering among children in low-income countries1,2,3,4. A Shiga toxin-producing EAEC caused a large and highly lethal European outbreak in 2011, highlighting the potential virulence of this pathotype5,6. Virulence gene expression in EAEC is activated in a coordinated fashion by a regulator called AggR, a member of the AraC/XylS family comprising more than 10,000 bacterial transcription factors7,8,9,10.
The AraC/XylS is one of the most prominent families of regulators used by many bacteria to control metabolism, stress response, and virulence. The AraC/XylS members involved in virulence include ToxT, which regulates the expression of the cholera toxin and the toxin-coregulated pilus in Vibrio cholerae11,12,13. In Salmonella enterica, AraC-like members HilC and HilD regulate the expression of HilA, which controls the expression of the pathogenicity island-1 involved in bacterial adhesion and invasion of the host gut epithelium14,15,16. The CS1 and CS2 fimbrial proteins of Enterotoxigenic E. coli (ETEC) are positively regulated by the AraC-like proteins Rns/CfaD17. In EAEC, the master transcriptional regulator AggR is required for the expression of at least 44 genes9, including the fimbriae (AAF/II)7, the lipoprotein dispersin18, and its secretion system AatPABCD10, the chromosomally encoded type VI secretion system8 and its negative regulator Aar19,20,21.
Most studies examining AggR have focused on its role in regulating downstream virulence genes. However, further research is necessary to comprehend the initial events and components essential for activating AggR, which could lead to new opportunities for therapeutic intervention. The cell envelope provides structural support to the cell and contains various sensor systems that respond to external stimuli. In Gram-negative bacteria, one of the most highly abundant proteins in the envelope is Lpp (the Braun lipoprotein), a small helical protein (5.8 kDa) that helps maintain envelope integrity and contributes to its mechanical properties by providing a covalent attachment between the OM and PG22,23. The Lpp attachment to PG is catalyzed by L, D-transpeptidases that link the C-terminal lysine of Lpp to a diaminopimelic acid residue in the PG24,25,26. Recent experimental findings suggest that Lpp functions as a cellular ruler that helps maintain the periplasm’s width. Modifications in its length were shown to affect the Rcs phosphorelay signaling pathway27.
Here, we show that Lpp anchoring to the peptidoglycan is a requisite for AggR activation. Chemical inhibition or genetic deletion of L, D-transpeptidases involved in the crosslink of Lpp to the peptidoglycan abolished AggR activation. Moreover, the 042Δlpp mutant exhibited reduced biofilm formation on abiotic surfaces and reduced colonization of human intestinal colonoids.
Results
Lpp is required to express the master virulence regulator AggR
Lipoproteins participate in various bacterial physiological processes, and some form part of external sensor systems that regulate stress responses and virulence27,28. To determine if lipoproteins play a role in regulating virulence in EAEC, we focused on the canonical triacylated lipoprotein Lpp (Braun’s lipoprotein) as the most abundant lipoprotein in E. coli and Aap, an AggR-regulated non-canonical lipoprotein monoacylated by the acyltransferase AatD29. We have previously shown that acylation of Aap by AatD drives the translocation of Aap to the outer membrane (OM). Thus, the deletion of aatD causes the accumulation of Aap in the periplasmic space (Fig. 1A, lane 4)29. We used the 042ΔaatD mutant to easily track levels of Aap by examining periplasmic fractions. Accordingly, we generated single and double mutants of aap, lpp, and aatD (042Δaap; 042Δlpp; 042ΔaatD; 042ΔlppΔaatD; 042ΔlppΔaap) in EAEC strain 042 and monitored AggR-induced virulence proteins, including Aap (lipoprotein) in the periplasm and AafA (fimbria major subunit) in the outer membrane (OM). For these experiments, periplasmic and outer membrane fractions for Aap and AafA were analyzed by SDS-PAGE and WB using anti-Aap (Fig. 1A) and anti-AafA (Fig. 1B) polyclonal antibodies. We found that the deletion of lpp affected the expression of several proteins in the periplasm and OM fractions (Fig. 1A and 1B, orange arrows). Surprisingly, the deletion of lpp also abolished the accumulation of Aap in the periplasmic space of strain 042ΔaatDΔlpp (Fig. 1A, lane 6) and AafA in OM (Fig. 1B, lane 5). Deletion of the gene encoding the Aap lipoprotein did not have any impact on the expression of other proteins or AggR-activated AafA since this was present in OM fractions at levels similar to those of the wild-type 042 strain (Fig. 1B, lane 4).
Lpp controls AggR expression. The expression of AggR-regulated Aap and AafA was determined by SDS-PAGE and WB in periplasmic (Panel A) and membrane fractions (Panel B). Transcriptional levels of lpp (panel C), aggR (Panel D), and aap (Panel E) were evaluated in 042, 042Δlpp, 042ΔlppΔaatD, and 042ΔlppΔaap by qRT-PCR. As negative controls, 042Δlpp (Panel C), 042ΔaggR (Panel D), and 042Δaap (Panel E) were included in our analysis. Asterisks indicate significant differences by ANOVA ( ***P < 0.0001).
We reasoned that perhaps Lpp acts upstream of AggR to activate AggR-regulated virulence factors. We, therefore, carried out a qRT-PCR analysis to confirm this possibility. As expected, lpp transcription was observed in the parental 042 strain but not the lpp mutant strains (Fig. 1C). Reduced transcriptional levels of aggR (Fig. 1D) and AggR-regulated aap (Fig. 1E) were observed in 042Δlpp. These results confirm that Lpp is required to transcribe AggR and AggR-regulated virulence factors.
A covalent link of Lpp to PG is required for AggR activation
L, D-transpeptidases control the periplasmic width between OM and IM by linking the Lpp to the PG24,25,30. It has been shown that the disruption of this spacing interferes with the proper assembly of periplasm-spanning structures and signal transduction from the environment to the cell. We, therefore, sought to investigate if the absence of L, D-transpeptidases results in the same effect as the absence of Lpp on AggR activation. For this study, five L, D-transpeptidases [Orf0241, Orf0908 (YbiS), Orf1183, Orf1845 (YnhG), and Orf2228 (ErfK)] were identified in EAEC strain 042 (Supplementary File 1). Since L, D-transpeptidase ErfK cross-links Lpp to PG25, we selected the three closely related to ErfK (YbiS and YnhG) in EAEC and generated single and double mutant strains. Expression of aggR was determined by qRT-PCR. We found that L, D-transpeptidases have redundant activity as single transpeptidase deletion are still able to induce AggR-expression (Fig. 2). However, 042 strain bearing double mutation in ynhG/erfK showed a significant reduction of aggR (Fig. 2A, bar 7) and AggR-regulated Aap (Fig. 2B, lane 9) expression compared to the parental 042 strain.
L, D- transpeptidases are required for AggR expression. Five L, D- transpeptidases were identified in the EAEC 042 strain. Single or double mutations in L, D- transpeptidases were generated by lambda red. AggR expression was determined by qRT-PCR in 042, 042ΔaggR, 042ΔaatD∆ybiS, 042ΔaatDΔynhG, 042ΔaatDΔerfK, 042ΔaatDΔynhGΔybiS and 042ΔaatDΔynhGΔerfK (Panel A). Periplasmic fractions were isolated and analyzed by SDS-PAGE and WB using an anti-Aap polyclonal antibody (Panel B). Asterisks indicate significant differences by ANOVA (**, P < 0.01; ***, P < 0.001, ****, P < 0.0001).
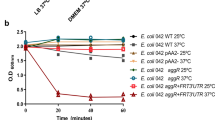
Since multiple L, D-transpeptidases can be inhibited by CuCl2 in bacterial cultures31, we used this second approach to confirm that cross-linking of Lpp to PG by L, D-transpeptidases is a determinant factor in the activation of AggR. Accordingly, we determine the expression of AggR-regulated Aap and AafA by WB in 042 derivatives [042, 042Δaap, 042ΔaafA, 042ΔaatD, and 042ΔaatD(pAatD)] grown in M9 medium in the presence of CuCl2 or the presence of MnCl2 as a negative control. We observed that inhibition of L, D-transpeptidases with CuCl2 abolishes the expression of Aap and AafA in periplasmic or membrane preps, respectively (Supplementary File 2). At the same time, MnCl2 did not affect AggR-regulated genes. These findings suggest that Lpp anchoring to the peptidoglycan is necessary for AggR activation, potentially facilitating contact with sensor proteins of the AggR signaling cascade.
Characterization of the Lpp regulon in EAEC
Since besides AggR and AggR-regulated genes, the expression of many other unidentified proteins was also affected by the lpp deletion (Fig. 1A, B, orange arrows), we sought to determine the global impact of lpp deletion in EAEC gene expression by RNAseq, which could reveal potential envelope sensor/elements associated with AggR activation. Total RNA was isolated from bacterial cultures of WT 042, 042Δlpp, and 042Δlpp(pLpp), prepared for sequencing, and analyzed by CD genomics (NY, USA). We found that the Lpp regulon comprises 102 differentially expressed genes (DEGs) (+ /- 1.5 fold, p < 0.002) (Fig. 3A, 3B). 88.23% of DEGs were found in the chromosome, and 11.76% in the pAA plasmid. Lpp-regulated genes were grouped into six major categories in Supplementary File 3. The highest hits of each category are listed in Table 1.
Analysis of Lpp regulon. EAEC042 and 042Δlpp were analyzed by RNAseq. 102 differentially expressed genes are represented in the volcano graph of Panel A. The genes were grouped into six major categories (Panel B): membrane/secreted proteins (Panel C), metabolic proteins (Panel D), transporter systems (Panel E), transcriptional factors (Panel F), and AggR-regulated virulence factors (Panel G).
The membrane/secreted proteins category includes 23 genes (22.54%) (Fig. 3C). Fourteen of these 23 proteins corresponded to hypothetical proteins. The major hits in this category include orf2242 (flu, Ag43), orf3550, orf1180, orf3310, orf1408, and orf2014.
Proteins involved in metabolic functions include genes associated with the metabolism of carbohydrates (fructose and mannose) and amino acids (alanine, arginine, glutamate, and aspartate), which were regulated by Lpp (18 genes, 17.64%) (Fig. 3D). We observed that 9 out of 102 (8.82%) Lpp-regulated genes were involved in the metabolism of glutamate/aspartate (orf0688, orf0687, orf0690, orf0689, orf0702, orf3806, orf4243, orf489, orf1911).
Transporter proteins including four ABC operons (glutamate/aspartate ABC transporter orf687-orf690; molybdate transporter orf782-orf783; orf3551-orf3554 (18 genes, 17.64%) were regulated by Lpp (Fig. 3E).
Lpp regulates transcriptional factors, including family members of the LysR (orf1456, orf2226, and orf2227), LuxR (orf3806 and orf0323), TetR/AcrR (orf1818), Rcs system which sense envelope stress (orf4414 and rcsA) and hypothetical transcriptional regulators (orf3309, orf2891, orf1951) (14 genes, 13.72%) (Fig. 3F).
AggR-regulated virulence genes encoded in the pAA virulence plasmid, including fimbria AAF/II genes (pAA046, pAA048), the lipoprotein Aap secretion system AAT (pAA008, pAA009, and pAA010), the non-canonical N-acyltransferase AatD (pAA011); the lipoprotein Aap (pAA055) and the putative isopentenyl-diphosphate delta-isomerase (pAA004) (Fig. 3G) and many other genes encoded in the chromosome were identified in the Lpp regulon (19.64%).
Of note, AggR-regulated AAF fimbrial genes and the Ag43 adhesin were down-regulated in the lpp mutant, suggesting that Lpp regulates adhesion and aggregation simultaneously in EAEC 042.
Given the relevance of Lpp to the master regulator of virulence, AggR, we validated the RNAseq by qRT-PCR and analyzed the expression of AggR-regulated genes (aatP, aatA, aatB, aatC, aatD, and aafA). In our analysis, we also included membrane/secreted proteins (orf1180, orf3550, and flu (Ag43) and transcriptional factors (aggR and nac) (Fig. 4). Our analysis was performed as previously published9.
Validation of transcriptomic database by qRT-PCR. The RNAseq data was validated by qRT-PCR. lpp (Panel A), AggR-regulated genes (aatP, aatA, aatB, aatC, aatD, aggR, and aafA) (Panels B-H), membrane/secreted proteins (orf1180, orf3550 and flu) (Panels I-K), and transcriptional factors nac and aggR (Panels G, L) were selected based on their relevance in virulence and metabolism. The transcriptional levels of each gene were quantitated in 042 (green bars), 042Δlpp (blue bars), and 042Δlpp(pLpp) (black bars). Expression levels for each queried gene were normalized to the constitutively expressed cat gene in EAEC042. Data are representative of at least three independent experiments. Asterisks indicate significant difference by ANOVA (*, P < 0.05; **, P < 0.01, ***, P < 0.001, ****P < 0.0001).
The nitrogen assimilation control protein (Nac) is a LysR-type transcriptional regulator that activates the transcription of σ70-dependent genes. The regulation of metabolic regulator nac in 042Δlpp could explain the expression of many genes associated with nitrogen metabolism and transport in the Lpp regulon (Fig. 4L).
As expected, expression of AggR and AggR-regulated genes was reduced (~ 3 to six fold) in the absence of lpp (Fig. 4B-H). Likewise, we confirm the downregulation of aggR and upregulation of nac transcriptional factors in the 042Δlpp mutant (Fig. 4). Our data confirm the regulatory role of Lpp on the AggR virulence regulator in EAEC.
Lpp regulates AAF-mediated biofilm formation on abiotic surfaces
We aimed to investigate the effects of deleting lpp in EAEC biofilm formation since Lpp is necessary to express AggR and AggR-regulated fimbrial AafA protein (Fig. 1B 1D, 4H). Accordingly, biofilm formation was assessed by growing 042, 042Δlpp, 042Δlpp(pLpp), and 042ΔaafA strains in DMEM-HG for eight hours as described in material and methods (Fig. 5). We observed that deletion of lpp reduced the biofilm formation of 042Δlpp in the abiotic surface (1 to 4 folds) (Fig. 5A, B). The reduced biofilm was not due to a bacterial growth defect but reduced AAF/II expression, as the analyzed strains showed similar growth rates (Supplementary File 4).
Lpp impairs biofilm formation on abiotic surfaces. 042, 042Δlpp, 42ΔaafA, and 042Δlpp(pLpp) strains were grown in a 96-well plate in DMEM-high glucose for eight hours. The bacterial biofilm was stained with crystal violet (Panel A). The cell-bound crystal violet was dissolved in 96% ethanol and measured at OD 600 ƞm in a microplate reader (Panel B). Asterisks indicate significant differences by ANOVA (**, P < 0.01, ****P < 0.0001).
Lpp is required for EAEC colonization of human intestinal colonoids
Human intestinal organoids have become the gold standard for studying host–pathogen interactions and have been successfully used to investigate essential features of EAEC pathogenesis32,33. We, therefore, used this relevant intestinal model to examine the role of Lpp in bacterial colonization. For these experiments, human intestinal colonoid monolayers were grown in transwells and infected with parental 042, 042Δlpp, and 042Δlpp(pLpp) at 37 °C for six hours (Fig. 6), and bacterial adherence was analyzed by confocal microscopy. 3D (XYZ) (6A-C) and 2D (XY) (6D-F) images of a representative experiment run in triplicate are shown in Fig. 6. The bacteria load was quantified in the images using the Java-based image processing program ImageJ (Fig. 6G). We observed that the deletion of lpp significantly decreases bacterial colonization in human colonoids compared to the parental strain. Complementation in trans of 042Δlpp restored bacterial colonization to comparable wild-type levels (Fig. 6C, 6F). Moreover, microscopic examination of bacterial adherence revealed decreased aggregation of 042Δlpp in colonoids compared to parental and complemented EAEC strains (Fig. 6D-F). Altogether, our findings demonstrate that Lpp is required for EAEC intestinal colonization.
Lpp modulates colonization of EAEC 042 in human colonoids. Human colonoids were infected with 042 (Panels A, D), 042Δlpp (Panels B, E) or 042Δlpp(pLpp) (Panels C, F). Anti-actin antibodies (for the human colonoid in red) and anti-044 antibodies (for the bacteria in green) were used in the experiment. The relative number of bacteria was measured in randomly selected Z-stack microscopic fields by ImageJ software (Panel G). Representative confocal 3D ( Panels A-C) and 2D (D-F) images from three independent samples are shown. Asterisks indicate significant differences by ANOVA (*, P < 0.05; **, P < 0.01).
Discussion
AggR is a member of the AraC/XylS family, which includes over 10,000 bacterial transcription factors. Although the AggR regulon is well-characterized7,8,9, the precise mechanism by which AggR is initially activated remains unclear. Our data provide experimental evidence indicating that the activation signal for AggR originates at the bacterial cell envelope. In this study, we offer insights into the global gene expression landscape associated with Lpp in EAEC and its essential role in activating the AggR master virulence regulator.
The cell envelope in Gram-negative bacteria consists of two membranes separated by a layer of peptidoglycan (PG)27,34. In E. coli, one of the most abundant lipoproteins is Lpp, which plays a crucial role in the mechanical properties of the envelope22,28. Lpp provides a covalent link between the outer membrane (OM) and the PG layer, facilitated by L,D-transpeptidases24,25. This connection also enables interactions among various sensor systems found in both layers that respond to external stimuli. Our research revealed that deleting the lpp gene, or inhibiting L,D-transpeptidases—either individually or in combination—results in the downregulation of AggR and AggR-regulated virulence genes.
To assess the global impact of Lpp in the EAEC strain 042, we characterized the Lpp regulon through RNA sequencing (Fig. 3). The deletion of lpp in EAEC affects the expression of over 100 genes related to metabolic, transport, and regulatory functions. As anticipated, the differentially expressed genes within the Lpp regulon correlated with the expression of virulence genes associated with AggR activity (Fig. 3 and 4). Our RNAseq analysis also identified fifteen transcriptional regulators, including AggR, Nac, glnK, LysR-type transcriptional regulators, and members of the Rcs system. Notably, our findings appear to be the first to establish a connection between Lpp and nitrogen metabolism, as many differentially expressed genes related to nitrogen assimilation and transport in the Lpp regulon are regulated by Nac and GlnK (Fig. 3, supplementary file 3). Nac is a LysR-type transcriptional regulator that activates the transcription of σ70-dependent genes, which encode proteins that provide the cell with ammonia or glutamate to meet the demand for biosynthetically available nitrogen35. In contrast, GlnK is a signal transduction protein that controls the activity of glutamine synthetase (GS), a crucial enzyme in nitrogen metabolism, as well as the transmembrane ammonia channel AmtB36. It’s important to note that genes associated with nitrogen metabolism have been also shown to be under AggR regulation20.
Interestingly, while Lpp is found in non-pathogenic E. coli, its expression has been linked to the virulence of various pathogens. For instance, a Δlpp mutant of Yersinia pestis is more quickly eliminated by macrophages compared to the wild-type (WT) strain37. In pathogenic E. coli strain O157:H7, the deletion of lpp reduces cell invasiveness38, and in the uropathogenic strain CFT073, lpp deletion increases sensitivity to serum killing39. Similarly, lpp deletion in Salmonella leads to reduced virulence and decreased host cell invasion due to downregulation of the type III secretion system and the flagellar apparatus40. Here, we show that deletion of lpp significantly decreased bacterial biofilm formation on abiotic surfaces (Fig. 5) and bacterial colonization of human intestinal colonoids (Fig. 6). Moreover, microscopic examination of infected colonoids revealed decreased adherence and aggregation of 042Δlpp compared to parental and complemented EAEC strains. These findings correlate with the reduced expression of AggR-regulated AAF/II fimbria and the Ag43 required for bacterial adherence and aggregation, respectively (Fig. 4).
It has been demonstrated that Lpp plays a crucial role in maintaining the distance between the inner and outer membranes, thereby influencing the size of the periplasm23,41. This suggests that other proteins in the outer and inner membranes, along with downstream elements of the Lpp regulatory cascade, may also regulate the AggR promoter. Additional transcriptional regulators, such as Fis and H-NS, have been shown to either activate or repress AggR by directly binding to its promoter42. Although the Lpp regulon has identified several transcription factors, it does not indicate that Lpp directly controls these regulators. We are currently focused on characterizing potential transcriptional regulators, including members of the LysR and Rcs families, as well as hypothetical outer and inner membrane proteins within the Lpp regulon. These proteins and regulators may play a significant role in the downstream signaling pathways that activate AggR.
In summary, our data indicate that Lpp and L,D-transpeptidases, which are located in the bacterial envelope, are crucial for the initial activation of AggR. Once AggR expression is activated, the regulator binds to its promoter, which enhances both its own expression42 and the expression of genes associated with virulence and intestinal colonization (Fig. 7). Our findings can be applied to other AggR homologs that regulate virulence factors in various pathogenic E. coli strains, potentially aiding in the development of anti-virulence strategies against enteric pathogens.
Materials and methods
Bacterial strain and growth conditions
Bacterial strains used in this study are shown in Supplementary File 5. EAEC 042 derivatives were routinely propagated in Luria Broth (LB) and Dulbecco’s modified Eagle’s medium with 0.4% glucose (DMEM high glucose) (Gibco, Grand Island, NY) as previously described9.
Generation of 042 derivatives
Mutagenesis of 042 derivatives was accomplished by the lambda red recombination43. The locus for lpp (CBG34670.1; 1,926,749—1,926,985), orf908 (CBG33733.1; 967,245—968,165), orf1845 (CBG34671.1; 1,927,049—1,928,053), orf2228 (CBG35054.1; 2,317,726—2,318,661), aatD (CBG27765.1; 8,237 – 9,451), and aap (CBG27807.1; 42,684 – 43,034) were replaced by the kanamycin (km) resistance marker. Primers used for the lambda-red procedure are indicated in Supplementary File 6. For complementation experiments, lpp was amplified by PCR and cloned into the pBAD30-Paar plasmid downstream of the Paar promoter19.
RNA-seq
RNA was extracted from EAEC 042 derivatives [042, 042Δlpp, and 042Δlpp(pLpp)] grown in DMEM-high glucose as previously published9,20. RNA was extracted with TRIzol (Invitrogen) and purified with RNeasy Mini kit columns (Qiagen). Purified RNA was used for library construction, Illumina stranded RNA-seq library preparation, sequencing (2 × 150 bp, 10 M read pairs), and data analysis (CD genomics NY, USA). Reads were mapped to the EAEC 042 chromosome and pAA plasmid with the BWA aligner44. Counts for each annotated genomic feature were determined by htseq-count (http://www-huber.embl.de/users/anders/HTSeq/doc/count.html). Differential expression between counts for each feature was then calculated with DESeq45 using the false-detection rate-adjusted Benjamini Hochberg P value. The p-value obtained from the test was corrected, and the false discovery rate (FDR) was used as a key indicator of differentially expressed genes (DEG). The DEG vs. P value was plotted using GraphPad Prism 6 (GraphPad Software, Inc., CA, USA). During the analysis, fold change ≥ 2, and FDR < 0.05 were set as screening criteria. Fold change indicates the ratio of expression levels between the samples.
Quantitative real-time PCR (qRT-PCR)
EAEC strains were grown in 14 ml of DMEM-High glucose for four h (log phase). Bacterial pellets were resuspended in 1 ml Trizol, and RNA was extracted following the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Contaminating DNA was removed using an RNase-Free DNase Set (Qiagen, Maryland, USA), and total RNA was quantified using a Nanodrop 1000 (ThermoFisher Scientific, Waltham, MA, U.S.A.). cDNA was generated and quantified using a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA). Primers used in this study are reported in Supplementary File 6. Reactions were duplicated using two different RNA samples for each strain. The expression level for each queried gene was normalized to the constitutively expressed cat gene as previously described9.
SDS-PAGE and Western blot analysis of Aap and AafA
EAEC strains were grown in 14 ml of DMEM-High glucose. The cultures were pelleted, and bacterial pellets were washed with 1 ml of PBS and resuspended in 1 ml of periplasmic solution (20% sucrose, 30 mM Tris–HCl pH 8.0, 1 mM EDTA pH 8.0). Bacterial preps were incubated for 20 min at room temperature, pelleted, and resuspended in 200 µl of ice-cold 5 mM MgSO4. Bacterial preparations were incubated for 20 min at 4 °C and centrifuged at 14,000 rpm for 5 min. Supernatants were isolated and analyzed using SDS-PAGE and WB. To isolate EAEC Aggregative Adherence Fimbriae II (AAF/II), strains were grown in 13 ml of DMEM high glucose to reach an OD600 of 0.8. Bacteria were pelleted, washed twice with sterile PBS, and resuspended in 200 µl of 0.5 mM Tris and 75 mM NaCl. The samples were heated for 30 min at 65 °C, centrifuged, and supernatants were isolated for SDS-PAGE and WB analysis.
For the SDS-PAGE and Western blot analysis, the protein samples were separated in acrylamide gels and transferred to Immobilon-P membranes (BioRad, Hercules CA, USA) using standard protocols. Membranes were incubated overnight with anti-AafA or anti-Aap antibodies. The next day, membranes were washed twice in PBS-0.1% tween and incubated for one hour with a horseradish peroxidase-conjugated goat anti-rabbit IgG antibody. Membranes were developed using TMB membrane peroxidase substrate (KPL, Gaithersburg, MD, USA).
Copper chloride assays
042 derivatives were grown overnight in LB and diluted 1:100 in 5 ml of M9 broth supplemented with different concentrations of CuCl2 (1 mM) or MnCl2 (1 mM). The bacterial cultures were incubated overnight. The next day, the cultures were pelleted and washed twice with sterile PBS, and periplasmic fractions and proteins associated with the membrane were isolated and analyzed by SDS-PAGE and WB, as indicated above.
Biofilm formation
042, 042Δlpp, 042ΔaafA, and 042Δlpp(pLpp) strains were grown overnight in LB and diluted 1:100 in DMEM-high glucose and plated in a 96-well plate (Costar). Strains were grown for eight hours with shaking at 37 °C. After the incubation period, the plates were washed thrice with sterile PBS. Then, 200 μl of crystal violet solution (0.2%) was added to all wells, incubated for 5 min, washed twice, and air dried. The cell-bound crystal violet was dissolved in 96% ethanol and measured at OD600ƞm in a microplate reader (Agilent BioTek).
Human intestinal organoid culture
Human intestinal organoids used in this study were derived from a colonoid repository previously established from deidentified biopsy specimens from healthy subjects who provided written informed consent at Johns Hopkins University by approved guidelines and regulations (IRB NA_00038329).
The maintenance of human organoids and preparation of colonoid monolayers were previously described32. Briefly, organoids were routinely cultured as 3D cysts embedded in Matrigel (Corning) and used to prepare cell monolayers in 24-well, 0.4 µm pore size polyester membrane cell culture inserts (Transwell supports, Corning) precoated with 100 µl of 34 µg/ml of human collagen IV solution (Sigma). Intestinal monolayers were routinely grown at 37 °C with 5% CO2 until confluency as assessed by the increase in transepithelial electrical resistance (TEER), measured using an epithelial volt/ohm meter (EVOM, World Precision Instruments). Confluent monolayers were differentiated for five days before infections.
Confocal microscopy
EAEC 042 derivatives were grown overnight in LB supplemented with appropriate antibiotics (Sigma Chemical Co, St. Louis, MO). The next day, overnight cultures were diluted 1:50 (V/V) in DMEM-HG medium (Invitrogen, USA) and incubated at 37 °C with shaking to the mid-log phase (OD600 = 0.6) to induce Lpp expression. Bacterial cultures were adjusted to 108 CFU/ml in PBS, and 10 µl (106 CFU) was added to the apical surface of colonoid monolayers. Cells were infected for six hours at 37 °C, 5% of CO2. Following the bacterial infection, the cells were fixed with Carnoy’s solution (90% methanol, 10% glacial acetic acid), washed three times with PBS, permeabilized with 0.1% saponin, and blocked with 2% bovine serum albumin/fetal bovine serum for 30 min (Sigma Aldrich, USA). Cells were rinsed with PBS and incubated overnight at 4 °C with primary antibodies diluted 1:100 in PBS containing 15% FBS and 2% BSA. We used anti-044 for EAEC staining as primary antibodies and Alexa-phalloidin for cell-actin staining. Stained cells were washed thrice with PBS and incubated with appropriate Alexa-conjugated secondary antibodies (Molecular Probes/Invitrogen, USA) diluted 1:500 in PBS. Hoechst (Vector Laboratories, USA) was used at a 1:1000 dilution in PBS for nucleus/DNA labeling. After incubation, cells were washed three times for 5 min each and mounted in ProLong Gold (Vector Laboratories, USA) overnight at 4 °C. Confocal imaging was carried out at the Imaging Core Facility at the University of Virginia using an LSM-710 Multiphoton laser-scanning confocal microscope (Zeiss, Germany) running ZEN 2012 (black and blue edition) imaging software (Zeiss, Germany). Images were captured with a 64X oil objective.
The same settings were used to image across samples for quantitative analysis of bacteria. The relative number of bacteria was measured in randomly selected Z-stack microscopic fields by ImageJ software (NIH) using the particle enumeration algorithm as previously reported32. Briefly, single images exhibiting green fluorescent bacteria were obtained using the Blue edition ZEN2012 software Zeiss (Zeiss, Germany). Images were opened as 16-bit type images with ImageJ. Threshold values were adjusted to eliminate the background. Bacteria were enumerated in images processed as Binary > Watershed images. This algorithm separates particles that are close together (e.g., aggregated bacteria). Lastly, images were analyzed as particles set as size (pixel2) = I0-infinite, which is relatively close to the size of E. coli in 64X confocal images. Particle counts in each image were input in an Excel sheet and plotted using the Prism software (Graph Pad).
Bioinformatics and statistical analysis
Statistical analysis of the data for qRT-PCR experiments was performed using the GraphPad Prism 6 (GraphPad Software, Inc., CA, USA). The statistical significance of the differences in the sample means was calculated using ANOVA with post hoc Tukey’s correction. Results were considered significant at P < 0.05.
Institutional guidelines
Human intestinal organoids used in this study were derived from a colonoid/enteroid repository previously established from deidentified biopsy specimens from healthy subjects who provided written informed consent at Johns Hopkins University (Protocol NA_00038329). All methods followed the University of Virginia-approved guidelines and regulations (IRB-HSR # 18,959). All experimental protocols were approved by the University of Virginia Review Board (IBC number 1329–11).
Data availability
The RNA-seq data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO accession number: GSE282211.
Reference Lists
Nataro, J. P. Enteroaggregative Escherichia coli pathogenesis. Curr. Opin. Gastroenterol. 21, 4–8 (2005).
Nataro, J. P. & Kaper, J. B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11, 142–201 (1998).
Nataro, J. P. & Sears, C. L. Infectious causes of persistent diarrhea. Pediatr. Infect. Dis. J. 20, 195–196 (2001).
Nataro, J. P., Steiner, T. & Guerrant, R. L. Enteroaggregative Escherichia coli. Emerg. Infect. Dis. 4, 251–261. https://doi.org/10.3201/eid0402.980212 (1998).
Rasko, D. A. et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N. Engl. J. Med. 365, 709–717. https://doi.org/10.1056/NEJMoa1106920 (2011).
Boisen, N. et al. The presence of the pAA plasmid in the German O104:H4 Shiga toxin type 2a (Stx2a)-producing enteroaggregative Escherichia coli strain promotes the translocation of Stx2a across an epithelial cell monolayer. J. Infect. Dis. 210, 1909–1919. https://doi.org/10.1093/infdis/jiu399 (2014).
Nataro, J. P., Yikang, D., Yingkang, D. & Walker, K. AggR, a transcriptional activator of aggregative adherence fimbria I expression in enteroaggregative Escherichia coli. J. Bacteriol. 176, 4691–4699 (1994).
Dudley, E. G., Thomson, N. R., Parkhill, J., Morin, N. P. & Nataro, J. P. Proteomic and microarray characterization of the AggR regulon identifies a pheU pathogenicity island in enteroaggregative Escherichia coli. Mol. Microbiol. 61, 1267–1282. https://doi.org/10.1111/j.1365-2958.2006.05281.x (2006).
Morin, N., Santiago, A. E., Ernst, R. K., Guillot, S. J. & Nataro, J. P. Characterization of the AggR regulon in enteroaggregative Escherichia coli. Infect. Immun. 81, 122–132. https://doi.org/10.1128/IAI.00676-12 (2013).
Nishi, J. et al. The export of coat protein from enteroaggregative Escherichia coli by a specific ATP-binding cassette transporter system. J. Biol. Chem. 278, 45680–45689. https://doi.org/10.1074/jbc.M306413200 (2003).
DiRita, V. J. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol. Microbiol. 6, 451–458 (1992).
DiRita, V. J., Parsot, C., Jander, G. & Mekalanos, J. J. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 88, 5403–5407. https://doi.org/10.1073/pnas.88.12.5403 (1991).
Higgins, D. E., Nazareno, E. & DiRita, V. J. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J. Bacteriol. 174, 6974–6980. https://doi.org/10.1128/jb.174.21.6974-6980.1992 (1992).
Akbar, S., Schechter, L. M., Lostroh, C. P. & Lee, C. A. AraC/XylS family members, HilD and HilC, directly activate virulence gene expression independently of HilA in Salmonella typhimurium. Mol. Microbiol. 47, 715–728. https://doi.org/10.1046/j.1365-2958.2003.03322.x (2003).
Schechter, L. M., Damrauer, S. M. & Lee, C. A. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol. Microbiol. 32, 629–642. https://doi.org/10.1046/j.1365-2958.1999.01381.x (1999).
Schechter, L. M. & Lee, C. A. AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella typhimurium hilA promoter. Mol. Microbiol. 40, 1289–1299. https://doi.org/10.1046/j.1365-2958.2001.02462.x (2001).
Caron, J., Coffield, L. M. & Scott, J. R. A plasmid-encoded regulatory gene, rns, required for expression of the CS1 and CS2 adhesins of enterotoxigenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 86, 963–967 (1989).
Sheikh, J. et al. A novel dispersin protein in enteroaggregative Escherichia coli. J. Clin. Investig. 110, 1329–1337. https://doi.org/10.1172/JCI16172 (2002).
Santiago, A. E. et al. A large family of antivirulence regulators modulates the effects of transcriptional activators in Gram-negative pathogenic bacteria. PLoS Pathog. 10, e1004153. https://doi.org/10.1371/journal.ppat.1004153 (2014).
Santiago, A. E. et al. The AraC negative regulator family modulates the activity of histone-like proteins in pathogenic bacteria. PLoS Pathog. 13, e1006545. https://doi.org/10.1371/journal.ppat.1006545 (2017).
Santiago, A. E. et al. A large family of anti-activators accompanying XylS/AraC family regulatory proteins. Mol. Microbiol. 101, 314–332. https://doi.org/10.1111/mmi.13392 (2016).
Asmar, A. T. & Collet, J. F. Lpp, the Braun lipoprotein, turns 50-major achievements and remaining issues. FEMS Microbiol. Lett. https://doi.org/10.1093/femsle/fny199 (2018).
Mathelie-Guinlet, M., Asmar, A. T., Collet, J. F. & Dufrene, Y. F. Lipoprotein Lpp regulates the mechanical properties of the E. coli cell envelope. Nat. Commun. 11, 1789. https://doi.org/10.1038/s41467-020-15489-1 (2020).
Magnet, S. et al. Identification of the L, D-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J. Bacteriol. 189, 3927–3931. https://doi.org/10.1128/JB.00084-07 (2007).
Magnet, S., Dubost, L., Marie, A., Arthur, M. & Gutmann, L. Identification of the L, D-transpeptidases for peptidoglycan cross-linking in Escherichia coli. J. Bacteriol. 190, 4782–4785. https://doi.org/10.1128/JB.00025-08 (2008).
Dramsi, S., Magnet, S., Davison, S. & Arthur, M. Covalent attachment of proteins to peptidoglycan. FEMS Microbiol. Rev. 32, 307–320. https://doi.org/10.1111/j.1574-6976.2008.00102.x (2008).
Asmar, A. T. et al. Communication across the bacterial cell envelope depends on the size of the periplasm. PLoS Biol. 15, e2004303. https://doi.org/10.1371/journal.pbio.2004303 (2017).
Braun, V. & Hantke, K. Lipoproteins: Structure, function Biosynthesis. Subcell. Biochem. 92, 39–77. https://doi.org/10.1007/978-3-030-18768-2_3 (2019).
Belmont-Monroy, L. et al. Characterization of a novel AraC/XylS-regulated family of N-acyltransferases in pathogens of the order Enterobacterales. PLoS Pathog. 16, e1008776. https://doi.org/10.1371/journal.ppat.1008776 (2020).
Bahadur, R., Chodisetti, P. K. & Reddy, M. Cleavage of Braun’s lipoprotein Lpp from the bacterial peptidoglycan by a paralog of l, d-transpeptidases LdtF. Proc. Natl. Acad. Sci. U. S. A. https://doi.org/10.1073/pnas.2101989118 (2021).
Peters, K. et al. Copper inhibits peptidoglycan LD-transpeptidases suppressing beta-lactam resistance due to bypass of penicillin-binding proteins. Proc. Natl. Acad. Sci. U. S. A. 115, 10786–10791. https://doi.org/10.1073/pnas.1809285115 (2018).
Liu, L. et al. Mucus layer modeling of human colonoids during infection with enteroaggragative E. coli. Sci. Rep. 10, 10533. https://doi.org/10.1038/s41598-020-67104-4 (2020).
Gonyar, L. A. et al. Aggregative adherence fimbriae II of enteroaggregative Escherichia coli are required for adherence and barrier disruption during infection of human colonoids. Infect. Immun. https://doi.org/10.1128/IAI.00176-20 (2020).
Silhavy, T. J., Kahne, D. & Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2, a000414. https://doi.org/10.1101/cshperspect.a000414 (2010).
Muse, W. B. & Bender, R. A. The nac (nitrogen assimilation control) gene from Escherichia coli. J. Bacteriol. 180, 1166–1173. https://doi.org/10.1128/JB.180.5.1166-1173.1998 (1998).
Blauwkamp, T. A. & Ninfa, A. J. Physiological role of the GlnK signal transduction protein of Escherichia coli: Survival of nitrogen starvation. Mol. Microbiol. 46, 203–214. https://doi.org/10.1046/j.1365-2958.2002.03153.x (2002).
Liu, T., Agar, S. L., Sha, J. & Chopra, A. K. Deletion of Braun lipoprotein gene (lpp) attenuates Yersinia pestis KIM/D27 strain: Role of Lpp in modulating host immune response, NF-kappaB activation and cell death. Microb. Pathog. 48, 42–52. https://doi.org/10.1016/j.micpath.2009.09.002 (2010).
Uhlich, G. A., Gunther, NWt., Bayles, D. O. & Mosier, D. A. The CsgA and Lpp proteins of an Escherichia coli O157:H7 strain affect HEp-2 cell invasion, motility, and biofilm formation. Infect. Immun. 77, 1543–1552. https://doi.org/10.1128/IAI.00949-08 (2009).
Diao, J. et al. Peptidoglycan association of murein lipoprotein is required for KpsD-dependent group 2 capsular polysaccharide expression and serum resistance in a uropathogenic Escherichia coli Isolate. MBio https://doi.org/10.1128/mBio.00603-17 (2017).
Fadl, A. A. et al. Global gene expression of a murein (Braun) lipoprotein mutant of Salmonella enterica serovar Typhimurium by microarray analysis. Gene 374, 121–127. https://doi.org/10.1016/j.gene.2006.01.034 (2006).
Mandela, E. et al. Adaptation of the periplasm to maintain spatial constraints essential for cell envelope processes and cell viability. Elife https://doi.org/10.7554/eLife.73516 (2022).
Morin, N. et al. Autoactivation of the AggR regulator of enteroaggregative Escherichia coli in vitro and in vivo. FEMS Immunol. Med. Microbiol. 58, 344–355. https://doi.org/10.1111/j.1574-695X.2010.00645.x (2010).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97, 6640–6645. https://doi.org/10.1073/pnas.120163297 (2000).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. https://doi.org/10.1093/bioinformatics/btp324 (2009).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106. https://doi.org/10.1186/gb-2010-11-10-r106 (2010).
Acknowledgements
The Child Health Research Center, Department of Pediatrics, NIAID NIH R01AI162858, and P01AI125181 funded this work.
Funding
National Institute of Allergy and Infectious Diseases,P01AI125181,R01AI162858
Author information
Authors and Affiliations
Contributions
D.R.V., N.L.M., and L.B.M. conducted the experiments. F.R.P. and N.L.M. established colonoid lines. F.R.P. and A.E.S. designed the experiments, compiled the data, wrote the manuscript, and participated in scientific discussion. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rodriguez-Valverde, D., Leon-Montes, N., Belmont-Monroy, L. et al. Lipoprotein Lpp and L, D-transpeptidases regulate the master regulator of virulence AggR in EAEC. Sci Rep 15, 13988 (2025). https://doi.org/10.1038/s41598-025-96373-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96373-0