Abstract
This study aimed to investigate the efficacy of different doses of Indocyanine Green (ICG) for fluorescence imaging during laparoscopic cholecystectomy, specifically between 0.5 to 3 h, to determine the optimal dose that would enhance clinical practice. A total of 80 patients with benign gallbladder diseases were enrolled fluorescence-guided laparoscopic cholecystectomy. Patients were randomly assigned to four groups based on the dose of ICG administered (n = 20 each): 0.25 mg, 0.50 mg, 1.00 mg, and 2.50 mg. Key clinical data were collected during the perioperative period, focusing on the delineation of the liver, identification of the cystic duct-common bile duct junction, and fluorescence intensity contrast analysis. The effectiveness of extrahepatic bile duct visualization was evaluated subjectively by three experienced hepatobiliary surgeons via surgical videos and images, categorized as “excellent,” “good,” or “poor.” Successful fluorescence imaging was achieved in all patients without the requiring conversion to open surgery. Significant differences were observed among the four groups in several metrics: intraoperative blood loss, bile duct identification time, fluorescence intensity contrast values, and the number of cases rated “excellent” by the surgeons (P < 0.05). No significant differences were observed for the remaining statistical indicators (P > 0.05). The findings based on the data suggest that a dose of 0.25 mg ICG within the 0.5–3 h window is optimal, yielding the highest fluorescence contrast between the extrahepatic bile duct and liver while garnering the best subjective evaluations. This study provides robust evidence to support the clinical application of ICG in surgical imaging, emphasizing its utility in clinical practice.
Similar content being viewed by others
Introduction
Fluorescence laparoscopy combining Indocyanine Green (ICG) technology is an emerging intraoperative imaging modality that leverages the advantages of both laparoscopic and fluorescence imaging techniques. The core mechanism relies on the fluorescence emitted by the ICG dye when exposed to near-infrared light, enabling the visualization of anatomical structures through a specialized imaging system1,2. Through precise control of the dose and timing of ICG injection, this technique can selectively highlight various structures, including blood vessels, bile ducts, and lymphatics3. In particular, within the biliary system, ICG is metabolized to facilitate real-time intraoperative visualization of biliary structures, with peak concentrations in bile typically occurring 0.5 to 2 h post-injection4,5,6. This property provides surgeons with an effective tool for intraoperative bile duct visualization, thus aiding in the prevention of bile duct injuries (BDI) and subsequently reducing the incidence of severe complications, such as bile leaks and abdominal infections7. Furthermore, studies have demonstrated that variations in ICG dosage significantly affect the quality of fluorescence imaging8. Specifically, lower doses of ICG can prolong overall imaging time, while higher doses enhance the intensity of the fluorescence signal9, providing valuable theoretical guidance for clinicians in selecting the optimal dose based on individual patient needs. However, despite the promising applications of fluorescence laparoscopy with ICG in the biliary system, its clinical use is currently largely exploratory10. In the academic community, there is an active debate concerning the optimal dosing regimen for ICG11,12,13. In clinical practice, the varying protocols for ICG use result in considerable variability in imaging outcomes14. Therefore, this study aims to establish the optimal preoperative dose of ICG within a timeframe of 0.5 to 3 h to achieve the best visualization of the extrahepatic biliary tract, thereby providing valuable reference points for clinical practice.
Methods
Study setting
In a prospective study conducted at People’s Hospital of Shijiazhuang from January to September 2024, data were collected from 90 patients suffering from benign gallbladder diseases who underwent fluorescence-guided laparoscopic cholecystectomy. Among these patients, 10 were excluded: specifically, 8 did not meet the inclusion criteria, and 2 were unable to participate in the follow-up. Further details are provided in Fig. 1.
Inclusion and exclusion criteria
Inclusion Criteria (1) Participants should be adults aged 18–80 years with complete clinical data and must have provided informed consent by signing an informed consent form; (2)The preoperative diagnosis of benign gallbladder disease should be established based on thorough medical history, physical examination, and at least one auxiliary diagnostic imaging procedure (abdominal ultrasound, CT, or MRI); (3) Eligibility for LC; (4) Child-Pugh classification of Grade A; (5) Patients should not have undergone any other abdominal surgical procedures during the current hospitalization period.
Exclusion Criteria (1) History of allergy to iodine or indocyanine green; (2) Liver function classified as Child-Pugh Grade B or C; (3) Patients with severe coagulation disorders or poor cardiopulmonary function who are unable to tolerate surgery or anesthesia; (4) Biliary obstruction or bile metabolism disorders; (5) Presence of malignant tumors in the gallbladder or liver.
Ethical issues
Eighty patients were assigned to four groups using a completely random assignment method, which incorporated both envelope randomization and a random number table prior to surgery: the 0.25 mg group (n = 20), the 0.50 mg group (n = 20), the 1.00 mg group (n = 20), and the 2.50 mg group (n = 20). Surgeries for all groups were performed by the same qualified and clinically experienced medical team specializing in LC. This study has been approved by the Medical Research Ethics Committee of Shijiazhuang People’s Hospital (No. 2025-047). This clinical trial has been registered and approved in the World Health Organization International Clinical Trials Registry Platform (NO.ChiCTR2500100138), with a registration date of 03/04/2025. All patients and their families were comprehensively informed during preoperative consultations and provided with signed informed consent form for surgery. All methods were conducted in accordance with the relevant guidelines and regulations.
Surgical equipment
Laparoscopic system and ICG solution
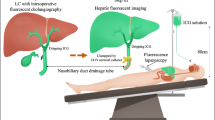
In this study, the near-infrared fluorescence imaging system developed by Beijing Precision Digital Medical Technology Co., Ltd. (DPM) was employed for intraoperative cholangiography. The fluorescent dye used was ICG for injection, specified a formulation of 25 mg produced by Dandong Yichuang Pharmaceutical Co., Ltd. (Drug Approval Number: H20055881).
Preparation of ICG solution
Under sterile conditions, 25 mg of ICG powder was dissolved in 10 ml of sterile water for injection to prepare a stock solution of 2.5 mg/ml. Subsequently, 1 ml of this stock solution was mixed with 9 ml of sterile water for injection to prepare a 0.25 mg/ml ICG solution, and 1 ml was mixed with 4 ml of sterile water to obtain a 0.50 mg/ml ICG solution. Additionally, 2 ml of the stock solution was diluted with 3 ml of sterile water to prepare a 1.00 mg/ml ICG solution. The detailed steps are illustrated in Fig. 2.
Surgical procedure
The surgical procedure was performed as follows: 1 mL of each of the four previously prepared ICG solutions was intravenously administered by a specialized nurse through a peripheral vein between 0.5 and 3 h prior to surgery, in accordance with the surgical schedule. During the operation, in fluorescence mode, the liver, gallbladder, and common bile duct were examined for green staining, or the bile duct was visualized by dissecting the adipose tissue above it. Subsequently, based on intraoperative conditions, the mode was alternated between fluorescence and white light to meticulously dissect Calot’s triangle, facilitating clear visualization of the cystic duct, common hepatic duct, and cystic artery. At a point 5.00 mm from the cystic artery toward the cystic duct, an absorbable clip was applied to occlude the cystic duct. After detaching the gallbladder from the gallbladder bed, it was excised and removed, and the peritoneal cavity was irrigated with saline to ensure the absence of bleeding or bile leakage. The entire surgical process incorporated sufficient time in the fluorescence view as required. Figure 3 illustrates the intraoperative fluorescence imaging at different ICG concentrations.
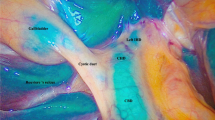
Real time fluorescence imaging of ICG with different concentrations during surgery. Figures A and C depict the initial fluorescence of 0.25 mg ICG during surgery, while Figures B and D show fluorescence imaging of the dissected gallbladder triangle and extrahepatic bile duct at the same dosage. Figures E and G illustrate the initial fluorescence of 0.50 mg ICG during surgery, while Figures F and H depict fluorescence imaging of the dissected gallbladder triangle and extrahepatic bile duct at this dosage. Figures I and K depict the initial fluorescence of 1.00 mg ICG during surgery, with Figures J and L illustrating fluorescence imaging of the dissected gallbladder triangle and extrahepatic bile duct at the same dosage. Figures M and O depict the initial fluorescence of 2.50 mg ICG during surgery, while Figures N and P illustrate fluorescence imaging of the dissected gallbladder triangle and extrahepatic bile duct at this dosage. (GB: Gallbladder, CD: Gallbladder duct, CHD: Common hepatic duct, CBD: Common bile duct)
Observational indicators
Preoperative Indicators General patient information, history of underlying diseases, prior abdominal surgeries, preoperative white blood cell count (WBC), and alanine aminotransferase levels (ALT).
Intraoperative Indicators Intraoperative blood loss, necessity for conversion to open surgery, operative duration, time taken to identify the bile duct intraoperatively (from the start of laparoscope manipulation), and results from the intraoperative fluorescent cholangiography assessment.
Postoperative Indicators Length of postoperative hospital stay (from the day of surgery to discharge), rate and duration of drainage tube placement, time to first postoperative flatus, postoperative complications (including abdominal effusion, bile leakage, abdominal infection, incision infection, and postoperative bleeding), WBC count on the first postoperative day, C-reactive protein level (CRP), ALT level, length of hospital stay, and hospitalization costs.
The incidence of complications was compared between the four groups of patients to assess the relative safety and effectiveness of the interventions. Follow-up will be performed one week after discharge to record the recovery status of all four groups of patients.
Evaluation data of imaging effect of biliary system
Three experienced hepatobiliary surgeons, demonstrating expertise in LC and independent surgical practice, assessed the imaging quality of the extrahepatic biliary system. These surgeons were blinded to the patients’ identities. The surgeons systematically reviewed surgical videos and images to evaluate and rate the imaging quality of the extrahepatic biliary system. Each evaluator had been previously trained on standardized evaluation criteria and provided ratings based on a comprehensive assessment of all factors impacting the imaging quality of the extrahepatic biliary system (see Table 1 for details).
Calculation of comparative values of fluorescence development intensity
During the surgical procedure, the operation should be recorded on video, ensuring that the clearest image is captured after successful visualization. Subsequently, five points should be uniformly selected at the interface between the liver and the cystic duct-common bile duct (CD-CBD) connection. Image J software is then utilized to measure the average fluorescence intensity at this interface. The fluorescence intensity ratio is calculated as follows: (CD-CBD connection fluorescence intensity - liver fluorescence intensity)/25515. A higher comparison value indicates a more optimal visualization of the extrahepatic bile duct during laparoscopic cholecystectomy. If visualization is unsuccessful, it is recorded as zero.
Postoperative management
LC is associated with relatively minimal surgical trauma, allowing for expeditious postoperative recovery. Ensuring patient safety and facilitating expedited recovery requires rigorous adherence to established postoperative management protocols. We implement an Evidence-Based Enhanced Recovery After Surgery (ERAS) Protocol, integrating multimodal postoperative care for all patients16. Continuous monitoring of vital signs is performed until clinical stability is attained. Postoperatively, we ensure timely replenishment of fluids, electrolytes, and energy, while administering appropriate analgesia. Patients are advised to mobilize early when feasible to promote recovery and minimize complications. On the first postoperative day, blood tests are conducted to evaluate the complete blood count and assess liver and kidney function, thereby monitoring the patient’s recovery trajectory and overall condition. A designated staff member conducts a follow-up assessment of patients’ post-discharge status one week after surgery, and regular outpatient follow-ups are scheduled thereafter. Among the all patients included in the study, 2 were lost to follow-up, with follow-up data from the remaining 80 patients indicating no postoperative complications.
Statistical analysis
Statistical analysis was conducted utilizing SPSS 25.0 software. Measurement data were assessed for normality using the Kolmogorov–Smirnov (K–S) test. Data that conformed to a normal distribution were expressed as mean ± standard deviation (\(\overline{x} \pm s\)) and analyzed via one-way ANOVA for group comparisons. In contrast, data that did not conform to a normal distribution were presented as median (interquartile range) and analyzed using the Kruskal–Wallis test. Categorical data were reported as frequency (%) and compared between groups employing chi-square or adjusted chi-square tests. When the assumptions of the chi-square test were not satisfied, Fisher’s exact test was applied. Subsequently, for intraoperative and postoperative variables that demonstrated statistical significance among the four groups, post hoc multiple comparison analyses were performed. The least significant difference (LSD) test was employed for normally distributed data, whereas the Kruskal–Wallis one-way ANOVA test (for k samples) was utilized for non-normally distributed data. A P-value of less than 0.05 was deemed statistically significant.
Results
General information and baseline characteristics of patients
In this study, comprehensive clinical data from 80 patients were analyzed to assess the outcomes of different doses of ICG in diagnosing biliary conditions. Table 2 provides a detailed overview of the baseline characteristics of the four patient groups, categorized by the ICG dosage administered. Each group presented distinct distributions of gallstone-related conditions, including chronic cholecystitis, acute cholecystitis, gallbladder polyps, and concurrent gallstones with polyps. Specifically, the 0.25 mg group had 11 cases (55.00%) of gallstones with chronic cholecystitis, 7 cases (35.00%) of gallstones with acute cholecystitis, 1 case (5.00%) of gallbladder polyps, and 1 case (5.00%) of gallstones with concurrent gallbladder polyps. In the 0.50 mg group, there were 7 cases (35.00%) of gallstones with chronic cholecystitis, 12 cases (60.00%) of gallstones with acute cholecystitis, no cases of gallbladder polyps, and 1 case (5.00%) of gallstones with concurrent gallbladder polyps. The 1.00 mg group included 8 cases (40.00%) of gallstones with chronic cholecystitis, 11 cases (55.00%) of gallstones with acute cholecystitis, 1 case (5.00%) of gallbladder polyps, and no cases of gallstones with concurrent gallbladder polyps. Lastly, the 2.50 mg group comprised 9 cases (45.00%) of gallstones with chronic cholecystitis, 10 cases (50.00%) of gallstones with acute cholecystitis, 1 case (5.00%) of gallbladder polyps, and no cases of gallstones with concurrent gallbladder polyps.
Statistical analysis indicated no significant differences in baseline characteristics among the four groups (P > 0.05). This suggests that the groups were well-matched and comparable at the outset of the study, ensuring that any observed differences in outcomes could be attributed to the varying ICG doses rather than pre-existing differences between the groups.
Patient intraoperative data
In this study, all 80 patients successfully underwent fluorescence imaging during laparoscopic surgery without the need for open surgery. Table 3 provides detailed data on the intraoperative conditions of the patients. A comparison of blood loss, time to achieve clear visualization of the bile duct, and fluorescence intensity contrast across cases revealed statistically significant differences among the four groups (P < 0.05). Following the editing and selection of appropriate surgical videos, three non-surgical physicians assessed the cases according to the table’s contents. The evaluation demonstrated that 18 cases (90.00%) in the 0.25 mg group, 15 cases (75.00%) in the 0.50 mg group, 12 cases (60.00%) in the 1.00 mg group, and 10 cases (50.00%) in the 2.50 mg group were rated as “excellent.” Statistical analysis revealed significant variations in the number of cases rated as “excellent” among the groups (P < 0.05), as shown in Fig. 4.
Intraoperative imaging demonstrated successful visualization of the gallbladder and liver in all patients. A detailed analysis of the imaging outcomes revealed that, for the 0.25 mg ICG group, visualization of the common hepatic duct, cystic duct, and common bile duct was achieved in 12, 16, and 19 cases, respectively. For the 0.50 mg group, visualization of these structures occurred in 15, 16, and 17 cases, respectively. In the 1.00 mg group, visualization was achieved in 8, 13, and 16 cases, respectively; similarly, the 2.50 mg group resulted in 9, 14, and 15 cases, respectively. Statistical analysis revealed that the differences in intraoperative visualization across the various ICG dosages did not reach statistical significance (P > 0.05).
A post hoc multiple comparison analysis was performed for the indicators that exhibited statistical significance. Furthermore, a detailed statistical analysis of intraoperative fluorescence imaging revealed the results shown in Fig. 5.
-
(1)
Intraoperative blood loss analysis indicated a statistically significant difference between the 0.25 mg and 2.50 mg groups (P = 0.006), while no significant differences were observed among the other groups.
-
(2)
Statistically significant differences in bile duct visualization time were observed between the 0.25 mg group and the 0.50 mg group (P = 0.046), 1.00 mg group (P < 0.001), and 2.50 mg group (P < 0.001). Additionally, statistically significant differences were found between the 0.50 mg group and the 1.00 mg group (P = 0.047) as well as the 2.50 mg group (P = 0.002). However, no significant difference was observed between the 1.00 mg and 2.50 mg groups.
-
(3)
Statistically significant differences in fluorescence intensity contrast were observed between the 0.25 mg group and the 0.50 mg (P < 0.001), 1.00 mg (P < 0.001), and 2.50 mg (P < 0.001) groups. Additionally, the 2.50 mg group displayed significant differences compared to the 0.50 mg (P < 0.001) and 1.00 mg (P = 0.007) groups. Notably, the 0.50 mg and 1.00 mg groups did not show any significant difference in fluorescence intensity contrast.
-
(4)
An analysis of cases rated as “excellent” in fluorescence intensity revealed a statistically significant difference between the 0.25 mg and 2.50 mg groups (P < 0.05), while no significant differences were identified among the other groups.
Postoperative indicators and recovery status of patients
Table 4 presents data on postoperative recovery outcomes across the four groups, which received doses of 0.25 mg, 0.50 mg, 1.00 mg, and 2.50 mg of ICG. Analysis revealed no significant differences in postoperative drain placement rates among the groups, with rates of 40% in the 0.25 mg group, 55.00% in the 0.50 mg group, and 70.00% in both the 1.00 mg and 2.50 mg groups. Furthermore, there were no significant differences (P > 0.05) in the duration of drain placement, time to first postoperative flatus, WBC levels on postoperative day 1, ALT levels, length of hospital stay, or hospitalization costs across the groups. These findings suggest that the varying doses of ICG did not influence the postoperative recovery outcomes in a statistically meaningful manner.
Discussion
To date, there remains no universally accepted standard for the administration of ICG fluorescence laparoscopy during LC14. ICG is a water-soluble dye that is fully metabolized by the liver and excreted exclusively through the bile duct17, without undergoing renal metabolism18. ICG is a commonly used fluorescent dye in medical imaging, common adverse reactions include mild nausea, rash, and itching, meanwhile severe reactions such as anaphylactic shock are rare19. Although the incidence of adverse reactions is relatively low20, we hope to achieve optimal intraoperative imaging using lower doses of ICG to facilitate smooth surgical procedures.This study seeks to explore the effects of varying ICG doses on intraoperative imaging and their influence on surgical and postoperative outcomes. By analyzing data from 80 patients, we identified that administering 0.25 mg ICG peripherally within a 0.5 to 3-hour window preoperatively yields optimal intraoperative effects. A more detailed discussion of these findings follows.
Previous research conducted by Pardo Aranda suggested that administering 2.5 mg of ICG to patients 2 to 6 h prior to surgery represents an optimal protocol21. Furthermore, the study by López-Sánchez analyzed dosages of both 2.5 mg and 0.05 mg/kg22. In our study, we further reduced the dosage to one-tenth of the commonly used amount in order to minimize potential risks associated with ICG in patients. Notably, the dosage was not adjusted based on the patient’s weight or body mass index (BMI)23 due to the considerable variability in patient weight, which could result in non-integer values. This variability would complicate the precise preparation of ICG solutions according to experimental design and significantly increase the workload for medical personnel.
This study investigates the optimal use of ICG in LC to achieve effective intraoperative imaging while minimizing potential side effects and costs. The key findings and implications of the study are as follows. (1)Safety and Efficacy: All cases achieved successful imaging without the need for conversion to open surgery, validating the effectiveness and safety of ICG for intraoperative imaging. The incidence of adverse reactions was low, with mild side effects such as nausea, rash, and itching being the most common.At the same time, no surgical patients in this study experienced adverse reactions related to ICG. (2)Imaging quality: After using Image J software and formula calculation, there was a significant difference in fluorescence imaging intensity comparison, with the 0.25 mg group showing enhanced clarity and reduced liver background fluorescence interference. This can more accurately identify and dissect the Carlot triangle. (3)Subjective Assessments: Surgeons rated the imaging quality as “excellent” more frequently in the 0.25 mg group, further supporting the advantage of lower ICG doses.Three surgeons, who were not involved in the surgical procedures, were selected to evaluate the imaging quality, thereby ensuring an unbiased and objective assessment. Their external perspective is expected to contribute to a more impartial analysis, enhancing the validity of the study’s findings.(4)Blood Loss and Surgical Efficiency: The 0.25 mg group experienced significantly less intraoperative blood loss compared to the 2.50 mg group (P = 0.006). This improvement in surgical efficiency is attributed to quicker identification and dissection of the bile ducts, potentially reducing surgical trauma.These findings may be attributed to the rapid distribution and metabolism of ICG in the body24, facilitating more efficient and accurate surgical interventions.Although differences were observed in the number of imaging cases for the common hepatic duct, cystic duct, and common bile duct among the dosage groups, statistical analysis indicated that these differences were not significant (P > 0.05). This suggests that within the dosage range of this study, variations in ICG dosage may not directly affect its imaging efficacy; however, this lack of significance could be influenced by various factors, including sample size and individual patient variability.
The precise dissection of Calot’s triangle to identify the CD, CHD, and CBD is a critical surgical step for preventing BDI during LC25,26. In severe cases, particularly those involving recurrent chronic cholecystitis, the dissection of Calot’s triangle and identification of the cystic duct can be exceedingly challenging27. In such situations, as well as in cases with variations in biliary anatomy, ICG demonstrates considerable clinical value28. Additionally, reducing the fluorescence intensity of the background liver while enhancing the contrast of the extrahepatic bile ducts can significantly aid in achieving a critical view of safety (CVS) during cholecystectomy, thereby ensuring the safe identification of the biliary anatomy29. In summary, this study found that lower doses of ICG, particularly 0.25 mg, offer significant benefits, including reduced intraoperative blood loss, shortened surgery time, expedited identification of the bile duct, and improved fluorescence imaging contrast. The intraoperative fluorescence evaluation results further endorse the application value of a 0.25 mg dose of ICG for imaging. These findings offer valuable insights for future clinical decisions and suggest directions for further research. Future studies should continue to explore the optimal dosing regimen of ICG while considering factors that may influence imaging effectiveness, such as individual patient differences, body mass index (BMI), inflammation status of the gallbladder, and liver function, which could affect the metabolism rate of ICG in the body30. Such research will contribute to a more comprehensive understanding of the application prospects of ICG in intraoperative imaging and help establish precise dosing regimens to maximize the benefits of ICG fluorescence imaging technology while minimizing potential risks, ultimately enhancing the quality of medical services provided to patients.
Conclusions
This study found that administering a 0.25 mg dose of ICG within 0.5 to 3 h produces the maximum fluorescence contrast between the extrahepatic bile ducts and the liver. This optimal imaging effect enhances the surgeon’s ability to operate and minimizes the potential adverse impact on the patient during surgery.
Limitations
This study has several limitations, including a small sample size and incomplete observation indicators. Therefore, future research should focus on expanding the sample size, improving observation indicators, exploring the optimal dosage and indication range of fluorescence laparoscopic technology, in order to strengthen guidance for clinical practice.
Data availability
The datasets utilized and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Tsallis, K. G. et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided laparoscopic surgery. Surg. Endosc. https://doi.org/10.1007/s00464-019-06704-2 (2019).
Zhong, Y. et al. Application of indocyanine green fluorescence imaging in complex laparoscopic cholecystectomy. J. Laparosc. Surg. 29 (08), 602–606. https://doi.org/10.13499/j.cnki.fqjwkzz.2024.08.602 (2024).
Papayan, G. & Akopov, A. Potential of indocyanine green near-infrared fluorescence imaging in experimental and clinical practice. Photodiagn. Photodyn. Ther. https://doi.org/10.1016/j.pdpdt.2018.10.011 (2018).
Zhou, Y. & Jian, Z. X. Application of indocyanine green fluorescence imaging technology in laparoscopic hepatectomy. Chin. J. Hepatobiliary Surg. 3 (2019).
Vlek, S. L. et al. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: Results of a systematic review. Surg. Endosc. 31 (7), 2731–2742. https://doi.org/10.1007/s00464-016-5318-7 (2017).
Ji, C. Y. et al. Application of indocyanine green fluorescence imaging technology in laparoscopic mesenteric surgery. J. Clin. Pediatr. Surg. 10 (2021).
Chen, Z. et al. Application of indocyanine green fluorescence imaging technology in laparoscopic difficult cholecystectomy. Chongqing Med. 51 (17), 2984–2987 (2022).
NCT05376540. Norwegian randomized trial on indocyanine green cholangiography utility for laparoscopic cholecystectomy, Prestudy. (2022). Retrieved from https://clinicaltrials.gov/show/NCT05376540
Pan, Q. L. Application effect of different doses of indocyanine green in combination with laparoscopic intrahepatic cholelithiasis surgery using indocyanine green fluorescence imaging technology. Mod. Med. Forum, 19(13). (2021).
Wang, H. Y. et al. Application research of indocyanine green fluorescence imaging-assisted laparoscopic common bile duct exploration. J. Laparosc. Surg. 10 (2022).
Wang, X. et al. Consensus guidelines for the use of fluorescence imaging in hepatobiliary surgery. Ann. Surg. 274 (1), 97–106. https://doi.org/10.1097/SLA.0000000000004718 (2021).
Ladd, A. D. et al. Low vs standard-dose indocyanine green in the identification of biliary anatomy using near-infrared fluorescence imaging: A multicenter randomized controlled trial. J. Am. Coll. Surg. 236 (4), 711–717. https://doi.org/10.1097/XCS.0000000000000553 (2023).
Liu, H. et al. Investigation of the optimal indocyanine green dose in real-time fluorescent cholangiography during laparoscopic cholecystectomy with an ultra-high-definition 4K fluorescent system: A randomized controlled trial. Updates Surg. 75 (7), 1903–1910. https://doi.org/10.1007/s13304-023-01557-w (2023).
Wang, G. M. et al. Research progress on the use of indocyanine green fluorescence imaging technology for liver resection. Chin. J. Surg. 10, 871–875. https://doi.org/10.3760/cma.j.cn112139-20201130-00828 (2021).
van den Bos, J., Schols, R. M., van Kuijk, S. M. J., Wieringa, F. P. & Stassen, L. P. S. Technical note: Are currently used measurements of fluorescence intensity in near-infrared fluorescence imaging during laparoscopic cholecystectomy comparable? J. Laparoendosc. Adv. Surg. Tech. 29 (12), 1549–1555. https://doi.org/10.1089/lap.2019.0103 (2019).
Ljungqvist, O. ERAS–enhanced recovery after surgery: Moving evidence-based perioperative care to practice. JPEN J. Parenter. Enter. Nutr. 38 (5), 559–566. https://doi.org/10.1177/0148607114523451 (2014).
Duan, R. & Li, S. The impact of sequential laparoscopic cholecystectomy following percutaneous transhepatic gallbladder puncture and drainage on trauma stress and energy metabolism in elderly patients with acute cholecystitis. Chin. J. Gerontol. 41 (23), 5210–5213 (2021).
Fu, Q. et al. Application of indocyanine green cholangiography in surgery for elderly patients with gangrenous cholecystitis. J. Surg. Electron. 9 (05), 13–18 (2022).
Meira, J. et al. Immediate reactions to fluorescein and indocyanine green in retinal angiography: Review of literature and proposal for patient’s evaluation. Clin. Ophthalmol. https://doi.org/10.2147/OPTH.S234858 (2020).
Sun, X. et al. Repurposing indocyanine green: Exploring the potential of an old drug in modern medicine. Nanoscale 16 (24), 11411–11428. https://doi.org/10.1039/d4nr00283k (2024).
Pardo Aranda, F. et al. Indocyanine green (ICG) fluorescent cholangiography in laparoscopic cholecystectomy: Simplifying time and dose. Dig. Liver Disease. 55 (2), 249–253. https://doi.org/10.1016/j.dld.2022.10.023 (2023).
López-Sánchez, J. et al. Dose and administration time of indocyanine green in near-infrared fluorescence cholangiography during laparoscopic cholecystectomy (DOTIG): Study protocol for a randomized clinical trial. BMJ Open. 13 (3), e067794. https://doi.org/10.1136/bmjopen-2022-067794 (2023).
Wang, S. Clinical application research of intravenous injection of indocyanine green in fluorescent laparoscopic cholecystectomy [Doctoral dissertation, Anhui Medical University]. (2024). https://doi.org/10.26921/d.cnki.ganyu.2024.000564
Song, W. et al. Study on the effect of laparoscopic cholecystectomy guided by indocyanine green fluorescence imaging in the treatment of patients with benign gallbladder diseases. J. Practical Hepatol. 26 (05), 746–749 (2023).
Ostapenko, A. & Kleiner, D. Challenging orthodoxy: Beyond the critical view of safety. J. Gastrointest. Surg. 27 (1), 89–92. https://doi.org/10.1007/s11605-022-05500-z (2023).
Yang, S., Hu, S., Gu, X. & Zhang, X. Analysis of risk factors for bile duct injury in laparoscopic cholecystectomy in China: A systematic review and meta-analysis. Med. (Baltim). 101 (37), e30365. https://doi.org/10.1097/MD.0000000000030365 (2022).
Kono, Y. et al. Techniques of fluorescence cholangiography during laparoscopic cholecystectomy for better delineation of the bile duct anatomy. Med. (Baltim). 94 (25), e1005. https://doi.org/10.1097/MD.0000000000001005 (2015).
Ji, W. et al. Application effect of indocyanine green fluorescence imaging technology in laparoscopic cholecystectomy. Med. Innov. China. 20 (16), 163–166 (2023).
Li, D. & Jiang, T. Experience in the application of critical safety vision in laparoscopic cholecystectomy. J. Hepatopancreatobiliary Surg. 36 (03), 172–175 (2024).
Wakabayashi, T. et al. Indocyanine fluorescence navigation liver surgery: A systematic review on dose and timing of administration. Ann. Surg. 275 (6). https://doi.org/10.1097/SLA.0000000000005406 (2022).
Acknowledgements
None.
Funding
The work was supported by financial grants from the S &T Program of Shijiazhuang(2412005703).The funder had no role in data curation, formal analysis, or writing of the report.
Author information
Authors and Affiliations
Contributions
Conception and design of study: J.B.GU, D.k.Liu; Acquisition of data: J.B.GU, D.k.Liu, Y.Wang, F.Xue, J.H.FU, X.Zhao, S.Song; Data analysis and interpretation: D.k.Liu; Drafting of manuscript and critical revision: D.k.Liu; Approval of final version of manuscript: J.B.GU.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, D., Wang, Y., Xue, F. et al. Comparative analysis of indocyanine green dosages for optimal fluorescence imaging in laparoscopic cholecystectomy. Sci Rep 15, 13491 (2025). https://doi.org/10.1038/s41598-025-97912-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97912-5