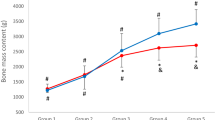
Abstract
While low-intensity blood flow restriction (LI-BFR) training has recently been shown to improve bone health, there remains limited evidence regarding its impact on older adults. This meta-analysis aimed to quantitatively identify the effects of LI-BFR training on bone mineral density (BMD) and bone biomarkers compared with conventional resistance training programs. Studies were identified through searches of four databases: PubMed, Scopus, SPORTDiscus, and Web of Science. R packages were utilized for this meta-analysis. The results indicated that compared to low-intensity (LI) training, LI-BFR significantly increased BMD (ES = 0.25, 95% Confidence Interval [CI]: [0.08, 0.41], p < 0.01), osteotropic hormones (i.e., GH, ES = 1.18, 95%CI: [0.66, 1.70]), p < 0.001; IGF-1, ES = 0.89, 95% CI: [0.44, 1.33], p < 0.001), but resulted in a smaller increase in bone resorption markers (i.e., CTX, ES = -0.77, 95%CI: [-1.16, -0.37], p < 0.001). LI-BFR training demonstrated similar effects on BMD improvement as high-intensity (HI) resistance training (ES = -0.1, 95%CI: [-0.66, 0.41], p = 0.64). Furthermore, sex and training frequency moderated the secretion of osteotropic hormones (male vs. female: IGF-1, 0.51 vs. 1.64, p < 0.01; ≤ 3 times per week vs. > 3 times per week: GH, 1.62 vs. 0.68, p < 0.01, and IGF-1, 1.13 vs. 0.39, p < 0.05). In conclusion, LI-BFR training shows promise for enhancing bone health in older adults, offering benefits comparable to HI training.
Similar content being viewed by others
Introduction
With the global trend of an aging population, health issues, particularly bone health, have garnered increasing attention1. Around 8.9 million osteoporotic fractures occur each year worldwide, primarily affecting individuals over 50 years old2. Osteoporosis is characterized by decreased bone density and deteriorating bone microstructure, which weakens bone strength and increases the risk of fractures3. While high-intensity (HI) training is effective for maintaining bone health4,5,6, it may present challenges for older adults due to the high mechanical loads involved. Low-intensity (LI) training presents an appealing alternative for older adults due to its reduced physical demands. However, it is less effective in enhancing bone density7,8. Evidence suggests that low-intensity blood flow restriction (LI-BFR) training (20–30% 1RM) may present a promising approach for stimulating bone adaptation9,10.
The application of pressurized cuffs at the proximal regions of the limbs in LI-BFR training partially limits venous return, leading to elevated local blood pressure and increased interstitial fluid accumulation, which consequently augments intramedullary pressure within bone and promotes bone metabolic activity11. Moreover, LI-BFR training induces local hypoxia conditions and a modest decrease in pH levels, stimulating angiogenesis and modulating the activities of bone-forming osteoblasts and bone-resorbing osteoclasts12,13. Additionally, BFR training increases the effects of hormones such as cortisol, testosterone, and insulin-like growth factor-1 (IGF-1), which regulate bone formation and resorption, effectively promoting bone health14,15. Therefore, BFR training may provide an effective alternative for older adults who are unable to participate in high-intensity exercise regimens.
However, current research presents controversies regarding the efficacy of LI-BFR training in improving bone health among older adults. Certain studies have suggested that LI-BFR is more effective than LI training alone in improving BMD and promoting bone formation16,17,18. Moreover, some findings have shown that the impact of LI-BFR training on BMD and growth factors is comparable to those of HI training19. However, other studies have reported similar outcomes with LI-BFR and LI training in bone health improvements7,20.
While some studies have explored the impact of LI-BFR training on bone health in older adults, a comprehensive meta-analysis is necessary to synthesize the current evidence and definitively determine its efficacy. Therefore, this study aimed to systematically assess the effects of LI-BFR training on bone health in older adults, ultimately informing evidence-based recommendations for preventing and treating age-related osteoporosis.
Materials and methods
Registration and literature search
This meta-analytical review was conducted following the PRISMA guidelines for systematic reviews and meta-analyses21 and the PRISMA statement (Prospero registration number: CRD42024520288).
The following databases were searched up to May 27, 2024: PubMed, Scopus, Web of Science, and SPORTDiscus. The search terms included “blood flow restriction”, “vascular occlusion”, “KAATSU”, “bone”, “osteo*”, " biomarkers”, “elderly”, “older”. The full search string is provided in Supplementary Material 1. After duplicates were removed, the titles and abstracts of the retrieved articles were screened, and the full texts were thoroughly reviewed (Fig. 1). Additionally, reference lists from included studies were examined. Two researchers (Y.L. and Y.Z.) independently retrieved articles and extracted data. Any discrepancies were resolved by a third researcher (Z.G.).
Eligibility criteria
Eligibility criteria for article inclusion were as follows: (a) age > 50 years; (b) pre- and post-training assessment of BMD, bone biomarkers (e.g., bone-specific alkaline phosphatase [BALP], C-terminal telopeptide of type I collagen [CTX], growth hormone [GH], and insulin-like growth factor 1 [IGF-1]); (c) comparisons between LI-BFR training and LI or HI resistance training; (d) studies with a physiotherapy evidence database (PEDro) scale score of ≥ 4 in the quality assessment.
Methodological quality assessment
The quality of the methodologies of the included studies was evaluated using the PEDro scale22. The PEDro scale evaluates the methodological quality based on 11 criteria (e.g., randomization, blinding, and outcome measures), with a maximum score of 10 points, excluding item 1 from the rating. Studies scoring below 4 on the PEDro scale are typically regarded as low quality. Therefore, the meta-analysis included studies that met a minimum PEDro score of 4. Two researchers independently performed the methodological quality assessment (Y.L. and Y.Z.), and any disagreements were settled by a third researcher (T.W.). Furthermore, potential bias was assessed using funnel plots.
Data extraction
Relevant data were extracted on population characteristics (i.e., age and sex), primary outcome measures, interventional characteristics (i.e., group, number, exercise mode, cuff pressure, training duration, and training frequency), and the main results of each study. The primary outcome measures included BMD, bone biomarkers (e.g., bone formation markers such as osteocalcin and BALP, and bone resorption markers such as CTX and N-terminal telopeptide [NTX]), and osteotropic hormones (e.g., GH, IGF-1, and testosterone). If the raw data (mean and standard deviation) were not available, they were requested directly from the authors; otherwise, the data were extrapolated from the figures.
Statistical analyses
R packages (R version 4.3.0 with R Studio version 2024.04.1 + 748) were utilized to conduct the statistical analyses. Effect sizes (standardized mean difference, SMD) with 95% confidence intervals were calculated based on measurements taken before and after the interventions, accounting for baseline differences across the included studies23. \(\:{SD}_{change}\) was calculated using the following equation:
The magnitude of effect size was classified according to the following scale: trivial (< 0.2), small (0.2−0.5), moderate (0.5−0.8), and large (> 0.8)24. A random effects model was applied to account for heterogeneity among the studies and to provide more generalizable and robust results. I2 statistics was used to assess the heterogeneity for between-study variability, with thresholds set at < 25% for low, 25−75% for moderate, and > 75% for high25. Sensitivity analyses were conducted to examine the robustness of the results by excluding studies with potential outlier effects or low quality.
A total of five meta-analyses were performed, examining the impact of LI-BFR versus LI training on BMD and bone biomarkers (i.e., BALP, CTX, GH, and IGF-1). Additionally, subgroup analyses were conducted to investigate the moderating effects of age (< 60 years old vs. ≥ 60 years old), sex, training duration (≤ 12 weeks vs. > 12 weeks), training frequency (≤ 3 days/week vs. > 3 days/week), and cuff pressure (≤ 150 mmHg vs. > 150 mmHg) on these training outcomes. Subgroup analyses were conducted if three or more relatively homogeneous data were available for each subgroup. Statistical significance was determined using a threshold of p < 0.05.
Results
Study selection
The initial search identified 1231 studies. Following duplicate removal, 594 studies were left for title and abstract examination. This screening excluded 561 studies, resulting in 33 studies for full-text review. Following the full-text review, 21 studies were excluded. Additionally, two more studies were identified through references of the included studies. Ultimately, 14 studies fulfilled the inclusion criteria (see Fig. 1). Detailed characteristics of these included studies are summarized in Table 1.
Methodological quality assessment
Among the included studies, three were of moderate quality, scoring between 4 and 5 points, while 11 were of high quality, scoring between 6 and 10 points. The median score was 6 out of 10, indicating a moderate to high level of overall quality, thereby ensuring the reliability of the studies. Detailed PEDro scale scores can be found in Supplementary material 3. Sensitivity analyses were conducted to assess the robustness of the results, and no significant changes in effect sizes were observed, confirming the consistency and reliability of the main findings (Supplementary material 4).
Meta-analysis results
Two studies examining the effects of LI-BFR vs. HI training on BMD reported an effect size of -0.13 (trivial effect, 95%CI: [-0.66, 0.41], p = 0.64) (see Fig. 2A). Eight studies investigating the effects of LI-BFR vs. LI training on BMD were included in this meta-analysis. The effect size was 0.25 (small effect, 95% Confidence Interval, CI: [0.08, 0.41], p < 0.01) (see Fig. 2B). Five studies, involving 166 participants, examined the effects of LI-BFR vs. LI training on BALP and reported an effect size of 0.36 (small effect, 95% CI: [-0.13, 0.84], p = 0.15) (see Fig. 3A). Four studies, involving 108 participants, examined the effects of LI-BFR vs. LI training on CTX and reported an effect size of -0.77 (medium effect, 95% CI: [-1.16, -0.37], p < 0.001) (see Fig. 3B). Seven studies, involving 268 participants, examined the effects of LI-BFR vs. LI training on GH and reported an effect size of 1.18 (large effect, 95% CI: [0.66, 1.70], p < 0.001) (see Fig. 3C). Ten studies, involving 311 participants, examined the effects of LI-BFR vs. LI training on IGF-1 and reported an effect size of 0.89 (large effect, 95% CI: [0.44, 1.33], p < 0.001) (see Fig. 3D). Additionally, the funnel plots from the five meta-analyses demonstrated a fairly uniform distribution, indicating no significant publication bias or selective reporting (see Supplementary Material 2).
Subgroup analyses were conducted if three or more relatively homogeneous data were available for each subgroup. A total of 13 subgroup analyses were performed for BMD, GH, and IGF-1 based on age, sex, training duration, training frequency, and cuff pressure (see Table 2). Training frequency had a significant moderating effect on GH (1.62 [0.98, 2.27] vs. 0.68 [0.32, 1.05], p < 0.01) and IGF-1 (1.13 [0.57, 1.69] vs. 0.39 [0.03, 0.74], p < 0.05). Specifically, training frequencies of >3 times per week were less effective than frequencies of ≤ 3 times per week. Additionally, sex had a significant moderating effect on IGF-1 (0.51 [0.16, 0.87] vs. 1.64 [1.05, 2.23], p < 0.01), with females showing a more pronounced IGF-1 response.
Discussion
The present meta-analysis compared the impact of LL-BFR training on bone health with that of LI and HI resistance training without BFR in older adults. The main findings indicated that LI-BFR resulted in more significant bone health improvements compared to LI training. Specifically, these improvements included enhancements in BMD, increased bone formation (BALP ↑), elevated secretion of osteotropic hormones (GH ↑ and IGF-1 ↑), and decreased bone resorption (CTX ↓). Additionally, LL-BFR had similar effects on BMD improvement as HI resistance training. Furthermore, the study found that training frequency moderated the secretion of osteotropic hormones (GH and IGF-1), with a frequency of ≤ 3 times per week being more effective than > 3 times per week. Sex also had a moderating effect on IGF-1, with women showing greater increases in IGF-1 levels with LL-BFR training compared to men. These findings highlight the promise of LL-BFR training as a low-load option for improving bone health in older adults, comparable to the benefits of HI resistance training.
The current meta-analysis found LI-BFR training yielded superior improvements in bone health compared to LI training, consistent with the meta-analysis by Wang, et al.9. However, several key differences underscore the novelty of our study. Wang et al. analyzed separate groups for young and older adults, but the older adult subgroup was based on a limited number of studies and included fewer outcome measures. In contrast, this meta-analysis specifically focuses on older adults, with a more robust sample and a wider range of outcome measures, providing a more comprehensive and nuanced evaluation of the relative effectiveness of LI-BFR versus LI training in this population.
There are several potential reasons for these discrepancies regarding LI-BFR versus LI training yielding superior improvements in bone health. Firstly, the increase in intramedullary pressure and interstitial fluid resulting from BFR training may enhance the activity of osteoblasts and osteoclasts, activate bone cell signaling, and may promote bone formation and remodeling26. Secondly, hypoxia and pH reductions caused by BFR can regulate the secretion of bone metabolic hormones, thereby promoting bone formation12,27. This meta-analysis suggests that the increased concentrations of osteogenic hormones (GH and IGF-1) in LI-BFR training may promote bone formation and remodeling. Additionally, the hypoxic and acidic environment induced by BFR training may lower parathyroid hormone (PTH) levels, thereby inhibiting bone resorption and further promoting bone formation28. Thirdly, the hypoxic conditions generated by BFR can stimulate hypoxia-inducible factor (HIF) and enhance the production of vascular endothelial growth factor (VEGF), promoting the formation of new blood vessels13, which is crucial for the transport of osteoblast precursors29. Through these mechanisms, LI-BFR training accelerates bone formation and inhibits bone resorption. This meta-analysis also confirmed these findings, as LI-BFR training resulted in higher levels of bone formation markers (i.e., BALP) and lower levels of bone resorption markers (i.e., CTX) compared to LI resistance training. Therefore, LI-BFR training appears to significantly enhances bone health through multiple mechanisms, possibly making it superior in promoting bone health.
Regarding BMD, the present meta-analysis found no significant difference between LI-BFR training and HI resistance training, but LI-BFR training was more effective than LI resistance training. The meta-analysis by Wang et al.9 also observed statistically significant results in BMD; however, the effect sizes were relatively small, with − 0.01 for LI-BFR vs. HI training and 0.04 for LI-BFR vs. LI training. One possible reason for this discrepancy is that the meta-analysis by Wang, et al.9 did not distinguish between different populations, such as young adults, older adults, and patients. In contrast, this present meta-analysis specifically focused on older adults. Another possible reason is the difference in the intervention periods of the included studies. Wang, et al.9 reported intervention periods ranged from 3 to 12 weeks, whereas in the present meta-analysis, intervention periods ranged from 4 to 36 weeks, with four studies exceeding 24 weeks. The bone remodeling process takes approximately 24 weeks (about 2 weeks for the resorption phase, 5 weeks for the reversal phase, and 16 weeks for the formation phase)30,31. Most of the included studies in the meta-analysis by Wang, et al.9 were in the reversal and early formation phases, while the included studies in the present meta-analysis were mostly in the formation phase or had completed a full bone remodeling cycle. As a result, the longer intervention periods in the studies included in this meta-analysis likely contributed to more significant BMD adaptation and improvement. The finding of no significant difference between LI-BFR and HI training suggests that LI-BFR could be as effective as HI resistance training. Hence, LI-BFR may provide a viable alternative for improving bone health in older adults.
Interestingly, our findings indicated that training frequency had a moderating effect on the secretion of osteogenic hormones (i.e., GH and IGF-1), with a frequency of ≤ 3 times per week resulting in higher secretion, and > 3 times per week leading to relatively lower secretion. This may be because high-frequency training (> 3 times per week) can lead to overtraining, putting the body into a state of stress. This state may affect the balance of the endocrine system and the bone repair process, thereby reducing the secretion of osteogenic hormones32. Therefore, it is essential to design training programs with a reasonable frequency to ensure that the body has enough recovery time to maintain bone health.
Additionally, our meta-analysis also found that sex had a moderating effect on the secretion of osteogenic hormones, with women producing more osteogenic hormones with LI-BFR training compared to men. Postmenopausal women may face greater challenges in maintaining bone density due to a significant decrease in estrogen levels33. However, the endocrine response to exercise differs between women and men. Despite the decrease in estrogen levels, women may compensate by increasing the secretion of osteogenic hormones, such as GH and IGF-1, to maintain bone health34.
Different levels of blood flow restriction lead to varying hemodynamic changes, which may differentially impact bone adaptation10. However, in the present meta-analysis, variations in occlusion pressure did not significantly moderate the training outcomes. One possible reason is the multiple forms of pressure presentation used in the included studies, such as specific pressure values, percentage of arterial occlusion pressure (AOP %), and cuff tightness. These variations complicate the moderation analysis and reduce the precision of the results. Additionally, due to individual differences, the same occlusion pressure may induce different degrees of blood flow restriction. Therefore, it is crucial to adopt personalized occlusion pressure prescriptions. The currently popular method of AOP % is recommended35, as it allows for personalized pressure settings based on individual arterial characteristics, thereby ensuring the precision and safety of the training.
Limitations
The meta-analysis has several potential limitations that necessitate cautious interpretation of the findings. Firstly, the small number of included studies may limit the statistical power and generalizability of the findings, particularly in the moderation analyses. More high-quality studies are needed in the future to enhance the robustness and applicability of the results. Secondly, in the subgroup analyses, the methods of representing occlusion pressure varied, including specific pressure values, AOP %, and cuff tightness. This variability increased the complexity of the analysis and may have reduced the precision and accuracy of the results, necessitating cautious interpretation. Thirdly, while many studies have not reported adverse reactions or injuries related to LI-BFR training, this does not imply that LI-BFR training is free of potential safety issues. The lack of reported issues may stem from limitations in study design or incomplete reporting. Therefore, it is crucial to implement LI-BFR training with caution, incorporating thorough risk assessments and careful monitoring to ensure participant safety.
Conclusion
In conclusion, LI-BFR training demonstrates superior efficacy in enhancing bone health compared to LI resistance training, while also exhibiting comparable benefits to HI resistance training. Additionally, women and low-frequency training (≤ 3 times per week) result in higher levels of osteogenic hormones (i.e., GH and IGF-1). Therefore, LI-BFR training appears to be a viable and effective alternative to HI resistance training for older adults.
Practical applications
When implementing LI-BFR training, several practical considerations are crucial to maximize effectiveness and ensure safety. Training sessions exceeding three times per week may reduce bone health improvements. Therefore, a low-frequency regimen is recommended to ensure effectiveness and prevent overtraining, which is crucial for allowing adequate recovery time in older adults. Additionally, due to the extended bone remodeling cycle, typically around six months, short-term interventions may not yield significant results. Thus, long-term engagement in LI-BFR training is necessary to observe meaningful improvements in bone health, highlighting the importance of sustained intervention.
Moreover, the application of LI-BFR should be carefully managed to avoid adverse events. It is essential to conduct a comprehensive risk assessment of the training program before initiating LI-BFR training, considering the individual health status and medical history of the participants. It is recommended to use a personalized AOP % method to ensure safety and maximize effectiveness. By adhering to these practices, LI-BFR training may serve as a safe and effective strategy for improving bone health in older adults, offering an alternative to high-load resistance training.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Wade, S., Strader, C., Fitzpatrick, L., Anthony, M. & O’Malley, C. Estimating prevalence of osteoporosis: examples from industrialized countries. Archives Osteoporos. 9, 1–10 (2014).
Ström, O. et al. (Springer, (2011).
Kanis, J. A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359, 1929–1936 (2002).
Kitsuda, Y., Wada, T., Noma, H., Osaki, M. & Hagino, H. Impact of high-load resistance training on bone mineral density in osteoporosis and osteopenia: a meta-analysis. J. Bone Miner. Metab. 39, 787–803 (2021).
Maddalozzo, G. & Snow, C. High intensity resistance training: effects on bone in older men and women. Calcif. Tissue Int. 66, 399–404 (2000).
Bloomfield, S. A., Little, K., Nelson, M. & Yingling, V. American college of sports medicine position stand: physical activity and bone health. Med. Sci. Sports Exerc. 195, 3611 (2004).
Linero, C. & Choi, S. J. Effect of blood flow restriction during low-intensity resistance training on bone markers and physical functions in postmenopausal women. J. Exerc. Sci. Fit. 19, 57–65 (2021).
Eslamipour, F., Gheitasi, M., Hovanloo, F. & Yaghoubitajani, Z. High versus Low-Intensity resistance training on bone mineral density and content acquisition by postmenopausal women with osteopenia: A randomized controlled trial. Med. J. Islamic Repub. Iran. 37, 126 (2023).
Wang, X. et al. Effects of blood flow restriction training on bone metabolism: a systematic review and meta-analysis. Front. Physiol. 14, 1212927 (2023).
Hughes, L. & Centner, C. Idiosyncratic bone responses to blood flow restriction exercise: new insights and future directions. J. Appl. Physiol. 136, 283–297 (2024).
Loenneke, J. P. et al. Blood flow restriction: rationale for improving bone. Med. Hypotheses. 78, 523–527 (2012).
McCarthy, I. The physiology of bone blood flow: a review. JBJS 88, 4–9 (2006).
Araldi, E., Schipani, E. & Hypoxia HIFs and bone development. Bone 47, 190–196 (2010).
Shigehara, K., Izumi, K., Kadono, Y. & Mizokami, A. Testosterone and bone health in men: a narrative review. J. Clin. Med. 10, 530 (2021).
Yinghao, L. et al. Effects of a blood flow restriction exercise under different pressures on testosterone, growth hormone, and insulin-like growth factor levels. J. Int. Med. Res. 49, 03000605211039564 (2021).
Kargaran, A. & Amani-Shalamzari, S. Effect of cognitive-walking training with blood flow restriction on muscle quality and bone density in elderly women. J. Practical Stud. Biosci. Sport. 10, 54–66 (2022).
Karabulut, M. et al. Effects of high-intensity resistance training and low-intensity resistance training with vascular restriction on bone markers in older men. Eur. J. Appl. Physiol. 111, 1659–1667 (2011).
Liu, Y., Ye, Q. & Liu, H. Effects of KAATSU and resistance training on bone mineral density, insulin sensitivity, muscle strength and hormone secretion in pre-diabetic state population. Chin. J. Osteoporos. 24, 56–63 (2018).
Park, S., Lee, J., Ahn, J., Son, W. & Yoon, S. -j. Effect of low-intensity resistance training with blood flow restriction on serum VEGF level, bone markers and bone mineral density in elderly women. Korean J. Sport Sci. 30, 459–469 (2019).
YE, Q. Effects of KAATUS training combined with vibration training on bone metabolism and bone mineral density in elderly men. Chin. J. Osteoporos. 24, 290–294 (2018).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71 (2021).
Verhagen, A. P. et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 51, 1235–1241 (1998).
Higgins, J. P. T., Li, T. & Deeks, J. J. Choosing effect measures and computing estimates of effect, in Cochrane Handbook for Systematic Reviews of Interventions (eds Higgins, J., Thomas, J., et al.) 143–176, 2nd ed. (Cochrane, 2021).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Routledge, 2013).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Bmj 327, 557–560 (2003).
Fritton, S. P. & Weinbaum, S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu. Rev. Fluid Mech. 41, 347–374 (2009).
Prisby, R. D. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J. Endocrinol. 235, R77–R100 (2017).
Copatti, S. L. et al. Acute effects of the resistance exercise associated with different blood flow restriction pressures on bone remodeling biomarkers. J. Exerc. Sci. Fit. 20, 155–160 (2022).
Lafage-Proust, M. H. & Roche, B. The vasculature and bone, in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (ed. Bileszkian, J. P.) 981–991, 9th ed. (John Wiley & Sons, 2019).
Agerbaek, M., Eriksen, E., Kragstrup, J., Mosekilde, L. & Melsen, F. A reconstruction of the remodelling cycle in normal human cortical Iliac bone. Bone Miner. 12, 101–112 (1991).
Eriksen, E., Gundersen, H., Melsen, F. & Mosekilde, L. Reconstruction of the formative site in Iliac trabecular bone in 20 normal individuals employing a kinetic model for matrix and mineral apposition. Metabolic Bone Disease Relat. Res. 5, 243–252 (1984).
Fry, A. C. & Kraemer, W. J. Resistance exercise overtraining and overreaching: neuroendocrine responses. Sports Med. 23, 106–129 (1997).
Burger, H. G., Hale, G., Robertson, D. & Dennerstein, L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne women’s midlife health project. Hum. Reprod. Update. 13, 559–565 (2007).
LANG, I. et al. Effects of sex and age on growth hormone response to growth hormone-releasing hormone in healthy individuals. J. Clin. Endocrinol. Metabolism. 65, 535–540 (1987).
Patterson, S. D. et al. Blood flow restriction exercise: considerations of methodology, application, and safety. Front. Physiol. 10, 448053 (2019).
Bittar, S. T. et al. Effect of walking with blood flow restriction in elderly women with osteoporosis/osteopenia. Fisioterapia Em Movimento. 36, e36116 (2023).
Centner, C., Zdzieblik, D., Roberts, L., Gollhofer, A. & König, D. Effects of blood flow restriction training with protein supplementation on muscle mass and strength in older men. J. Sports Sci. Med. 18, 471 (2019).
Karabulut, M., Sherk, V. D., Bemben, D. A. & Bemben, M. G. Inflammation marker, damage marker and anabolic hormone responses to resistance training with vascular restriction in older males. Clin. Physiol. Funct. Imaging. 33, 393–399 (2013).
Lopes, K. G. et al. Exercise with blood flow restriction improves muscle strength and mass while preserving the vascular and microvascular function and structure of older adults. Clin. Hemorheol. Microcirc. 82, 13–26 (2022).
Seo, D., So, W. Y. & Sung, D. J. Effect of a low-intensity resistance exercise programme with blood flow restriction on growth hormone and insulin-like growth factor-1 levels in middle-aged women. South. Afr. J. Res. Sport Phys. Educ. Recreation. 38, 167–177 (2016).
Shimizu, R. et al. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur. J. Appl. Physiol. 116, 749–757 (2016).
TANG, L. & PENG, X. Effect of exercise under blood flow restriction on risk of fragility fractures in postmenopausal patients with chronic obstructive pulmonary disease. Chin. J. Rehabilitation Theory Pract. 29, 761–769 (2023).
Zaravar, L., Nemati, J., Rezaei, R., Jahromi, M. K. & Daryanoosh, F. Effect of eight weeks water exercise with blood flow restriction on growth hormone, insulin-like growth factor-1 and bone metabolism in elderly women. Sport Physiol. 13, 69–92 (2021).
Author information
Authors and Affiliations
Contributions
Y.L. and Y.Z. conceptualized the review and registered the protocol. Y.L., Y.Z., and T.W. searched the online databases and extracted the data. Z.G. and T.W. performed data analysis. Y.L., Y.Z., Z.G., and X.W. drafted the primary manuscript. Y.L., Y.Z., Z.G., X.W., and T.W. revised and confirmed the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Zhang, Y., Wang, T. et al. Systematic review and meta-analysis of the effects of blood flow restriction training on bone health in older adults. Sci Rep 15, 12800 (2025). https://doi.org/10.1038/s41598-025-98053-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98053-5