Abstract
In this study, a novel hydrophobic polysulfide (NPS) reagent was synthesized using Pistacia atlantica gum and sulfur powder under hydrothermal conditions. Boehmite (γ-AlOOH) functionalized with curcumin (BCur) and TiO2 nanoparticles were coated with the NPS reagent. Subsequently, cotton fabrics were dip-coated separately with NPS, NPS-BCur, and NPS-TiO2, resulting in the fabrication of superhydrophobic filters with water contact angles (WCA) exceeding 150°. The reagents and coated fabrics were thoroughly characterized using FT-IR, XRD, TGA, SEM, X-ray mapping, and WCA measurements. The filters demonstrated exceptional performance in separating oil-water mixtures, achieving separation efficiencies of 91–98.6%. Even after 20 recycling cycles, negligible changes were observed in WCA and separation efficiency. The use of natural materials, eco-friendly synthesis, high durability, and outstanding separation performance underscore the sustainability and practicality of these superhydrophobic cotton fabrics.
Similar content being viewed by others
Introduction
Oily pollution has become a major global environmental challenge, with industries such as textiles, petrochemicals, food processing, mining, and steel production generating vast amounts of oily wastewater1,2. For instance, a simple mining operation can produce up to 140,000 L of oily wastewater daily, posing significant environmental concerns3. Furthermore, oil production and transportation often lead to catastrophic spills that threaten marine ecosystems1,2. Common solutions include mechanical equipment like skimmers and booms, which require substantial energy and operational pressures4. Absorbent materials such as foams5,6, sponges7,8, and textiles9,10,11 are also employed, particularly during emergency oil spill responses. Recently, superhydrophobic materials have gained attention for their exceptional stability, durability, efficiency, and recyclability in oil-water separation12,13,14,15,16. However, many superhydrophobic systems suffer from poor durability and resistance to environmental stressors, such as mechanical forces, high temperatures, pressures, and corrosive chemicals17,18. For instance, Sebastian et al.19 prepared a superhydrophobic coatings on aluminum using PDMS/SiO2 which showed a drastic reduction in water contact angle ( about 50°) after mechanical tests highlighting mechanical vulnerability. Similarly, Xu et al.20 observed a significant change in water contact angle of their prepared superhydrophobic TiO2−Polymer nanocomposite surface after prolonged UV exposure. Additionally, He and Zhou21 found that graphene oxide/PGMA composite coatings lost superhydrophobicity after exposure to acidic and alkaline conditions for extended periods. These limitations reduce their practicality in real-world conditions. To address this, various materials including aerogels, textiles, polymers, and metal meshes have been explored for building robust superhydrophobic systems22,23,24,25. Among these materials, cellulosic cotton fibers, an abundant natural polymer widely used in the textile industry, have emerged as a promising candidate for oil-water separation. While cotton fibers possess both lipophilic and hydrophilic functional groups, their hydrophilic nature limits their application in selective oil-water separation. Hydrophobic surface modifications are therefore essential to enhance their performance21. Materials such as TiO226,27, ZnO28,29, and SiO230,31 nanoparticles, as well as synthetic reagents like polydimethylsiloxane (PDMS)32,33 and tetraethyl orthosilicate (TEOS)34,35, have been used to coat cotton fibers. Despite these advancements, sustainable and eco-friendly solutions are increasingly preferred. For example, Shang et al.36 developed a hydrophobic castor oil-based nanocomposite coating for cotton textiles. In another research, Cheng et al. utilized TA-APTES nanoparticle coating on the surface of PET fabric to obtain superhydrophilicity37.
Turpentine gum is a natural oleoresin extracted from the Pistacia Atlantica tree, which is native to Iran, Iraq, and Turkey. It contains over 30 organic components, primarily comprising alkene and alkane functional groups38,39. The two main components of this gum are abietic acid and α-pinene. With its sticky and hydrophobic structure, turpentine gum is a promising compound for coating cotton fibers. Although Pistacia Atlantica gum has been utilized in various applications40,41,42,43, it has never been employed as a hydrophobic agent to produce the reagent described in our work. Polysulfides, synthesized from elemental sulfur, represent another class of sustainable materials. These solvent-free compounds are produced through a reaction between sulfur powder and alkene-based renewable monomers44. Such polysulfides hold potential as coatings for cotton fabrics.
In this study, a novel hydrophobic polysulfide (NPS) reagent was synthesized using Pistacia Atlantica gum and elemental sulfur through a solvent-free reaction. This polysulfide (NPS) reagent was then applied as a coating on nanoparticles and cotton fabrics to produce superhydrophobic materials. These materials were subsequently used as filters for efficient oil-water separation, demonstrating high stability, durability, and environmental compatibility. Also, a comprehensive analysis of the reagents and coated fabrics was conducted using a variety of techniques, including Fourier Transform Infrared Spectroscopy (FT-IR), X-ray Diffraction (XRD), Thermogravimetric Analysis (TGA), Scanning Electron Microscopy (SEM), X-ray mapping, and Water Contact Angle (WCA) measurements.
Materials and methods
Materials
(3-Aminopropyl)triethoxysilane (APTES), petroleum ether, toluene, ethanol (98%), normal hexane, sulfur powder, (3-Chloropropyl)triethoxysilane (CPTES), Sodium Hydroxide (NaOH), Hydrochloric Acid (HCl) and acetone were all purchased from Sigma-Aldrich. TiO2 nanoparticles (Aeroxide P-25 with an average particle size of 21 nm) were purchased from Degussa AG, Germany. Boehmite nanoparticles with an average particle size of 25 nm were synthesized according to the method described in previous work45.Turmeric powder and fresh natural Pistacia Atlantica gum were purchased from the local market of Kermanshah, Iran. The textile fabric, made from 100% cotton fibers, was purchased and washed with acetone before use. Gasoil, kerosene, gasoline and crude oil were all obtained from the Kermanshah Oil Company.
Synthesis of BCur nanoparticles
First, curcumin was extracted from turmeric powder. To achieve this, 20 g of turmeric powder was dispersed in 100 ml of ethanol and stirred for 60 min. The solution was then filtered to obtain an ethanolic curcumin solution. Next, 15 g of boehmite nanopowder was added to 250 ml of a solvent mixture (H₂O: EtOH, 20:80) and sonicated in an ultrasonic bath for 15 min. Subsequently, 15 ml of CPTES was added to the mixture, which was transferred to a three-necked flask and refluxed under nitrogen purging at 50 °C for 8 h. Afterward, 50 ml of the curcumin solution was added to the flask, and refluxing was continued for 14 h at 70 °C. Finally, the product (BCur) was filtered using a centrifuge, washed several times with ethanol, and dried in an oven at 70 °C46.
Synthesis of natural polysulfide (NPS)
To synthesize the NPS hydrophobic agent, 11.5 g of Pistacia Atlantica gum was dissolved in 10 ml of ethanol. Then, 1 g of sulfur was added to the mixture and sonicated for 20 min in an ultrasonic bath. The mixture was then transferred to the Teflon-lined hydrothermal autoclave and heated in an oven for 10 h at 170 °C. Finally, the synthesized dark brown solution was dried in an oven to obtain natural polysulfide (NPS).
Synthesis of NPS coated TiO2 and BCur
11.5 g of Pistacia Atlantica gum was dissolved in 10 ml of ethanol, then 1 g of sulfur and 0.5 g of TiO2 (or BCur) were added to them. The mixture was then placed in an ultrasonic bath for 10 min. The resulting mixture was transferred to the teflon-lined hydrothermal autoclave and put in the oven for 10 h at 170 °C. At the end, the precipitate of NPS coated TiO2 (or BCur) was centrifuged and washed several times with acetone, then dried at 70 °C in the oven. The two hydrophobic products were NPS-TiO2 and NPS-BCur.
Functionalization of cotton textile
A circular cotton fabric was cut to a diameter of 5 cm. To remove surface contaminants, it was washed with acetone in an ultrasonic bath and dried in an oven at 70 °C for 20 min. The cotton fabric was then placed inside a three-necked flask containing 100 ml of a 1% (w/v) APTES solution in toluene. The flask was then refluxed under stirring and nitrogen purging at 60 °C for 8 h. Subsequently, the fabrics were removed from the flask and washed several times with acetone in an ultrasonic bath for 10 min to remove unreacted materials. Finally, the fabric was dried in an oven at 60 °C for 15 min to obtain amine-functionalized cotton textile (Cotton-NH2).
Surface treatment of functionalized cotton fabric
In three separate beakers, 1 g of each obtained hydrophobic reagent (NPS, NPS-TiO2, or NPS-BCur) was dispersed in 20 ml of acetone and sonicated for 15 min. Then, three circular Cotton-NH2 fabrics (diameter: 5 cm) were separately dip-coated in the respective solutions for 5 min. Afterward, the cotton fabrics were removed from the solutions and placed on a Petri dish for heating in an oven at 85 °C for 8 h. Finally, to remove unreacted materials, the coated fabrics were washed several times with acetone in an ultrasonic bath and dried at 70 °C for 60 min. The three NPS-coated cotton fabrics were obtained and labeled as Cott-NPS, Cott-TiO2-NPS, and Cott-BCur-NPS.
Overall mechanism of superhydrophobic cotton filter fabrication
The synthesis of natural polysulfide (NPS) is based on a solvent-free inverse vulcanization reaction, where Pistacia atlantica gum reacts with sulfur powder under hydrothermal conditions. The high temperature promotes crosslinking between alkene functional groups in the gum and elemental sulfur, resulting in the formation of a stable polysulfide polymer network. The NPS reagent is then used to coat boehmite (BCur) and TiO₂ nanoparticles, where sulfur-containing species in NPS interact with hydroxyl groups on the nanoparticle surfaces, ensuring uniform coverage. To enhance adhesion to cotton fabric, the textile is first functionalized APTES, introducing amine (-NH2) groups that form strong hydrogen bonds and covalent interactions with the NPS coating, leading to a durable superhydrophobic layer.
Oil–water separation test
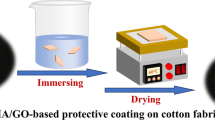
To perform the oil-water separation test, a simple setup including a filter holder (shown in Fig. 1) was used. Biphasic solutions of water and oily compounds (gasoline, toluene, petroleum ether, kerosene, gasoil, and n-hexane) were prepared with a volume ratio of 50:50. For better visual recognition, a few drops of methyl orange were added to 25 ml of water to obtain orange-colored water. Then, 25 ml of the oily compound was added to the colored water. These mixtures were then attempted to be separated by the prepared cotton fabric filters. The resulting biphasic solutions were separated in the filter setup based on gravity. Oily compounds passed through the prepared superhydrophobic cotton filter, while water was rejected by the filter (Fig. 1).
To evaluate the stability and durability of the hydrophobic coating on the cotton fabrics, we repeated the separation of biphasic mixtures of water-gasoline for 20 cycles. Separation efficiencies and WCAs of the filters were measured after each cycle. After each cycle, the fabrics were thoroughly washed with acetone and dried in an oven.
The set-up for oil-water separation using NPS coated superhydrophobic cotton filters (image created with Adobe photoshop Version 2019 https://www.adobe.com/products/photoshop.html).
Characterization
The surface morphology of the cotton fabric was identified before and after coating with NPS, NPS-TiO2, and NPS-BCur reagents using a scanning electron microscope (SEM) (TSCAN Company, Check). The chemical structures of NPS, NPS-TiO2, and NPS-BCur were identified using Fourier Transform Infrared Spectroscopy (FTIR) (IFS 48 FTIR spectrometer, Bruker Ettlingen, Germany) and X-ray Diffraction (XRD) (Herzog company, Germany) analyzers. To confirm the presence of functional groups on the surface of the fabrics, X-ray mapping analysis was conducted.
The water contact angle of the fabrics was measured with a Contact Angle meter (XCA-50, Iran), using the average contact angles of 5 water droplets (4 µl). The commercial SPIP Version 6.7.3 (Scanning Probe Image Processor) software (https://www.imagemet.com/products_/spip/) was utilized for the surface topography analysis of the SEM images of the cotton fabrics. Thermogravimetric analysis (TGA) was conducted using a Pyris Diamond analyzer under an air flow of 200 ml/min in the temperature range of 27–700 °C at a heating rate of 10 °C/min.
Results and discussion
Natural gum from Pistacia Atlantica is extracted from Persian turpentine trees in the western Zagros region of Kermanshah province in Iran. This natural gum primarily consists of turpentine and colophony. Turpentine contains more than 30 organic molecules, with α-pinene constituting the major part (41% w)47. While abietic acid is the major component of colophony. Therefore, the primary components of Pistacia Atlantica gum are α-pinene and abietic acid.
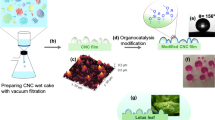
The hydrothermal reaction between sulfur and natural gum at 170 °C resulted in the formation of a sticky polysulfide (NPS). Almost all constituents of the gum possess an alkene functional group and form a polysulfide structure through a reverse vulcanization reaction with sulfur at high temperatures44. General trends in the synthesis of superhydrophobic reagents and nanoparticles are illustrated in Fig. 2. In Fig. 2a, the possible molecular structure of the red-brown polysulfide (NPS) is shown. It appears that the polymeric chains of NPS are primarily composed of three monomers: sulfur, α-pinene, and abietic acid. Figure 2b schematically illustrates the procedure for synthesizing BCur nanoparticles from extracted curcumin and boehmite nanoparticles. Functionalization of boehmite with CPTES reagent can create an active surface for covalent bonding with curcumin. Figure 2c depicts the surface coating of BCur nanoparticles through in situ hydrothermal formation of an NPS layer. Sulfur components within the NPS chains can react with functional groups of curcumin. In situ hydrothermal NPS coating of TiO2 nanoparticles is also illustrated in Fig. 2d. The synthesized NPS exhibits superhydrophobic nature and also induces hydrophobicity in both NPS-TiO2 and NPS-BCur nanoparticles.
Characterization of NPS, NPS-TiO2 and NPS-BCur reagents
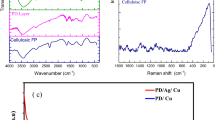
FTIR, XRD, and TGA analyses were utilized to identify the types of functional groups and the chemical structures of NPS, NPS-TiO2, and NPS-BCur. Figure 3 shows the FTIR spectra of these superhydrophobic reagents.
In the NPS spectrum, two peaks appearing at 2948 and 2863 cm−1 are attributed to alkane C–H stretching48. Additionally, peak broadening in the range of 2500–3500 cm−1 can be attributed to stretching vibrations of carboxylic acid OH groups49. The peak at 1705 cm−1 is attributed to C=O stretching vibrations50, primarily from the abietic acid component in the NPS structure. The two peaks at 1456 and 1377 cm−1 indicate alkane C-H bending vibrations for CH2 and CH3 groups51. C-O stretching vibrations appear at 1024 and 1246 cm−1 for carboxylic acid groups52. The peak at 1114 cm−1 can also be attributed to C=S vibrations for thiocarbonyl groups53 resulting from the reaction between sulfur and carbonyl groups in gum components. The absorption peak at 816 cm−1 corresponds to alkene = C–H out-of-plane vibrations54. The two peaks at 586 and 665 cm−1 can be attributed to C–S and S–S vibrations55 in the polysulfide structure. The sharp peak at 756 cm−1 corresponds to the vibration of S–O–R thioester groups56, which can be formed through the reaction between abietic acid and sulfide groups.
In the NPS-BCur spectrum, several characteristic peaks are observed: 1070, 3280, and 3056 cm−1 for Al–OH, and 637 and 480 cm−1 for Al–O–Al, which are indicative of the boehmite structure45. The broad peak at 3416 cm−1 corresponds to the phenolic OH57 of the curcumin structure. Another broad peak at 1638 cm−1 can also be attributed to C=C and C=O vibrations58 in the curcumin structure.
The presence of several NPS characteristic peaks in the NPS-BCur spectrum, such as those at 2948, 2863, 1705, 1456, 1377, and 756 cm−1, confirms the effective formation of an NPS layer on the surface of BCur nanoparticles. A decrease in the peak intensity at 1705 cm−1 in curcumin could be attributed to the reaction of NPS acidic carbonyl groups with functional groups in BCur nanoparticles.
In the spectrum of NPS-TiO2, the peak observed at 3454 cm−1 corresponds to the vibration of the O–H bond. The peak at 1631 cm−1 is attributed to absorbed water. The broad peak at 570 cm−1 corresponds to Ti–O–Ti bonds in TiO2 nanoparticles59. The broadening of the peak at 756 cm−1, along with the presence of some frequency peaks in the range of 100–1600 cm−1, as well as two peaks at 2924 and 2863 cm−1, can be attributed to the NPS coating.
.
XRD analysis is a suitable technique for accurately determining the phase composition of the nanoparticles used in this study. Figure 4 shows the XRD patterns of NPS-BCur and NPS-TiO2 nanoparticles. The sharp 2θ peaks observed at 27°, 36°, 54°, 55°, 63°, 69°, 71°, and 74° are indicative of the rutile phase of TiO2, while the peaks at 28°, 36°, 41°, 54°, and 69° correspond to the anatase phase of TiO2 [60].
The XRD pattern of NPS-BCur exhibits characteristic 2θ peaks at 28°, 32°, 40°, 50°, 65°, and 72°, which are attributed to the boehmite phase (γ-AlOOH)45. Compared to the NPS-TiO2 spectrum, the peaks in the NPS-BCur spectrum are slightly broader. This broadening suggests a higher amount of organic components (curcumin and NPS) on the surface of the boehmite nanoparticles compared to the NPS layer on the surface of TiO2. The higher amount of organic components on the boehmite surface may contribute to a more amorphous character in the NPS-BCur sample.
Thermogravimetric analysis (TGA) effectively reveals the thermal stability of the synthesized materials and also determines the coverage of organic compounds on the surface of boehmite and TiO2 nanoparticles. Figure 5 shows the thermograms of NPS, NPS-BCur, and NPS-TiO2.
For the NPS reagent, two stages of weight loss were observed. The first stage, up to 320 °C, resulted in a weight loss of approximately 30%, likely due to the degradation and breakdown of organic compounds within the polysulfide structure. In the second stage, the remaining 70% of the NPS was combusted and completely destroyed. The NPS reagent exhibited stability up to 100 °C.
For the NPS-BCur nanoparticles, three stages of weight loss were observed. The first stage, up to 290 °C, resulted in a weight loss of approximately 10%, likely due to the degradation and breakdown of organic compounds within the NPS-BCur structure. In the second stage, another 20% of NPS-BCur was combusted and completely destroyed. In the third stage, a further 12% weight loss occurred, which can be attributed to the phase transition of alumina. As a result, approximately 30% of the NPS-BCur structure consists of organic compounds, including curcumin and NPS polysulfide.
For the NPS-TiO2 nanoparticles, two stages of weight loss were observed. The first stage, up to 550 °C, resulted in a weight loss of approximately 5%, likely due to the degradation, breakdown, and combustion of the NPS organic layer. In the second stage, approximately 1.7% of the NPS-TiO2 weight decreased, which is associated with the phase transition of TiO2. It appears that NPS-TiO2 exhibits thermal stability up to 278 °C. The amount of NPS coating in NPS-TiO2 is approximately 5%, which is lower than that in NPS-BCur.
Figure 6 schematically illustrates the coating process for cotton textile fabrics using superhydrophobic reagents and nanoparticles. To enhance the adhesion forces between the NPS reagent and the surface of the cotton fibers, the fabric surface was initially functionalized with the APTES reagent. As shown in Fig. 6, the –NH2 groups introduced onto the cotton surface can form stronger hydrogen bonds with the –S-S-S– groups in the NPS polymer. These strong hydrogen bonds contribute to the durability and greater stability of the hydrophobic textile fabric. Hydrogen bonding between the NPS coating on the nanoparticle surface and the –NH2 groups on the cotton surface facilitates the strong adsorption of nanoparticles.
In cotton fabrics coated with NPS-BCur and NPS-TiO2 nanoparticles, both surface roughness and superhydrophobicity were achieved simultaneously. In contrast, the fabric coated with NPS alone exhibited only hydrophobic properties without any significant surface roughness. The incorporation of coated nanoparticles offers the advantage of creating surface roughness, which is a crucial factor in achieving superhydrophobicity on surfaces.
The schematic for preparation of cotton treated with NPS, NPS-BCur and NPS-TiO2 (image created with Adobe photoshop Version 2019 https://www.adobe.com/products/photoshop.html).
Creating surface roughness is one of the effective and common methods for achieving superhydrophobic surfaces61. The chemical nature and surface morphology of the textile fabric are two crucial factors that, when combined, can significantly enhance the level of surface hydrophobicity. As shown in Fig. 7, the surface morphology of the cotton textiles was investigated using SEM analysis. Figure 7a presents SEM images of untreated cotton fabric. The cotton fabric is composed of an interwoven texture of very smooth fibers with a diameter of approximately 10 μm. Figure 7b shows that the surface of the treated cotton fibers is covered with a thin layer of NPS polysulfide. This layer appears relatively smooth and lacks significant roughness. In contrast, Fig. 7c reveals that the surface of the cotton fibers is completely covered by a uniform and rough layer of NPS-TiO2 nanoparticles.
These nanoparticles have sizes ranging from approximately 20–30 nm, with some agglomerated nanoparticles observed in the range of 50–70 nm. As shown in Fig. 7d, the surface of the cotton fibers is completely and uniformly coated with NPS-BCur nanoparticles, resulting in a significantly increased surface roughness. The size of NPS-BCur nanoparticles ranges from 30 to 40 nm, with some agglomerated nanoparticles observed in the range of 80–100 nm. Using the SPIP analysis software, Fig. 8 illustrates the topographic views of cotton fabrics before and after treatment with the hydrophobic reagent (NPS) and nanoparticles (NPS-BCur, NPS-TiO2). The surface roughness of the cotton fabric was significantly increased after coating, with this increase being more pronounced in samples coated with nanoparticles (NPS-BCur, NPS-TiO2).
To quantify the effect of coatings on surface morphology, surface roughness parameters were determined using Scanning Probe Image Processor (SPIP) software based on SEM image analysis. The average roughness (Ra) and root mean square roughness (Rq) values were measured for both untreated and coated cotton fabrics. The results showed a significant increase in surface roughness after coating. The Ra values increased from 45.3 nm (untreated cotton) to 132.8 nm (Cotton-NPS), 168.5 nm (Cotton-NPS-TiO2), and 182.1 nm (Cotton-NPS-BCur). Similarly, Rq values followed the same trend, increasing from 52.1 nm (untreated cotton) to 150.4 nm (Cotton-NPS), 189.2 nm (Cotton-NPS-TiO2), and 201.7 nm (Cotton-NPS-BCur). The highest roughness values observed in Cotton-NPS-BCur can be attributed to the presence of boehmite nanoparticles, which enhance the formation of micro/nanoscale hierarchical structures. These findings confirm that the increase in roughness significantly contributes to the superhydrophobic behavior of the coatings by facilitating the formation of air pockets, reducing solid-liquid contact, and enhancing water repellency.
Topography of the cotton fabric surface (a) representative and self-assembled with the hydrophobic reagent (b) NPS (c) NPS-TiO2 (d) NPS-BCur using the SPIP software (SPIP Version 6.7.3 (Scanning Probe Image Processor) software (https://www.imagemet.com/products_/spip/).
The presence and distribution of key elements within the superhydrophobic coating layer on the surface of cotton fibers were investigated using X-ray mapping analysis. Figure 9 comparatively shows the distribution of key elements (C, O, N, S, Al, and Ti) in the X-ray mapping images of coated and non-coated cotton fabrics.
Carbon and oxygen are the primary constituents of all cotton fabrics. The presence of nitrogen (N) confirms the existence of the amine (–NH2) functional group introduced by the APTES coating on the cotton fabric surface. In the raw cotton fabric, a small amount of nitrogen was observed in the mapping image, likely due to the presence of small amounts of natural nitrogenous compounds such as amino acids or other organic compounds within the raw cotton structure. In the mapping images of the coated cotton fabrics, a significant increase in the intensity of the nitrogen signal was observed, confirming the uniform distribution of the APTES coating across the fabric surface.
The presence of sulfur (S) confirms the presence of the NPS polysulfide coating. Similarly, the presence of titanium (Ti) and aluminum (Al) indicates the presence of TiO2 and boehmite nanoparticles, respectively, on the fabric surface. In all of the mapping images for coated cotton fabrics, a uniform and abundant distribution of sulfur (S), titanium (Ti), and aluminum (Al) was observed, confirming the uniform coating of hydrophobic reagents across the cotton fabric surface.
The degree of hydrophobicity and hydrophilicity of cotton fabrics can be determined by measuring the water contact angles (WCA). Figure 10 presents the results of WCA measurements for non-coated and coated cotton fabrics. The raw cotton fabric exhibited a zero-degree water contact angle, indicating its completely superhydrophilic nature.
Cotton fabric coated with NPS exhibited complete hydrophobicity, although the water contact angle (WCA) was slightly below 150°. In contrast, coating with NPS-BCur and NPS-TiO2 nanoparticles resulted in the formation of superhydrophobic fabrics with WCAs exceeding 150°. The synergy between the hydrophobic nature of NPS and the surface roughness created by the nanoparticles is the primary factor contributing to the achievement of superhydrophobicity on the fabric.
Investigation of oil water separation
Cotton fabrics coated with hydrophobic reagents were investigated for their potential application as superhydrophobic filters for oil-water separation of biphasic mixtures. The schematic of the separation setup is shown in Fig. 1.
The superhydrophobic cotton fabrics can rapidly absorb the oily phase and act as an impenetrable barrier, preventing the water phase from passing through. The oil separation efficiency was calculated using the following equation13:
Separation Efficiency (%) = 100 × (1 - m / m₀).
Where m is the weight of the permeated hydrocarbon and m₀ is the initial weight of the hydrocarbon. It is important to note that the weight of the solvent adsorbed by the filter was not included in the calculations. If this factor is considered, the separation efficiency would likely be higher.
Figure 11 compares the separation efficiencies of various coated cotton fabrics for different biphasic hydrocarbon mixtures. The separation efficiencies follow the order: Cotton-NPS < Cotton-NPS-TiO2 < Cotton-NPS-BCur.
Filters coated with the NPS reagent exhibited lower hydrophobicity compared to those coated with nanoparticles, resulting in lower separation efficiency. Surface coating with nanoparticles enhances surface roughness, leading to superhydrophobic properties. The higher separation efficiency of Cotton-NPS-BCur compared to Cotton-NPS-TiO2 can be attributed to the presence of curcumin molecules within the BCur nanoparticles. Curcumin possesses a relatively hydrophobic nature.
Heavy oil–water separation performance
To evaluate the effectiveness of the superhydrophobic coatings in separating heavy oil-water mixtures, additional experiments were conducted using crude oil. The coated fabrics (Cotton-NPS, Cotton-NPS-TiO2, and Cotton-NPS-BCur) were used to separate a crude oil/water mixture (1:1 v/v) under gravity-driven filtration. The separation efficiency was determined gravimetrically by weighing the collected oil phase before and after filtration. Cotton-NPS-BCur and Cotton-NPS-TiO2 achieved 98.1% and 97.6% separation efficiency, respectively, while Cotton-NPS exhibited 95.8% efficiency. The slightly lower efficiency compared to light oil separation is attributed to the higher viscosity and stronger adhesion of crude oil to the fabric surface. These results confirm that the superhydrophobic coatings are highly effective in separating heavy oils from water, making them promising candidates for oil spill remediation and industrial wastewater treatment.
These findings demonstrate the exceptional durability, chemical stability, and broad applicability of the coated fabrics, reinforcing their potential for real-world environmental applications.
Durability and stability
To evaluate the stability and durability of the hydrophobic coating on the cotton fabrics, the separation of biphasic mixtures of water and gasoline was repeated for 20 cycles. Separation efficiencies and WCAs of the filters were measured after each cycle. After each cycle, the fabrics were thoroughly washed with acetone and dried in an oven. The results of the recycling process are presented in Fig. 12.
Figure 12a shows that the separation efficiency of the cotton-NPS filter decreased slightly from 92.29 to 91.02% after 20 separation cycles, a negligible change. Concurrently, the WCA of cotton-NPS decreased from 148° to 138°, suggesting the dissolution of some NPS polysulfide from the fabric surface by the oil phase. However, this 10-degree reduction in WCA had a minimal impact on the separation efficiency of the cotton-NPS filter.
Figure 12b demonstrates that the reduction in separation efficiency for the cotton-NPS-TiO2 fabric was 0.65% after 20 cycles of recycling. The WCA also decreased slightly, from 157° to 153°. According to Fig. 12c, the reduction in separation efficiency for the cotton-NPS-BCur fabric was 0.53% after 20 cycles of recycling, from 98.73 to 98.2%. The WCA for cotton-NPS-BCur also decreased slightly, from 162° to 157°. The very minor changes in WCA and separation efficiencies for the superhydrophobic coated fabrics, particularly those coated with NPS-BCur and NPS-TiO2, indicate the high stability and durability of these superhydrophobic filters.
The utilization of natural compounds such as curcumin, gum, sulfur powder, and cotton fibers in the synthesis and fabrication of superhydrophobic fabrics is a significant advantage of this work, aligning with the principles of green chemistry and sustainable development. These superhydrophobic reagents and filters have the potential to be highly efficient and effective in addressing environmental pollution.
Effect of nanoparticles on the stability of NPS coating
The slight decrease in separation efficiency observed for Cotton-NPS (from 92.29 to 91.02% after 20 cycles) can be attributed to the partial dissolution of NPS polysulfides in the oil phase over repeated use. However, for Cotton-NPS-TiO₂ and Cotton-NPS-BCur, a similar dissolution effect is expected to be significantly lower due to the stronger interactions between the NPS layer and the nanoparticles. The presence of TiO2 and BCur nanoparticles enhances coating stability by increasing adhesion forces and reducing the mobility of the polysulfide network. This is supported by the minimal reduction in separation efficiency observed for Cotton-NPS-TiO2 (from 98.2 to 97.6%) and Cotton-NPS-BCur (from 98.73 to 98.2%) after 20 cycles, which is lower than the decline seen in Cotton-NPS. These results indicate that the incorporation of nanoparticles improves coating stability, effectively mitigating the dissolution of NPS polysulfides in the oil phase and enhancing long-term performance.
Textile tensile breaking strength test
The mechanical strenght of all coated and non-coated cotton fabrics was evaluated through tensile breaking strength test according to the standard of GB/T 3923.1–1997. The maximum stress results, which indicate the strength of the fabrics, are shown in Fig. 13 for comparison. The results indicate that coating cotton fabric with NPS reagent and nanoparticles led to an increase in the tensile strength of the fabric, with the highest value corresponding to the fabric coated with BCur-NPS nanoparticles, which could be due to stronger interactions of this nanoparticle with the surface of cellulose fibers.
Mechanical durability of superhydrophobic cotton fabrics
The mechanical robustness of the coated cotton fabrics was evaluated through cyclic tensile strain tests at deformation levels of 10%, 20%, and 50% using a universal tensile testing machine (model 5567 from Instron Co., Ltd). Each sample underwent 50 and 100 stretching cycles, followed by measurements of the water contact angle (WCA) and oil-water separation efficiency. At lower strain levels (10% and 20%), the filters maintained WCAs above 161°, 155°, and 145° for NPS-BCur, NPS-TiO2, and NPS, respectively, indicating minimal impact on surface hydrophobicity. Even at 50% strain, the WCAs for NPS-BCur, NPS-TiO2, and NPS remained around 159°, 153°, and 143°, respectively, suggesting only minor structural disruptions. The separation efficiency consistently exceeded 98% for all filters, demonstrating strong adhesion of the NPS coating to the cotton substrate. These results highlight the excellent mechanical durability of the superhydrophobic coating, making it highly suitable for real-world applications where flexibility and repeated mechanical deformation are expected.
Abrasion resistance of superhydrophobic cotton fabrics
To assess mechanical durability under frictional forces, the coated fabrics were subjected to abrasion tests using 800-grit sandpaper under a 100 g applied load for 100 cycles. Additionally, a controlled blade scratching test was performed to simulate mechanical wear. After every 20 cycles, the water contact angle (WCA) and oil-water separation efficiency were measured. The results showed that even after 100 abrasion cycles, the WCA remained above 140° for Cotton-NPS, 150° for Cotton-NPS-TiO₂, and 155° for Cotton-NPS-BCur, indicating strong retention of hydrophobic properties. Oil-water separation efficiency exhibited only a minor decrease, remaining above 98%, confirming the excellent abrasion resistance of the NPS coating. These findings demonstrate that the superhydrophobic coatings maintain their functionality even after repeated mechanical wear, highlighting their suitability for real-world applications where durability under friction is essential.
Water impact resistance of superhydrophobic cotton fabrics
To investigate the water impact resistance of the superhydrophobic filters, a high-pressure water jet (1.5 L/min for 5 min) was applied to the fabrics to simulate environmental water exposure. No significant changes in the water contact angle (WCA) or separation efficiency were observed, confirming the strong adhesion of the coatings.
Chemical stability (acidic and alkaline resistance)
To investigate the chemical resistance of the coated fabrics (Cotton-NPS, Cotton-NPS-TiO2, and Cotton-NPS-BCur), the samples were immersed in acidic (pH 3, HCl solution) and alkaline (pH 11, NaOH solution) for 24 h. After exposure, the water contact angle (WCA) and oil-water separation efficiency were measured. Cotton-NPS exhibited a slight decrease in WCA (148° to 138°) and a 3% reduction in efficiency, indicating moderate sensitivity to harsh pH conditions. However, Cotton-NPS-TiO2 and Cotton-NPS-BCur remained highly stable, retaining WCA values above 150° and separation efficiencies > 98%, demonstrating excellent resistance to extreme pH environments.
Environmental impact and advantages of Pistacia atlantica gum-based coatings
The sustainability and environmental impact of superhydrophobic coatings are crucial considerations for large-scale applications62. Conventional synthetic coatings, such as fluorinated polymers63, polydimethylsiloxane (PDMS)64, and tetraethyl orthosilicate (TEOS)65, offer excellent superhydrophobicity and durability but often rely on toxic precursors, costly reagents, and complex fabrication processes, limiting their eco-friendliness and scalability. Additionally, silane coupling agents such as APTES and CPTES are commonly used to enhance adhesion between organic and inorganic phases; however, they may pose environmental concerns due to potential toxicity and persistence. In this study, their use was minimized, with Pistacia Atlantica gum serving as a natural, eco-friendly alternative, reducing reliance on synthetic reagents. The natural polysulfide (NPS)-based coatings developed in this work utilize abundant, renewable, and biodegradable materials, offering high performance with water contact angles exceeding 150° and oil-water separation efficiency above 98%, comparable to or even superior to synthetic alternatives. Additionally, the use of low-cost natural materials significantly reduces production expenses, making them a viable option for sustainable oil-water separation technologies. More importantly, the biodegradable and non-toxic nature of Pistacia Atlantica gum ensures environmental sustainability, aligning with green chemistry principles and reducing the ecological impact often associated with synthetic coatings. These advantages highlight the potential of natural polysulfide-based coatings as a viable and sustainable solution for oil-water separation applications, promoting eco-friendly functional material development.
Conclusion
In this study, a novel NPS polymer reagent, a hydrophobic and sticky polysulfide, was synthesized utilizing natural compounds, including Pistacia Atlantica gum and sulfur powder, under hydrothermal conditions. Boehmite nanoparticles were synthesized and subsequently functionalized with natural curcumin and then coated with the NPS reagent. Additionally, TiO2 nanoparticles were separately coated with the NPS reagent. Both types of coated superhydrophobic nanoparticles were thoroughly characterized. Subsequently, cotton fabrics were individually dip-coated with three reagents: NPS, NPS-BCur, and NPS-TiO2, resulting in the fabrication of hydrophobic filters with water contact angles exceeding 150 degrees.
The performance of these superhydrophobic filters in separating biphasic oil-water mixtures was evaluated, demonstrating separation efficiencies ranging from 91 to 98.6%. After 20 cycles of water-gasoline separation, minimal changes were observed in both water contact angles and separation efficiencies for these superhydrophobic coated fabrics, particularly those coated with NPS-BCur and NPS-TiO2, indicating their exceptional stability and durability.
The utilization of natural materials in the synthesis process, coupled with the observed eco-friendliness, high durability, and high separation efficiency, highlights the significant advantages of these superhydrophobic cotton fabrics. These fabrics exhibit strong potential for effective application in addressing environmental pollution, particularly in oil-water separation.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Chen, P. C. & Xu, Z. K. Mineral-coated polymer membranes with superhydrophilicity and underwater superoleophobicity for effective oil/water separation. Sci. Rep. 3 (1), 2776 (2013).
Wang, L. K. Waste chlorination and stabilization. Adv. Physicochem. Treat. Process. 403–440 (2006).
Guerin, T. F. Heavy equipment maintenance wastes and environmental management in the mining industry. J. Environ. Manag. 66 (2), 185–199 (2002).
Ventikos, N. P. et al. A high-level synthesis of oil spill response equipment and countermeasures. J. Hazard. Mater. 107 (1–2), 51–58 (2004).
Calcagnile, P. et al. Magnetically driven floating foams for the removal of oil contaminants from water. ACS Nano. 6 (6), 5413–5419 (2012).
Dong, X. et al. Superhydrophobic and superoleophilic hybrid foam of graphene and carbon nanotube for selective removal of oils or organic solvents from the surface of water. Chem. Commun. 48 (86), 10660–10662 (2012).
Nguyen, D. D. et al. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 5 (7), 7908–7912 (2012).
Zhu, Q. et al. Robust superhydrophobic polyurethane sponge as a highly reusable oil-absorption material. J. Mater. Chem. A. 1 (17), 5386–5393 (2013).
Li, J. et al. One-step fabrication of robust fabrics with both-faced superhydrophobicity for the separation and capture of oil from water. Phys. Chem. Chem. Phys. 17 (9), 6451–6457 (2015).
Zhang, J. & Seeger, S. Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv. Funct. Mater. 21 (24), 4699–4704 (2011).
Zhang, L., Zhang, Z. & Wang, P. Smart surfaces with switchable superoleophilicity and superoleophobicity in aqueous media: toward controllable oil/water separation. NPG Asia Mater. 4 (2), e8–e8 (2012).
Rostami, A. et al. Fabrication of durable superhydrophobic nanofibrous filters for oil-water separation using three novel modified nanoparticles (ZnO–NSPO, AlOO–NSPO, and TiO2–NSPO). Polym. Adv. Technol. 31 (5), 941–956 (2020).
Rostami, A. & Sharifnia, S. Fabrication of robust and durable superhydrophobic fiberglass fabrics for oil–water separation based on self-assembly of novel N-TESPO and N-TESPS reagents. J. Mater. Chem. A. 5 (2), 680–688 (2017).
Tian, D. et al. Micro/nanoscale hierarchical structured ZnO mesh film for separation of water and oil. Phys. Chem. Chem. Phys. 13 (32), 14606–14610 (2011).
Wang, S., Li, M. & Lu, Q. Filter paper with selective absorption and separation of liquids that differ in surface tension. ACS Appl. Mater. Interfaces. 2 (3), 677–683 (2010).
Zhou, X. et al. Robust and durable superhydrophobic cotton fabrics for oil/water separation. ACS Appl. Mater. Interfaces. 5 (15), 7208–7214 (2013).
Spaeth, M. & Barthlott, W. Lotus-effect®: biomimetic super-hydrophobic surfaces and their application. Adv. Sci. Technol. 60, 38–46 (2009).
Zhao, Y. et al. Photoreactive azido-containing silica nanoparticle/polycation multilayers: durable superhydrophobic coating on cotton fabrics. Langmuir 28 (15), 6328–6335 (2012).
Sebastian, D., Yao, C. W. & Lian, I. Abrasion resistance of superhydrophobic coatings on aluminum using PDMS/SiO2. Coatings 8 (11), 414 (2018).
Xu, Q. F. et al. Superhydrophobic TiO2–polymer nanocomposite surface with UV-induced reversible wettability and self-cleaning properties. ACS Appl. Mater. Interfaces. 5 (18), 8915–8924 (2013).
He, X. & Zhou, W. Hydrophobic modification and durability protection of cotton garment fabric surfaces by graphene oxide/pgma composite coatings. Sci. Rep. 14 (1), 1–10 (2024).
Chen, X., Weibel, J. A. & Garimella, S. V. Continuous oil–water separation using polydimethylsiloxane-functionalized melamine sponge. Ind. Eng. Chem. Res. 55 (12), 3596–3602 (2016).
Feng, L. et al. A super-hydrophobic and super‐oleophilic coating mesh film for the separation of oil and water. Angew. Chem. 116 (15), 2046–2048 (2004).
Wu, L. et al. Magnetic, durable, and superhydrophobic polyurethane@ Fe3O4@ SiO2@ fluoropolymer sponges for selective oil absorption and oil/water separation. ACS Appl. Mater. Interfaces. 7 (8), 4936–4946 (2015).
Zhang, A. et al. Poly (dimethylsiloxane) oil absorbent with a three-dimensionally interconnected porous structure and swellable skeleton. ACS Appl. Mater. Interfaces. 5 (20), 10201–10206 (2013).
Huang, J. et al. Robust superhydrophobic TiO 2@ fabrics for UV shielding, self-cleaning and oil–water separation. J. Mater. Chem. A. 3 (6), 2825–2832 (2015).
Yang, J. et al. Rapid dipping preparation of superhydrophobic TiO2 cotton fabric for multifunctional highly efficient oil-water separation and photocatalytic degradation. Colloids Surf. A. 657, 130590 (2023).
Xu, B. & Cai, Z. Fabrication of a superhydrophobic ZnO Nanorod array film on cotton fabrics via a wet chemical route and hydrophobic modification. Appl. Surf. Sci. 254 (18), 5899–5904 (2008).
Xu, W. et al. Robust ZnO/HNTs-based superhydrophobic cotton fabrics with UV shielding, self-cleaning, photocatalysis, and oil/water separation. Cellulose 29 (7), 4021–4037 (2022).
Du, B. et al. Superhydrophobic surfaces with pH-induced switchable wettability for oil–water separation. ACS Omega. 4 (15), 16508–16516 (2019).
Hou, C., Fan, Z. & Yang, J. Preparation and properties of super hydrophobic cotton fabric constructed by modified nano-SiO2 hybrid fluoro-epoxy copolymer. Surf. Interfaces. 45, 103814 (2024).
Guo, H. et al. A robust cotton textile-based material for high-flux oil–water separation. ACS Appl. Mater. Interfaces. 11 (14), 13704–13713 (2019).
Meng, X. et al. ZIF-8/GO/PDMS modified cotton fabric to form a hierarchical-structure coating for fast oil/water separation. J. Water Process. Eng. 60, 105158 (2024).
Ahmad, W. et al. Silica-Based superhydrophobic and superoleophilic cotton fabric with enhanced self-cleaning properties for oil–water separation and methylene blue degradation. Langmuir 40 (11), 5639–5650 (2024).
Bae, G. Y. et al. Superhydrophobicity of cotton fabrics treated with silica nanoparticles and water-repellent agent. J. Colloid Interface Sci. 337 (1), 170–175 (2009).
Shang, Q. et al. Sustainable and robust superhydrophobic cotton fabrics coated with castor oil-based nanocomposites for effective oil–water separation. ACS Sustain. Chem. Eng. 8 (19), 7423–7435 (2020).
Cheng, J. et al. One-step, low-cost and environmentally friendly method to prepare superhydrophilic polyester fabric for oil-water separation. Surf. Coat. Technol. 476, 130198 (2024).
Daneshrad, A. & Aynehchi, Y. Chemical studies of the oil from pistacia nuts growing wild in Iran. J. Am. Oil Chem. Soc. 57 (8), 248–249 (1980).
Delazar, A., Reid, R. & Sarker, S. GC-MS analysis of the essential oil from the Oleoresin of pistacia Atlantica Var. Mutica. Chem. Nat. Compd. 40, 24–27 (2004).
El Zerey-Belaskri, A., Belyagoubi-Benhammou, N. & Benhassaini, H. From traditional knowledge to modern formulation: potential and prospects of pistacia Atlantica Desf. Essential and fixed oils uses in cosmetics. Cosmetics 9 (6), 109 (2022).
Mahjoub, F. et al. Effect of pistacia Atlantica Kurdica gum on diabetic gastroparesis symptoms: a randomized, triple-blind placebo-controlled clinical trial. Electron. Phys. 10 (7), 6997 (2018).
Mohammadi, G. et al. Preparation and characterization of pistacia Atlantica oleo-gum-resin-loaded electrospun nanofibers and evaluating its wound healing activity in two rat models of skin Scar and burn wound. Front. Pharmacol. 15, 1474981 (2024).
Rahbar Saadat, Y. et al. Cyto/genotoxic effects of pistacia Atlantica resin, a traditional gum. DNA Cell Biol. 35 (6), 261–266 (2016).
Worthington, M. J., Kucera, R. L. & Chalker, J. M. Green chemistry and polymers made from sulfur. Green Chem. 19 (12), 2748–2761 (2017).
Rajabi, L. & Derakhshan, A. Room temperature synthesis of boehmite and crystallization of nanoparticles: effect of concentration and ultrasound. Sci. Adv. Mater. 2 (2), 163–172 (2010).
Jani, M. A. & Bahrami, K. BNPs@ Cur-Pd as a versatile and recyclable green nanocatalyst for Suzuki, heck and Stille coupling reactions. J. Exp. Nanosci. 15 (1), 182–201 (2020).
Minaiyan, M., Karimi, F. & Ghannadi, A. Anti-inflammatory effect of pistacia Atlantica subsp. Kurdica volatile oil and gum on acetic acid-induced acute colitis in rat. Res. J. Pharmacognosy. 2 (2), 1–12 (2015).
Hill, I. R. & Levin, I. W. Vibrational spectra and carbon–hydrogen stretching mode assignments for a series of n-alkyl carboxylic acids. J. Chem. Phys. 70 (2), 842–851 (1979).
Max, J. J. & Chapados, C. Infrared spectroscopy of aqueous carboxylic acids: comparison between different acids and their salts. J. Phys. Chem. A. 108 (16), 3324–3337 (2004).
Sato, H. et al. FT-IR and near-infrared FT-Raman study of aggregation of bacteriochlorophyll C in solutions: evidence for involvement of the ester group in the aggregation. Biochemistry 34 (24), 7854–7860 (1995).
Bellamy, L. The Infra-red Spectra of Complex Molecules (Springer Science & Business Media, 2013).
Cotton, F. A. & Kraihanzel, C. Vibrational spectra and bonding in metal carbonyls. I. Infrared spectra of phosphine-substituted group VI carbonyls in the CO stretching region. J. Am. Chem. Soc. 84 (23), 4432–4438 (1962).
Rao, C. & Venkataraghavan, R. The C = S stretching frequency and the – N – C = S bands in the infrared. Spectrochim. Acta Part. A Mol. Spectrosc. 45, 299–305 (1989).
Smith, B. The Infrared Spectroscopy of Alkenes. (2016).
Bastian, E. J. Jr & Martin, R. B. Disulfide vibrational spectra in the sulfur-sulfur and carbon-sulfur stretching region. J. Phys. Chem. 77 (9), 1129–1133 (1973).
Sheppard, N. The vibrational spectra of some organic sulphur compounds and the characteristic frequencies of C—S linkages. Trans. Faraday Soc. 46, 429–439 (1950).
Kleinermanns, K. et al. Infrared spectroscopy of resonantly ionized (Phenol)(H2O) N++. J. Phys. Chem. A. 103 (27), 5232–5239 (1999).
Ţucureanu, V., Matei, A. & Avram, A. M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 46 (6), 502–520 (2016).
Viana, M., Soares, V. & Mohallem, N. Synthesis and characterization of TiO2 nanoparticles. Ceram. Int. 36 (7), 2047–2053 (2010).
Zhao, Y. et al. Synthesis and optical properties of TiO2 nanoparticles. Mater. Lett. 61 (1), 79–83 (2007).
Zhang, X. et al. Superhydrophobic surfaces: from structural control to functional application. J. Mater. Chem. 18 (6), 621–633 (2008).
Xu, C. L. et al. Dual superlyophobic materials for Under-Liquid microfluid manipulation, immiscible solvent separation, and CO2 blockage. ACS Appl. Mater. Interfaces. 15 (15), 19761–19772 (2023).
Bayer, I. S. Superhydrophobic coatings from ecofriendly materials and processes: a review. Adv. Mater. Interfaces. 7 (13), 2000095 (2020).
Gong, X. & He, S. Highly durable superhydrophobic Polydimethylsiloxane/silica nanocomposite surfaces with good self-cleaning ability. ACS Omega. 5 (8), 4100–4108 (2020).
Bakar, N. H. A. et al. Synthesis of a water-based TEOS–PDMS sol–gel coating for hydrophobic cotton and polyester fabrics. New J. Chem. 48 (2), 933–950 (2024).
Author information
Authors and Affiliations
Contributions
Z.S. was the primary author of this study. A.A.D. proposed the novelty of this work. L.R. supervised the project. A.R. reviewed and edited the data for this research. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Seifi, Z., Derakhshan, A.A., Rostami, A. et al. Green synthesis of superhydrophobic cotton filters using Pistacia atlantica gum for efficient oil and water separation. Sci Rep 15, 11536 (2025). https://doi.org/10.1038/s41598-025-96721-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96721-0