Abstract
Objective: Remimazolam is a novel benzodiazepine sedative that provides effective sedation, stable haemodynamics, and minimal adverse effects during intravenous general anaesthesia. The aim of this study was to determine the 50% effective dose (ED50) of remimazolam combined with different doses of esketamine for painless gastroscopy and to evaluate the efficacy and safety of this combination. Methods: This was a randomised, double-blind, up-and-down sequential allocation study. Patients undergoing painless gastroscopy who met all the inclusion criteria and did not meet any of the exclusion criteria were randomised in a 1:1:1 ratio into the ES0 group (0 mg/kg of esketamine), ES1 group (0.2 mg/kg of esketamine), and ES2 group (0.4 mg/kg of esketamine). The initial dose of remimazolam was 0.3 mg/kg in each group, with the dose increased or decreased by 0.05 mg/kg for the subsequent patient based on the success or failure of sedation in the previous patient. The trial was concluded when seven successful failure crossovers were achieved. The ED50 and 95% confidence intervals (CI) of remimazolam were calculated using Probit regression. Haemodynamic parameters, time to induction of anaesthesia, time to gastroscopy, time to awakening from anaesthesia, and adverse events were recorded. Results: A total of 59 patients were included in the final analysis: 19 in the ES0 group, 23 in the ES1 group, and 17 in the ES2 group. The ED50 (95% CI) of remimazolam in the ES0, ES1, and ES2 groups was 0.344 (0.302–0.389) mg/kg, 0.289 (0.249–0.328) mg/kg, and 0.193 (0.145–0.239) mg/kg, respectively. Additionally, the ES1 and ES2 groups exhibited more stable haemodynamics compared to the ES0 group. However, the ES1 and ES2 groups had significantly longer recovery times than the ES0 group. The incidence of hypotension was higher in the ES0 group compared to the ES1 and ES2 groups. Conclusion: The ED50 of remimazolam combined with 0 mg/kg, 0.2 mg/kg, and 0.4 mg/kg of esketamine for induction of anaesthesia during painless gastroscopy was 0.344 mg/kg, 0.289 mg/kg, and 0.193 mg/kg, respectively. Combining esketamine with remimazolam for induction of anaesthesia during painless gastroscopy offers advantages in terms of haemodynamic stability and reduced adverse effects.
Similar content being viewed by others
Introduction
With the advancement of medical care, the demand for painless gastroscopy among patients has increased year by year1,2,3. Gastroscopy has significant diagnostic value for conditions such as gastritis, peptic ulcers, and gastro-oesophageal tumours4. However, traditional gastroscopy procedures often result in patient discomfort due to painful stimuli, which can lead to interruptions and, consequently, missed or inaccurate diagnoses. Numerous studies have shown that painless gastroscopy significantly shortens operation time, reduces stress responses, and minimises pain during the examination compared to conventional gastroscopy5,6.
Painless gastroscopy typically requires an appropriate depth of anaesthesia. Currently, commonly used drug regimens include propofol or etomidate in combination with opioids7,8. Although propofol is widely used, it is associated with adverse effects such as hypotension, bradycardia, respiratory depression, and injection pain9,10. Etomidate, while causing less respiratory and circulatory depression, carries risks such as muscle tremors, postoperative nausea and vomiting, and suppression of adrenocortical function11,12. Sufentanil, although effective for analgesia, increases the risks of hypotension, respiratory depression, and postoperative cognitive dysfunction13,14. Therefore, it is crucial to identify an anaesthetic drug combination that ensures safe and effective painless gastroscopy.
Remimazolam, a novel benzodiazepine sedative, has the advantages of a rapid onset of action, fast metabolism, minimal accumulation, and a low impact on cognitive brain function15,16. It is hydrolysed to inactive metabolites by plasma esterases and has minimal effects on hepatic and renal function. Clinical studies have demonstrated that remimazolam’s anaesthetic effect during painless gastroscopy is comparable to propofol, but with fewer respiratory and circulatory adverse effects17,18.
Esketamine, an isomer of ketamine, has stronger analgesic and sedative effects than ketamine, while causing fewer psychiatric and circulatory adverse effects19,20. Studies have shown that esketamine can provide effective analgesia while maintaining stable haemodynamics and causing fewer respiratory adverse events21,22.
Recent studies suggest that the combination of remimazolam and esketamine for intravenous general anaesthesia provides effective sedation and analgesia with fewer adverse events. However, there remains a paucity of data regarding the appropriate dosage for this combination23,24. Therefore, the present study was designed to address this gap. This study aimed to determine the 50% effective dose (ED50) of remimazolam combined with different doses of esketamine for painless gastroscopy and to evaluate its anaesthetic effects, providing guidance for clinical practice.
Methods
Ethical approval
This was a single-centre, randomised, double-blind, up-down sequential allocation trial. The study was approved by the Ethics Committee of Nanchong Central Hospital (2022, trial (003) No.) and registered in the China Clinical Trial Registry (Registration time: 21/02/2023; Available at https://www.chictr.org.cn/bin/project/edit?pid=178987; Registration Number: ChiCTR2300068488). Each patient provided informed consent before painless gastroscopy. We confirm that our study complies with the Declaration of Helsinki.
Participants
A total of 84 adult patients who underwent painless gastroscopy between June 2023 and October 2023 were recruited for this study. Inclusion criteria were: age 18–65 years, body mass index (BMI) 19–28 kg/m2, and American Society of Anesthesiologists (ASA) class I–II. Exclusion criteria included: complex endoscopic operations such as gastric polypectomy, severe cardiopulmonary disease, abnormalities in liver and kidney function, a history of allergy to the study medications, and anticipated difficult airway.
Randomisation and blinding
Eligible patients were randomly allocated to the ES0 group (0 mg/kg of esketamine), ES1 group (0.2 mg/kg of esketamine), and ES2 group (0.4 mg/kg of esketamine) in a 1:1:1 ratio. Allocation details were concealed in opaque sealed envelopes. A non-blinded nurse prepared the study medication in uniform looking 10 mL syringes according to the patient’s weight, labeled only with the patient’s number.The syringes in the ES0 group were filled with saline in the same volume as in the ES1 and ES2 groups. All patients, surgeons, and other investigators were blinded to the group assignment.
Anaesthesia
All patients fasted for 8 h before the procedure. Upon entering the gastroscopy room, monitoring was initiated to continuously measure the electrocardiogram, pulse oximetry (SpO₂), non-invasive arterial blood pressure, and heart rate (HR). Peripheral venous access was secured, and Ringer’s lactate solution was infused. The patient was placed in the left lateral position, and oxygen was administered via a nasal cannula at a flow rate of 3 L/min. Anaesthesia induction began after the patient was oxygenated to an oxygen saturation of 100%. Intravenous esketamine and remimazolam were administered sequentially. Gastroscopy was initiated when the Modified Observer’s Alertness/Sedation scale (MOAA/S) was ≤ 1. During the procedure, MOAA/S was assessed every minute to ensure sustained sedation (score ≤ 1). If the score exceeded 1 at any time, additional remimazolam (2.5–5 mg) was administered. Vital signs continued to be monitored after the examination, and the time at which the patient’s MOAA/S score reached 5 was recorded as the point of awakening.
Determination of ED50
The ED50 of remimazolam was determined using Dixon’s up-and-down sequential allocation method25. The initial dose of remimazolam in each group was 0.3 mg/kg. Sedation failure was defined as an MOAA/S score > 1 within 3 min of starting gastroscopy, after which the dose for the next patient was increased by 0.05 mg/kg. Otherwise, the dose was decreased by 0.05 mg/kg. The trial was concluded when there were seven successful and failed crossovers, at which point the sample size was considered sufficient26.
Study outcomes
The primary outcome was the ED50 of remimazolam. Secondary outcomes included the following: patient baseline characteristics (age, gender, height, weight, BMI, ASA classification, etc.), anaesthetic induction time (Dosing was initiated until MOAA/S score ≤ 1), operative time, awakening time (End of gastroscopy to MOAA/S score = 5), endoscopist satisfaction, and patient satisfaction. Haemodynamic parameters were recorded at T1 (baseline), T2 (start of gastroscopy), T3 (after 2 min), T4 (at the end of the procedure), and T5 (at awakening). Adverse events occurring between the start of the examination and the patient’s awakening were also recorded. These included hypertension, hypotension, tachycardia, bradycardia, and hypoxaemia. Blood pressure was classified as hypertension or hypotension if it increased or decreased by more than 20% of the baseline value. Bradycardia or tachycardia was defined as an HR of < 50 or > 100 beats/min27. Hypoxaemia was defined as an SpO₂ level of less than 90% and was managed using mandibular support, increased oxygen flow, mask pressure oxygenation, or, if necessary, emergency intubation.
Statistical analysis
The sample size for this study was based on the sequential allocation method. According to this principle, ED50 can be accurately calculated when the sample size reaches seven crossings28. ED50 and ED95 were estimated using probabilistic regression analysis of SPSS. Normally distributed data were expressed as mean ± standard deviation. Between-group comparisons were performed using one-way ANOVA, while repeated measures ANOVA was used for within-group comparisons. Non-normally distributed data were expressed as median and interquartile range, analysed using the Kruskal-Wallis H test. Qualitative data were expressed as frequencies and percentages, analysed using the chi-square test. Ordinal data (e.g., satisfaction scores) were analyzed using the Kruskal-Wallis test. A P-value of < 0.05 was considered statistically significant.
Results
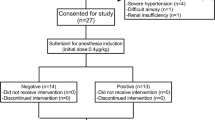
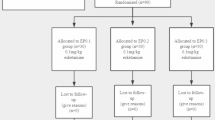
The flow chart of this study is shown in Fig. 1. We assessed 84 patients for eligibility between April and June 2023. Twenty-five patients were excluded, of whom 16 did not meet the eligibility criteria, and nine patients were withdrawn from the study. A total of 59 patients were included in the final analysis, with 19 in the ES0 group, 23 in the ES1 group, and 17 in the ES2 group, and seven crossovers occurring in each group.
The baseline characteristics for the three groups of patients are shown in Table 1. There were no significant differences in the baseline characteristics of gender, age, ASA classification, BMI, hypertension, and diabetes among the three groups (P > 0.05).
Figure 2 shows the ED50 determination of remimazolam in the three groups. The ED50 of remimazolam for painless gastroscopy was 0.344, 0.289, and 0.193 mg/kg in the ES0, ES1, and ES2 groups, respectively (Table 2). The ED50 of remimazolam was significantly lower in the ES2 group compared to the ES0 and ES1 groups (P < 0.05).
The hemodynamic data of the patients are shown in Tables 3 and 4. HR results for the three groups of patients are as follows. In the intragroup comparisons of the ES0, ES1, and ES2 groups, the HR at time points T2 and T3 were significantly increased compared to T1 (P < 0.05). In the intergroup comparisons, the HR of the ES1 and ES2 groups at time point T2 were significantly lower than that of the ES0 group (P = 0.016). There were no statistically significant differences in HR at the other time points (P > 0.05).
The results of MAP during painless gastroscopy for the three patient groups are as follows. In the ES0 group, MAP at time points T2, T3, T4, and T5 was significantly lower compared to T1 (all P < 0.001). In the ES1 group, MAP at time points T4 and T5 was significantly lower compared to T1 (P < 0.001). In the ES2 group, there were no statistically significant differences in MAP across all time points (P > 0.05). In between-group comparisons, at time point T2, MAP in the ES0 group was significantly lower than that in the ES1 group (P = 0.027). At time point T4, MAP in the ES0 group was significantly lower than that in both the ES1 and ES2 groups (P = 0.02, P = 0.003). At time point T5, MAP in the ES0 group was significantly lower than that in the ES2 group (P = 0.008).
The secondary outcome indicators of this study are shown in Table 5. In terms of time to awaken from anaesthesia, patients in the ES1 and ES2 groups had significantly longer awakening times compared to the ES0 group (P = 0.004). Regarding endoscopist satisfaction, there was no statistically significant difference between the ES0 and ES1 groups, but the ES2 group had significantly lower satisfaction compared to both the ES0 and ES1 groups. In terms of patient satisfaction, there was no statistically significant difference between the ES0 and ES1 groups, but the ES2 group had significantly lower satisfaction compared to the ES0 group. In the comparison of adverse events, there was a significant reduction in the incidence of hypotension in the ES1 and ES2 groups compared to the ES0 group, and no statistically significant differences in other adverse effects.
Discussion
The use of painless gastroscopy techniques is becoming more widespread as the need for comfort medicine in clinical practice increases. Moderate to deep sedation must be maintained during painless gastroscopy. Currently, a combined anaesthetic regimen of propofol with opioid analgesics is predominantly used in clinical practice. This combination usually achieves good sedation and analgesia; however, both propofol and opioids have strong inhibitory effects on respiration and circulation. This increases the likelihood of respiratory depression, hypoxaemia, and hypotension during the examination29,30. The anaesthetic regimen of remimazolam combined with esketamine, which has a rapid onset of action, good sedation and analgesia, as well as fewer inhibitory effects on respiration and circulation, is a potentially excellent alternative for painless gastroscopy31.
The sequential allocation method is effective for evaluating the ED50 of drugs in small sample cases, so it has been widely used in anaesthesia study design32. In the present study, by designing three different doses of esketamine, it was found that a higher dose of esketamine reduced the ED50 of remimazolam for anaesthesia induction during painless gastroscopy. In Hua et al.‘s study, a higher dose of esketamine in geriatric painless gastroscopy reduced the ED50 of propofol, which was similar to the results obtained in the present study33.
The haemodynamic results of this study demonstrate positive implications. HR increased significantly in all three groups of patients after the start of gastroscopy, but the increase in HR was significantly greater in the ES0 group than in the ES1 and ES2 groups. This indicates that gastroscopic manipulation stimuli lead to an increase in HR in patients, but esketamine’s analgesic effect mitigates this response, resulting in a relatively smaller increase and more stable HR. In certain time comparisons, both the ES1 and ES2 groups exhibited a more stable MAP than the ES0 group. The possible mechanism is that esketamine indirectly excites the sympathetic nervous system, reducing the fall in blood pressure34. Therefore, we recommend the use of remimazolam combined with esketamine for more stable haemodynamics during painless gastroscopy. However, because of the higher incidence of hypertension in the ES2 group, we wish to emphasize the unique advantages of the ES1 group (0.2 mg/kg esketamine), which balances hemodynamic stability with clinical utility.
Our study found that patients in the ES1 and ES2 groups had a longer time to awaken. Previous studies have reported that the half-life of remimazolam is 0.6–0.9 h, which is comparable to propofol35. In contrast, esketamine has a half-life of 3–5 h, which may have contributed to the significant prolongation of awakening time in patients in the ES1 and ES2 groups. In addition, esketamine is slowly metabolised in vivo, and after conversion to desmethyl ketamine by hepatic enzymes, it retains a potency equivalent to 1/5 to 1/3 that of esketamine, along with a longer half-life (8–9 h). Given the dose-dependent prolongation of awakening time with esketamine, we recommend using doses below 0.2 mg/kg for short procedures like gastroscopy. This approach balances the benefits of hemodynamic stability with the need for rapid recovery, though further studies are needed to confirm optimal dosing.
Overall, remimazolam in combination with esketamine demonstrated positive clinical significance in anaesthesia for painless gastroscopy. The combination of remimazolam and esketamine not only meets the requirements for appropriate sedation and analgesia but also provides stable haemodynamic parameters. However, esketamine is metabolised more slowly, thus increasing patient recovery time. Wei et al. found that esketamine combined with remimazolam achieved good sedation and stable haemodynamics, but recovery time was prolonged, which is consistent with the results of this study36.
There are some limitations to this study. The primary objective was to determine the ED50 of remimazolam for painless gastroscopy at different esketamine doses. Therefore, the sample size was generated based on the sequential method, which may have been insufficient for comparisons of secondary outcome indicators. Future studies with larger sample sizes are warranted to validate the secondary outcomes. In additions, our study observed that a small dose (0.2 mg/kg) of esketamine significantly increased the time to awakening in patients, and future studies should further explore the effects of lower doses (< 0.2 mg/kg) of esketamine on time to awakening and hemodynamics. In addition, while our study focused on hemodynamic stability and common perioperative adverse events, we did not systematically monitor psychiatric adverse effects of esketamine (e.g., hallucinations, nightmares, or dissociative reactions). This oversight limits the comprehensive safety evaluation of the esketamine-remimazolam combination. Future studies should incorporate validated tools to quantify psychotomimetic effects and correlate them with esketamine doses.
Conclusion
The ED50 of remimazolam combined with 0 mg/kg, 0.2 mg/kg, and 0.4 mg/kg of esketamine for induction of anaesthesia during painless gastroscopy was 0.344, 0.289, and 0.193 mg/kg, respectively. Esketamine combined with remimazolam for induction of anaesthesia during painless gastroscopy was advantageous with respect to haemodynamics and adverse effects.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Travis, A. C., Pievsky, D. & Saltzman, J. R. Endoscopy in the elderly. Am. J. Gastroenterol. 107, 1495 – 501, 1494, 1502. (2012). https://doi.org/10.1038/ajg.2012.246
Ferreira, A. O. & Cravo, M. Sedation in Gastrointestinal endoscopy: where are we at in 2014? World J. Gastrointest. Endosc. 7, 102–109. https://doi.org/10.4253/wjge.v7.i2.102 (2015).
Moon, S. Sedation regimens for Gastrointestinal endoscopy. Clin. Endosc. 47, 135–140. https://doi.org/10.5946/ce.2014.47.2.135 (2014).
Ristikankare, M. et al. Sedation, topical pharyngeal anesthesia and cardiorespiratory safety during gastroscopy. J. Clin. Gastroenterol. 40, 899–905 (2006).
Qiu, L., Yao, L., Hu, P. & He, T. Analysis of the detection rate and clinical characteristics of early gastric cancer by painless gastroscopy and ordinary gastroscopy. Med. (Baltim). 103, e38120. https://doi.org/10.1097/MD.0000000000038120 (2024).
Men, F. et al. Comparison of the safety of the application of painless gastroscopy and ordinary gastroscopy in chronic hypertension patients combined with early gastric cancer. Oncol. Lett. 15, 3558–3561. https://doi.org/10.3892/ol.2018.7737 (2018).
Guo, Y. et al. Ed50 and ed95 of remimazolam tosilate combined with different doses of Fentanyl in elderly patients for painless gastroscopy. Drug. Des. Devel. Ther. 18, 2347–2356. https://doi.org/10.2147/DDDT.S462607 (2024).
Tang, S., Zheng, Y., Li, X., Zhang, Y. & Zhang, Z. Optimizing sedation in gastroscopy: a study on the Etomidate/propofol mixture ratio. Front. Med. (Lausanne). 11, 1392141. https://doi.org/10.3389/fmed.2024.1392141 (2024).
Garewal, D. & Waikar, P. Propofol sedation for Ercp procedures: a Dilemna?? Observations from an anesthesia perspective. Diagn. Therapeutic Endoscopy. 2012, 639190. https://doi.org/10.1155/2012/639190 (2012).
Eer, A. S., Padmanabhan, U. & Leslie, K. Propofol dose and incidence of dreaming during sedation. Eur. J. Anaesthesiol. 26, 833–836. https://doi.org/10.1097/EJA.0b013e32832c500c (2009).
Liu, Y. et al. Sedation with a 1:1 mixture of etomidate and Propofol for gastroscopy in hypertensive elderly patients. J. Clin. Hypertens. (Greenwich Conn. 25, 778–783. https://doi.org/10.1111/jch.14693 (2023).
Liu, G. & Xiong, Y. Analysis of stress response and analgesic effect of remazolam combined with etomidate in painless gastroenteroscopy. Contrast Media Mol. Imaging 2022 (4863682). https://doi.org/10.1155/2022/4863682 (2022).
Albores-García, D. & Cruz, S. L. Fentanyl and other new psychoactive synthetic opioids. Challenges to prevention and treatment. Revista De Investigacion Clinica; Organo Del. Hosp. De Enfermedades De La. Nutricion. 75, 93–104. https://doi.org/10.24875/RIC.23000109 (2023).
Maciejewski, D. Sufentanil in anaesthesiology and intensive therapy. Anaesthesiol. Intensive Ther. 44, 35–41 (2012).
Chen, G., Lin, S., Lin, P., Tseng, W. & Lu, C. A narrative review of remimazolam in procedural sedation. Asian J. Anesthesiology. 61, 39–45. https://doi.org/10.6859/aja.202306_61(2).0001 (2023).
Lee, A. & Shirley, M. Remimazolam: a review in procedural sedation. Drugs 81, 1193–1201. https://doi.org/10.1007/s40265-021-01544-8 (2021).
Chang, Y., Huang, Y., Chi, K. & Huang, Y. Remimazolam versus Propofol for procedural sedation: a meta-analysis of randomized controlled trials. Peerj 11, e15495. https://doi.org/10.7717/peerj.15495 (2023).
Zhang, J. et al. Remimazolam versus Propofol for procedural sedation and anesthesia: a systemic review and meta-analysis. Minerva Anestesiol. 88, 1035–1042. https://doi.org/10.23736/S0375-9393.22.16817-3 (2022).
Mion, G. & Himmelseher, S. Esketamine: less drowsiness, more analgesia. Anesth. Analg. 139, 78–91. https://doi.org/10.1213/ANE.0000000000006851 (2024).
Zhang, X. et al. Research advances in the clinical application of Esketamine. Ibrain 8, 55–67. https://doi.org/10.1002/ibra.12019 (2022).
Yu, Y. et al. Efficacy and safety of Esketamine for pediatric Gastrointestinal endoscopy: a meta-analysis and trial sequential analysis. Front. Pharmacol. 15, 1379101. https://doi.org/10.3389/fphar.2024.1379101 (2024).
Fu, M. et al. Postoperative Esketamine improves ventilation after video-assisted thoracoscopic lung resection: a double-blinded randomized controlled trial. Heliyon 10, e25100. https://doi.org/10.1016/j.heliyon.2024.e25100 (2024).
Chu, T. et al. Comparison of remimazolam and Propofol combined with low dose Esketamine for pediatric same-day painless bidirectional endoscopy: a randomized, controlled clinical trial. Front. Pharmacol. 15, 1298409. https://doi.org/10.3389/fphar.2024.1298409 (2024).
Zhang, K. et al. Effects of opioid-free Propofol or remimazolam balanced anesthesia on hypoxemia incidence in patients with obesity during Gastrointestinal endoscopy: a prospective, randomized clinical trial. Front. Med. (Lausanne). 10, 1124743. https://doi.org/10.3389/fmed.2023.1124743 (2023).
Dixon, W. J. Staircase Bioassay: the up-and-down method. Neurosci. Biobehav Rev. 15, 47–50 (1991).
Ni, M. et al. Effective dose of intranasal remimazolam for preoperative sedation in preschool children: a dose-finding study using Dixon’s up-and-down method. Front. Pharmacol. 15, 1372139. https://doi.org/10.3389/fphar.2024.1372139 (2024).
Bang, J. et al. Impact of remote ischemic preconditioning conducted in living kidney donors on renal function in donors and recipients following living donor kidney transplantation: a randomized clinical trial. J. Clin. Med. 8. https://doi.org/10.3390/jcm8050713 (2019).
Görges, M., Zhou, G., Brant, R. & Ansermino, J. M. Sequential allocation trial design in anesthesia: an introduction to methods, modeling, and clinical applications. Paediatr Anaesth. 27, 240–247. https://doi.org/10.1111/pan.13088 (2017).
Chen, D., Liao, M., Wu, X., Zhao, T. & Sun, H. Comparison of efficacy and safety of equivalent doses of remimazolam versus Propofol for gastroscopy anesthesia in elderly patients. Sci. Rep. 14, 7645. https://doi.org/10.1038/s41598-024-58294-2 (2024).
Lu, M., Liu, J., Wu, X. & Zhang, Z. Ciprofol: A novel alternative to Propofol in clinical intravenous anesthesia? Biomed. Res. Int. 2023 (7443226). https://doi.org/10.1155/2023/7443226 (2023).
International, B. R. Retracted: the clinical application of remimazolam benzenesulfonate combined with esketamine intravenous anesthesia in endoscopic retrograde cholangiopancreatography. Biomed. Res. Int. 2024, 9758358 (2024). https://doi.org/10.1155/2024/9758358
Pace, N. L. & Stylianou, M. P. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose Estimation in anesthesia research. Anesthesiology 107, 144–152 (2007).
Yang, H. et al. The median effective concentration of Propofol with different doses of Esketamine during Gastrointestinal endoscopy in elderly patients: a randomized controlled trial. Br. J. Clin. Pharmacol. 88, 1279–1287. https://doi.org/10.1111/bcp.15072 (2022).
Deng, J., Yu, Y., Tang, Z., Lei, H. & Tan, C. Efficacy and safety of low-dose Esketamine for painless Gastrointestinal endoscopy in adults: a systematic evaluation and meta-analysis. Front. Pharmacol. 15, 1364546. https://doi.org/10.3389/fphar.2024.1364546 (2024).
Gao, Y. et al. Pharmacokinetics of remimazolam after intravenous infusion in anaesthetised children. Br. J. Anaesth. 131, 914–920. https://doi.org/10.1016/j.bja.2023.08.019 (2023).
Li, W. et al. The efficacy and safety of remimazolam besylate combined with Esketamine for outpatient colonoscopy: a prospective, randomized, controlled clinical trial. Drug. Des. Devel. Ther. 17, 2875–2887. https://doi.org/10.2147/DDDT.S425860 (2023).
Funding
This study was supported by research funds from the Nanchong Science and Technology Bureau (22SXQT0293).
Author information
Authors and Affiliations
Contributions
ZL, ZXL, and CLL were responsible for the conception and design of the article, the collection and organization of research data, and the writing of the paper responsible for the article as a whole. GYP was responsible for data analysis and processing. MW, LXC, and ZLY were responsible for editing and organizing the tables.XY and LLJ were responsible for the revision of the paper, quality control and proofreading of the article, supervision, and management.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, L., Zhou, X., Chen, L. et al. The 50% effective dose of remimazolam combined with different doses of esketamine for painless gastroscopy. Sci Rep 15, 12770 (2025). https://doi.org/10.1038/s41598-025-97649-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97649-1