Abstract
Post-prostatectomy continence status, in addition to lower urinary tract symptoms, is a major concern among patients after robotic-assisted radical prostatectomy (RaRP). In this prospective study, we enrolled patients undergoing RaRP to evaluate subjective urinary symptoms and objective urodynamic parameters before and after surgery. Patients were recruited before RaRP surgery between January 2019 and August 2020. One day before surgery, the participants completed three questionnaires and pressure-flow studies, which were repeated approximately 3 months postoperatively. Of the total 135 patients initially enrolled, 85 (63.0%) completed the entire follow-up period. Three months after RaRP, the International Prostate Symptom Score showed significant increases in storage symptoms. Similar trends were observed in the Urinary Distress Inventory Short Form, and Overactive Bladder Symptom Score questionnaires. More than half of the patients regained continence within 2 months, although 9.6% remained incontinent after 1 year. Postoperative urodynamic studies indicated increased bladder hypersensitivity and significantly decreased detrusor pressure at peak flow. Furthermore, the bladder contractility index and bladder outlet obstruction index were reduced postoperatively. Ten patients (11.8%) developed de novo detrusor overactivity. The multivariate analysis identified age and cross-sectional area of the bladder neck as predictors of immediate continence after RaRP.
Similar content being viewed by others
Introduction
Robotic-assisted radical prostatectomy (RaRP) has become the predominant surgical approach for treating localized prostate cancer, potentially providing beneficial oncological and functional outcomes1,2,3. However, a common postoperative concern encountered with this approach is post-prostatectomy incontinence (PPI), particularly stress urinary incontinence (SUI)4,5. According to the 2015 LAPPRO study, approximately 21.3% of patients continued to experience incontinence 1 year after undergoing RaRP6. Beyond SUI, urgency urinary incontinence (UUI) and other storage symptoms such as frequency, urgency, and nocturia deserve attention as well5,7. Generally, lower urinary tract symptoms worsen immediately after prostatectomy but gradually improve, sometimes surpassing baseline conditions within several months8,9,10. Individuals with severe baseline symptoms tend to experience substantial improvements compared with their counterparts11.
Various studies focusing on urodynamic investigations before and after prostatectomy have contributed to an objective understanding of the functional changes after RaRP. Universally observed improvements include enhanced uroflow and reduced residual urine volume12,13,14. Patients with lower uroflow rates before RaRP tend to experience more considerable improvement, although their post-RaRP uroflow rates may still be lower than those in healthy patients15. Urethral pressure profile studies have indicated an initial decrease in the maximum urethral closure pressure and functional profile length after surgery, followed by partial recovery16,17.
Nevertheless, studies on urodynamic surveys in patients undergoing RaRP have common limitations, particularly because of the more invasive examinations used, such as those encountered in pressure-flow studies13,16. Owing to the invasive nature of these examinations, only a small number of patients are recruited in most studies, and a high withdrawal rate leads to attrition bias. In addition, urodynamic studies conducted at different time points after the operation may yield disparate results, leading to inconsistencies among studies. Furthermore, there was a lack of data on the chronological changes observed in these studies. Finally, the heterogeneity of patient background characteristics, including operative methods and the involvement of single or multiple operators, complicates interpretation of the findings. Therefore, in this study, we aimed to investigate symptomatic and urodynamic changes before and 3 months after RaRP within a single Taiwanese institute.
Methods
Ethics approval, patient recruitment, and background data collection
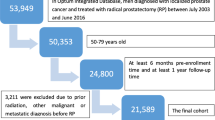
This prospective study adhered to the principles outlined in the Declaration of Helsinki, and was reviewed and approved by the 104th meeting of the Institutional Review Board of Taipei Veterans General Hospital (IRB-TPEVGH No.: 2018-08-007B). Between January 2019 and August 2020, we prospectively enrolled patients with localized prostate cancer scheduled to undergo RaRP. All participants in the study provided informed consent. In addition to age and body mass index, we meticulously documented the characteristics of prostate cancer for each patient, including initial prostate-specific antigen (PSA) levels, Gleason score, and tumor, node, and metastasis stage, as determined by magnetic resonance imaging (MRI) and whole-body bone scanning.
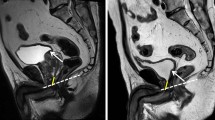
We conducted a comprehensive review of the MRI findings of each patient, incorporating specific measurements of interest. Prostate volume was calculated using the following ellipsoid volume formula: length × width × height × π / 6. The prostate-urethral angle determined from the sagittal view was defined as the angle formed by the proximal and distal prostatic urethra18,19. The cross-sectional area of the bladder neck was approximated as an ellipse at the junction of the bladder and prostate, using the following formula: length × width × π / 4. Membranous urethral length and the presence of intravesical prostatic protrusion (IPP) were measured from the sagittal view. We also evaluated whether the patient had undergone transurethral resection of the prostate using MRI, in addition to conducting a thorough history review. The surgical procedures were performed by multiple surgeons, all of whom used a transperitoneal approach with posterior reconstruction. Preservation of neurovascular bundles and the decision to perform bladder neck reconstruction were also documented for further analysis.
Study design, urinary questionnaires, and urodynamic investigation
On the day preceding RaRP, patients were instructed to complete three questionnaires pertaining to their existing urinary symptoms. These included the International Prostate Symptoms Score (IPSS), Urinary Distress Inventory, Short Form (UDI-6), and Overactive Bladder Symptom Score (OABSS). Pressure-flow studies were conducted on these patients. Approximately 3 months post-RaRP, the patients underwent a second pressure-flow study and again completed the three aforementioned questionnaires. Continence status was recorded during the outpatient clinic follow-up, with continence defined as the absence of observed urine leakage and non-use of incontinence pads.
The IPSS comprises three questions related to storage symptoms, four questions related to voiding symptoms, and an independent item for assessing quality of life. The UDI-6 further investigates urinary incontinence, whereas the OABSS focuses on three storage symptoms and UUI. Pressure-flow studies encompassed the filling and voiding phases. Normal saline was infused into the bladder during the filling phase. Patients reported their first sensation of bladder filling, referred to as the first desire (FD), as well as their strong desire (SD). The presence of involuntary detrusor contractions indicates detrusor overactivity. In the voiding phase, the peak flow (Qmax), detrusor pressure at peak flow (PdetQmax), voided volume, and post-void residual urine volume (PVR) were documented. These parameters allowed for the calculation of the bladder contractility index (BCI) and bladder outlet obstruction index (BOOI). The BCI is calculated as PdetQmax + 5Qmax, whereas the BOOI is calculated as PdetQmax – 2Qmax.
Statistical analyses
Paired t-tests were used to assess changes in patient symptoms and urodynamic parameters before and after RaRP. Additionally, univariate and multivariate logistic regression analyses were used to identify potential predictive factors of continence status at various time points. A p-value < 0.05 was deemed statistically significant, and all analyses were performed using SPSS (version 22.0; IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Initially, 135 patients were enrolled in this study; however, only 85 completed the entire follow-up period. The most common reason for participants withdrawing from the study was the discomfort caused by preoperative urodynamic testing, making them decline to participate in further postoperative assessments. A total of 33 patients, especially those with better postoperative recovery, withdrew for this reason. Among these 85 patients, the median age was 67 years (interquartile range [IQR], 62–72 years), and the median initial PSA level was 11.8 ng/mL (IQR, 7.3–16.7 ng/mL). Final pathology revealed that 40.0% and 20.0% of patients had Gleason groups 2 and ≥ 4 disease, respectively. From the pre-surgery MRI investigation, 45.9% of patients were diagnosed with stage T3a disease; however, pathological staging showed that T2 disease constituted the largest proportion of patients (Table 1). The preoperative characteristics of the 50 withdrawn patients showed no statistically significant differences compared to the 85 patients recruited in the study.
Magnetic resonance imaging findings and operative factors
Before RaRP, MRI findings were recorded. The mean prostate volume was 42.2 ± 18.5 mL, and the mean prostate urethral angle was 57.3 ± 13.5°. The mean cross-sectional area of the bladder neck was 8.2 ± 3.1 cm2, and the mean membranous urethra length was 1.5 ± 0.4 cm. Regarding operative factors, 50.6% of the patients had preserved bilateral neurovascular bundles, and 22.3% underwent bladder neck reconstruction during surgery. While the patients were operated on by seven different surgeons at our institute, one surgeon was dedicated to performing robotic surgery for more than half of the patients (Table 1).
Comparison of urinary symptoms
Urinary symptoms before and 3 months after RaRP were evaluated using three questionnaires. According to the IPSS, all three storage symptoms deteriorated significantly 3 months post-RaRP (frequency: from 1.96 ± 1.48 to 2.60 ± 1.52; urgency: from 1.25 ± 1.39 to 1.71 ± 1.26; nocturia: from 1.82 ± 1.13 to 2.45 ± 1.06). The total storage scores increased from 5.04 ± 3.25 to 6.75 ± 3.01. The total voiding scores decreased, but the difference was not statistically significant. Owing to the improvement in voiding symptoms, the total IPSS increased at 3 months but lacked statistical significance (from 11.40 ± 7.61 to 12.21 ± 6.88). According to the UDI-6, SUI and dribbling leakage deteriorated at 3 months (SUI: from 0.34 ± 0.74 to 1.69 ± 1.14; dribbling leakage: from 0.44 ± 0.76 to 1.59 ± 0.99). Notably, UUI became apparent postoperatively (UUI: from 0.64 ± 0.99 to 1.14 ± 1.11). Consequently, total UDI-6 scores increased by 3.51 (total UDI-6 score: from 4.16 ± 3.30 to 7.67 ± 4.60). According to the OABSS, nocturia and UUI worsened significantly (nocturia: from 1.56 ± 1.00 to 2.16 ± 0.81; UUI: from 0.59 ± 0.97 to 1.96 ± 1.63). The total OABSS increased from 3.80 ± 2.80 to 6.81 ± 3.50 (Table 2).
Change in continence status
The median time for post-RaRP Foley catheter removal at our institute was 4 days. Continence status was documented during hospitalization and at each outpatient follow-up visit after discharge. Approximately 21.7% of patients achieved continence immediately after Foley catheter removal. More than half of the patients regained continence within 2 months. One year after RaRP, eight patients (9.6%) still reported incontinence (Table 3). Among the eight patients with persistent incontinence, one presented with mixed urinary incontinence at the one-year postoperative time point, whereas the other seven may have experienced varying degrees of mixed urinary incontinence during the follow-up period and only exhibited pure SUI at the one-year postoperative mark. Also, six patients had only mild SUI, requiring less than one pad per day, whereas the remaining two needed more than one pad per day.
Comparison of pressure-flow study parameters
All pressure-flow study parameters were compared before and 3 months after RaRP. In the filling phase, FD and SD both occurred significantly earlier after RaRP than before (FD: from 134.11 ± 84.52 mL to 112.06 ± 60.69 mL; SD: from 220.18 ± 121.78 mL to 174.40 ± 79.87 mL). A trend of hypersensitivity was observed, with approximately 11.8% of the patients showing de novo detrusor overactivity. In the voiding phase, PdetQmax decreased to less than half after RaRP (PdetQmax: from 52.79 ± 21.29 cmH2O to 23.61 ± 12.94 cmH2O). Qmax showed a mild increase, whereas voided volume and post-void residual urine volume decreased significantly (Qmax: from 8.94 ± 3.98 mL/s to 10.20 ± 5.82 mL/s; voided volume: from 218.98 ± 111.94 mL to 188.06 ± 90.31 mL; post-void residual urine from 38.42 ± 45.50 mL to 11.54 ± 14.01 mL). The BCI decreased from 99.67 ± 20.79 to 74.84 ± 33.95, indicating post-RaRP detrusor underactivity. The BOOI decreased from 35.59 ± 24.33 to 3.18 ± 15.85, which is consistent with the theoretical removal of bladder outlet obstruction (Table 4). The International Continence Society (ICS) nomograms, before and after RaRP, showed a horizontal left shift in the dot distribution after surgery (Fig. 1); thus, most patients exhibited detrusor underactivity.
Predictors of immediate continence after RaRP
An analysis of the factors related to these patients aimed to identify potential predictors of immediate continence after Foley catheter removal. Age and cross-sectional area of the bladder neck were statistically significant factors in the univariate analysis. In the multivariate analysis, age and cross-sectional area of the bladder neck remained meaningful predictors of immediate continence (odds ratio = 0.841, p = 0.014 and odds ratio = 0.393, p = 0.000, respectively). Based on the odds ratio, we found that older age and a larger cross-sectional area of the bladder neck were associated with a lower likelihood of immediate urinary continence after catheter removal. Final pathological results and pre-RaRP pressure-flow study parameters failed to predict the likelihood of immediate continence (Table 5). Additionally, attempts to identify the potential predictors of long-term continence did not yield significant results.
Discussion
In this study, we discovered that 3 months after RaRP, storage symptoms, including UUI, deteriorated significantly. However, voiding symptoms showed some improvement but did not achieve statistical significance. At this juncture, more than half of the patients had regained continence. The pressure-flow study parameters indicated a trend of bladder hypersensitivity in the third month, along with a noteworthy decrease in detrusor pressure and the BCI. Age and cross-sectional area of the bladder neck emerged as potential predictors of immediate continence after RaRP.
Our study has several notable strengths. First, while most of the existing literature on urodynamic changes before and after RaRP focused on noninvasive assessments, such as uroflowmetry or post-void residual volume, studies using invasive pressure-flow or video urodynamic evaluations are comparatively rare10,13,16. Thus, our findings are valuable, and were difficult to obtain. Second, among the limited studies on invasive urodynamics, our study stands out with a large sample size of nearly 100 patients, enhancing the robustness of our analysis10,13,16. Finally, we identified the potential use of preoperative imaging to calculate the cross-sectional area of the bladder neck as a predictor of postoperative continence, a novel finding not previously reported.
The post-RaRP pressure-flow studies have been substantially explored in previous research, with most involving Asian populations and fewer than a hundred patients. A Korean study initially recruited 70 patients, of whom 61 completed the study procedure13. Four months after surgery, the proportion of detrusor overactivity decreased, and the peak flow rate increased. SUI was observed in 18.3% of patients at this time point. A Japanese study conducted in 2016 enrolled 111 patients, of whom 84 completed the urodynamic examination16. Urodynamic studies were conducted immediately after surgery and 1 year later. The response to stress-induced detrusor overactivity initially occurred earlier and recovered within 1 year. The peak flow rate increased gradually, whereas the detrusor pressure decreased immediately after operation and remained stable. A Taiwanese study involving 74 patients reported that the volume of stress-induced detrusor overactivity increased at 3 months postoperatively and continued to increase at 1 year10. The detrusor pressure at peak flow decreased to approximately half of its baseline value by 3 months and remained stable at 12 months. The incontinence rate at 1 year after surgery was 12.2%, which is consistent with our findings.
Our study observed a tendency toward bladder hypersensitivity or de novo overactive bladder/detrusor overactivity after RaRP. Several hypotheses have been proposed to explain this phenomenon, including postoperative bladder wall inflammation, geometric alterations in the bladder wall, and denervation of the bladder20. It is worth noting that this may be a temporary change, as storage symptoms were found to improve to baseline status at 1 year9,10. The incidence of de novo overactive bladder/detrusor overactivity varies among studies. A Japanese study reported that approximately 37.8% of patients had de novo overactive bladder at 3 months postoperatively21, whereas a Spanish study reported that only 9.3% of patients had de novo detrusor overactivity22. In our study, the incidence of de novo detrusor overactivity was approximately 11.8%, and patients responded to stress-induced detrusor overactivity earlier after RaRP. Different studies have employed varying definitions for overactive bladder or detrusor overactivity and investigated different post-RaRP time points.
Our study, along with other related studies, identified a significant reduction in detrusor pressure10,16. Additionally, the BCI decreased following RaRP. Traditionally, a low BCI suggests impaired bladder contractility. However, the absence of changes in the peak flow rate and post-void residual urine contradicts our understanding of detrusor underactivity, which is known for reduced bladder sensation, and large post-void residual volume23. Another study revealed that the Watts factor did not change significantly after prostatectomy24. The Schafer nomogram or ICS nomogram was developed for male patients with intact prostate. For patients undergoing RaRP, a new standard of impaired bladder contractility should be considered.
Predictors of post-RaRP continence remain a topic of great interest and have been investigated in several studies. However, a consensus is yet to be reached. Age was a common factor in various studies25,26, including ours. Some studies focused on the patient’s original anatomy, such as the thickness of the levator ani muscle or the length of the membranous urethra27,28. According to Kadono et al., urodynamic studies performed before RaRP failed to predict long-term continence results16. In contrast, urodynamic parameters have been used to predict the outcomes of male sling29. Several surgical techniques seemed to improve continence recovery, including nerve-sparing procedures, bladder neck preservation or reconstruction, Retzius-sparing approach, musculofascial reconstruction, and preservation of the urethra, puboprostatic ligaments, and even seminal vesicles30,31,32. However, our study did not observe any effect of the surgical approach on postoperative urinary continence. The relationship between the bladder neck and post-RaRP continence was mentioned in only one study and measured in one dimension33. Our study might be the first to measure this using a cross-sectional area.
Our study has several limitations. First, the dropout rate remained high, a common phenomenon observed in related studies. Owing to the invasive nature of pressure-flow studies, patients with mild urinary symptoms may be less inclined to undergo repeated invasive examinations after the operation, whereas those with more severe symptoms may be more willing to repeat the examination. Second, post-RaRP urodynamic studies were conducted 3 months after the operation. On the basis of Jiang and Kuo’s findings10, examinations at different time points may yield different results. Conducting examinations at multiple time points would help to capture chronological changes, if possible. Finally, patients in our study were treated by multiple operators. Although the heterogeneity of operative methods may be a concern, the distribution of patients among different surgeons is likely to be more representative of real-world situations.
In conclusion, age and cross-sectional area of the bladder neck may serve as predictors for early recovery of continence following RaRP. Postoperative urodynamic studies in this research revealed increased bladder hypersensitivity, and significantly decreased detrusor pressure at peak flow. Notably, 11.8% of patients developed de novo detrusor overactivity after prostatectomy. Moving forward, the focus should be on optimizing surgical techniques to enhance the recovery of continence. Additionally, future research may explore the development of a new bladder contractility index for patients undergoing prostatectomy.
Data availability
The data supporting the conclusions of this article will be made available by the corresponding author, Chih-Chieh Lin, upon reasonable request.
References
Jung, J., Bae, G. H., Kim, J. H. & Kim, J. Outcomes of prostate cancer patients after robot-assisted radical prostatectomy compared with open radical prostatectomy in Korea. Sci. Rep. 13, 7851 (2023).
Gagnon, L. O., Goldenberg, S. L., Lynch, K., Hurtado, A. & Gleave, M. E. Comparison of open and robotic-assisted prostatectomy: The university of British Columbia experience. Can. Urol. Assoc. J. 8, 92–97 (2014).
Du, Y. et al. Robot-assisted radical prostatectomy is more beneficial for prostate cancer patients: A system review and meta-analysis. Med. Sci. Monit. 24, 272–287 (2018).
Mottet, N. et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 71, 618–629 (2017).
Fujimura, T. et al. Longitudinal change of comprehensive lower urinary tract symptoms and various types of urinary incontinence during robot-assisted radical prostatectomy. Neurourol. Urodyn. 38, 1067–1075 (2019).
Haglind, E. et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: A prospective, controlled, nonrandomised trial. Eur. Urol. 68, 216–225 (2015).
Kan, K. M. et al. De Novo urinary storage symptoms are common after radical prostatectomy: Incidence, natural history and predictors. J. Urol. 207, 601–608 (2022).
Prabhu, V. et al. Radical prostatectomy improves and prevents age dependent progression of lower urinary tract symptoms. J. Urol. 191, 412–417 (2014).
Da-Cruz, J. A. S. et al. Assessment of the lower urinary tract symptoms after robotic-assisted radical prostatectomy: The behavior of voiding, storage and post micturition symptoms. Rev. Col Bras. Cir. 47, e20202605 (2020).
Jiang, Y. H. & Kuo, H. C. Changes of lower urinary tract function after robot-assisted radical prostatectomy: An urodynamic follow-up within 1 year. Tzu Chi Med. J. 35, 158–164 (2023).
Gordon, A. et al. Quantification of long-term stability and specific relief of lower urinary tract symptoms (LUTS) after robot-assisted radical prostatectomy. Urology 93, 97–103 (2016).
Takeshima, Y. et al. The association between the parameters of uroflowmetry and lower urinary tract symptoms in prostate cancer patients after robot-assisted radical prostatectomy. PLOS ONE. 17, e0275069 (2022).
Lee, D. S. & Lee, S. J. Urodynamic evaluation of patients with localized prostate cancer before and 4 months after robotic radical prostatectomy. Sci. Rep. 11, 3632 (2021).
Skarecky, D. et al. Analysis of improved urinary peak flow rates after robot-assisted radical prostatectomy. J. Endourol. 29, 1152–1158 (2015).
Gordon, A., Skarecky, D., Babaian, K. N., Dhaliwal, H. & Ahlering, T. E. Diminished long-term recovery of peak flow rate (PFR) after robotic prostatectomy in men with baseline PFR < 10 mL/s and incidental association with high-risk prostate cancer. Low Urin Tract. Symptoms. 11, 78–84 (2019).
Kadono, Y. et al. Use of preoperative factors including urodynamic evaluations and nerve-sparing status for predicting urinary continence recovery after robot-assisted radical prostatectomy: Nerve-sparing technique contributes to the reduction of postprostatectomy incontinence. Neurourol. Urodyn. 35, 1034–1039 (2016).
Kadono, Y. et al. Urodynamic evaluation before and immediately after robot-assisted radical prostatectomy. Urology 84, 106–111 (2014).
Hou, C. P. et al. Impact of the static prostatic urethral angle on men with lower urinary tract symptoms. Urol. Sci. 27, 47–50 (2016).
Cho, K. S. et al. Relationship between prostatic urethral angle and urinary flow rate: Its implication in benign prostatic hyperplasia pathogenesis. Urology 71, 858–862 (2008).
Porena, M., Mearini, E., Mearini, L., Vianello, A. & Giannantoni, A. Voiding dysfunction after radical retropubic prostatectomy: More than external urethral sphincter deficiency. Eur. Urol. 52, 38–45 (2007).
Matsukawa, Y. et al. De Novo overactive bladder after robot-assisted laparoscopic radical prostatectomy. Neurourol. Urodyn. 37, 2008–2014 (2018).
Barnoiu, O. S. et al. Urodynamic assessment of bladder and urethral sphincter function before and after robot-assisted radical prostatectomy. Actas Urol. Esp. 38, 78–83 (2014).
Lee, C. L. et al. Therapeutic outcome of active management in male patients with detrusor underactivity based on clinical diagnosis and videourodynamic classification. Sci. Rep. 12, 362 (2022).
Kitta, T. et al. Radical prostatectomy restores detrusor contraction pattern according to pressure flow parameters. Int. J. Urol. 24, 301–307 (2017).
Novara, G. et al. Evaluating urinary continence and preoperative predictors of urinary continence after robot assisted laparoscopic radical prostatectomy. J. Urol. 184, 1028–1033 (2010).
Shao, I. H., Chang, Y. H., Hou, C. M., Lin, Z. F. & Wu, C. T. Predictors of short-term and long-term incontinence after robot-assisted radical prostatectomy. J. Int. Med. Res. 46, 421–429 (2018).
Sadahira, T. et al. Pelvic magnetic resonance imaging parameters predict urinary incontinence after robot-assisted radical prostatectomy. Low Urin Tract. Symptoms. 11, 122–126 (2019).
Kim, L. H. C. et al. Association between preoperative magnetic resonance imaging-based urethral parameters and continence recovery following robot-assisted radical prostatectomy. Eur. Urol. Focus. 6, 1013–1020 (2020).
Toia, B. et al. Is pre-operative urodynamic bladder function the true predictor of outcome of male sling for post prostatectomy incontinence? World J. Urol. 39, 1227–1232 (2021).
Pacik, D. & Fedorko, M. Literature review of factors affecting continence after radical prostatectomy. Saudi Med. J. 38, 9–17 (2017).
Urkmez, A., Ranasinghe, W. & Davis, J. W. Surgical techniques to improve continence recovery after robot-assisted radical prostatectomy. Transl Androl. Urol. 9, 3036–3048 (2020).
Kadono, Y. et al. Investigating the mechanism underlying urinary continence using dynamic MRI after Retzius-sparing robot-assisted radical prostatectomy. Sci. Rep. 12, 3975 (2022).
Kohjimoto, Y., Higuchi, M., Yamashita, S., Kikkawa, K. & Hara, I. Bladder neck size and its association with urinary continence after robot-assisted radical prostatectomy. BJUI Compass. 4, 181–186 (2023).
Acknowledgements
This research was sponsored by the academic funds of Taipei Veterans General Hospital (fund reference ID: V113C-194). We would like to thank Editage (www.editage.com.tw) for English language editing.
Author information
Authors and Affiliations
Contributions
Ping-Hsuan Yu: Methodology, software, validation, formal analysis, investigation, data curation, writing—original draft preparation, visualization, project administration. Chih-Chieh Lin: Conceptualization, methodology, validation, resources, writing—review and editing, supervision, project administration. Hsiao-Jen Chung: resources, supervision. Tzu-Ping Lin: resources. Eric Yi-Hsiu Huang: resources. Tzu-Hao Huang: resources. William J. Huang: supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, PH., Lin, CC., Chung, HJ. et al. A prospective study using questionnaires and urodynamic assessments to evaluate bladder function and continence changes after robotic assisted radical prostatectomy. Sci Rep 15, 12135 (2025). https://doi.org/10.1038/s41598-025-97234-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97234-6