Abstract
The KRAS G12D mutation is the most prevalent type of pancreatic cancer and is found in about 35% of patients. Numerous natural chemicals are frequently investigated in cancer treatment to decrease side effects. Resveratrol (RVT) is a polyphenol that can promote cancer cell apoptosis and improve chemotherapy efficacy in cancers. To enhance delivery rate and efficacy, the size of about 30 nm gold nanoparticles (GNPs) was synthesized and conjugated to resveratrol via polyvinylpyrrolidone (GRs) for high bioavailability. Compared to RVT and GNPs, GRs had less inflammatory response and less toxicity on RAW 264.7 cells. This suggests that the toxicity of resveratrol can be alleviated by conjugation with gold nanoparticles. The viability of the human pancreatic cancer cell line (AsPC-1) decreased in sequence of GRs > RVT > GNPs, suggesting an enhanced anticancer effect of the GRs compared to resveratrol (RVT) alone. In addition, the extent of apoptosis was much bigger with GRs compared to RVT and GNPs. The apoptotic effects were confirmed with cell cycle arrest and expression of apoptosis-related genes and proteins. Thus, GRs had a better extent of anticancer effect than RVT, suggesting that GRs be considered as one of the prospective anti-cancer drugs for pancreatic cancer treatment.
Similar content being viewed by others
Introduction
Pancreatic cancer is a dangerous type of cancer because it is difficult to diagnose early, and the cancer cells infiltrate and spread easily surrounding tissues1. According to the reports in 2023, there could be approximately 64,050 people with new cases of pancreatic cancer in the United States2. The disease has no certain cure, and its exact cause is still unknown. Even though the symptoms are identified, they are mostly getting worse and not responding well to common therapies including radiation, chemotherapy, and targeted drugs. The search for advanced pancreatic cancer therapies has been continued3. Many anti-cancer agents have been investigated4,5. Among them, resveratrol (trans-3,5,4' -trihydroxystilbene) is a phytoalexin derived from the skin of grapes, raspberries, and other food sources. There have been reports that resveratrol has antioxidant and anti-inflammatory effects. Resveratrol has limitations for usage, such as low water solubility, poor bioavailability, and a short biological half-life. However, by conjugating resveratrol with gold nanoparticles (GNPs), these limitations can be overcome, significantly enhancing its therapeutic effectiveness6.
Drug delivery methods based on nanoparticles have a promising potential in cancer therapy. There have been numerous attempts to employ nanoparticles or nanomaterials as medication carriers7. Potential options have included metals, polymers, and hydrogels. One of the metal transporters is the gold nanoparticles (GNPs)8. Because of biocompatibility and comparatively low toxicity, GNPs has been widely utilized. Their size and shape can be precisely controlled, making them ideal for drug delivery. It is also known that GNPs can effectively penetrate cellular membranes and enable targeted delivery to cancer cells, thereby maximizing therapeutic efficacy. In addition, GNPs can be produced more easily than other metal nanoparticle carriers9. It was possible to manage their size and shape with ease compared to other metal nanoparticles. GNPs have been investigated for photothermal therapy, cancer diagnostics, and cancer treatment10. With the advancement of nanotechnology, therapeutic efficacy for cancer treatment could be improved by using nano-carrier-conjugated with anti-tumor medications while bioavailability is increased.
Previous study have shown that the action mechanism of GNPs conjugated with resveratrol involves multiple facets8. The gold nanoparticles enter cancer cells through endocytosis, where they release the conjugated resveratrol. Resveratrol, once inside the cell, modulates various intracellular signaling pathways, such as inhibiting the PI3K/Akt and MAPK/ERK pathways, which are commonly activated by the KRAS mutation. Additionally, the small size and surface charge of GNPs enable them to penetrate tumor vasculature and deliver resveratrol directly to cancer cells with higher specificity, potentially improving the overall therapeutic outcome8.
K-ras is the gene most commonly mutated in pancreatic cancer, with mutation rates reported between 75 and 95%11. Over 80% of KRAS mutations occur at codon 12, with the G12D substitution being particularly prevalent—present in approximately 70% of pancreatic ductal adenocarcinoma (PDAC) cases and nearly 50% of colorectal carcinoma (CRC) cases12,13. In the present study, AsPC-1 cells were employed as a pancreatic cancer cell line that has a G12D-mutated KRAS gene sequence. Then, gold nanoparticles conjugated with resveratrol (GRs) was synthesized and examined for anti-cancer activities in AsPC-1 cells.
Materials and methods
Cell lines
The human pancreatic cancer cell line (AsPC-1) and murine macrophage cell line (RAW 264.7) were purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). AsPC-1 cells were cultured in Roswell Park Memorial Institute 1640 (RPMI–1640) supplemented with 10% fetal bovine serum (FBS; Welgene, Daegu, Korea), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Welgene). RAW 264.7 murine macrophage cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Welgene) supplemented with 10% FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. All cells were incubated at 37 °C in a 5% CO2 humidified incubator.
GNPs synthesis
Gold nanoparticles (GNPs) were synthesized based on the seed-growth method14,15,16. To prepare the gold seed solution, the 20 mL solution containing 2.5 × 10–4 M gold chloride (HAuCl4) and 2.5 × 10–4 M trisodium citrate was mixed gently in the flask at room temperature for 15 ~ 20 min. Then, 0.6 mL of 0.1 M NaBH4 was added to the solution, and the solution turned pink immediately. Growth solution A was made by adding 5.831 g of cetyltrimethylammonium bromide (CTAB) to 200 mL of the aqueous solution containing 2.5 × 10–4 M HAuCl4. Then, it was mixed with the gold seed solution at 45 °C for 10 min until it became clear orange. The resulting solution received growth solutions B, C, and D in sequence according to the previous method14,15. The final growth solution was washed thrice with distilled water to remove CTAB (12,000 × g, 10 min, 30 °C). Thus, the GNPs of the desired size (30 ~ 40 nm) was made with the sequential treatment of the growth solutions.
Resveratrol conjugation
16.67 μM polyvinyl pyrrolidone (PVP, M.W. 40,000; PVP amount of 15 mg) was added to the 80 mL washed final solution E and stirred for 50 min at 60 °C. After cooling down, 20 mg of resveratrol (RVT) dissolved in 5 mL acetone was added and mixed for the next 3 h at 60 °C. Gold nanoparticles conjugated with resveratrol (GRs) were made by washing the mixture twice with distilled water (8000 × g, 10 min, 25 °C). GRs synthesis was repeated at least 3 times for assurance of conjugation14,15. All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Characterizations
Transmission electron microscopy (TEM) images of synthesized GNPs and GRs were acquired by an FEI Tecnai 20 (FEI Company; Hillsboro, OR, USA) using a 200 kV filament. The diameter, polydispersity, and zeta potential of the GNPs and GRs were measured using Nanobrook 90 plus (Brookhaven Instrument; Brookhaven, GA, USA)14,15,16.
Cell viability assay
For cell viability assay, EZ-Cytox kit was used (DoGenBio, Seoul, Korea). AsPC-1 cells (5 × 103 cells/well) were plated into 96-well cell culture plates for 24 h. After cell attachment, the cells were treated with GNPs, RVT, or GRs at various concentrations for 72 h. After treatment, AsPC-1 cells were added into the medium for 3 h according to the manufacturer’s instructions. Then, the absorbance value was measured at 490 nm using a Microplate Reader (EL-800, Bio-Tek; Abington, MA, USA).
Nitric oxide assay
NO production was measured by nitrite concentration in the medium using a Griess reagent (Sigma-Aldrich)5. RAW 264.7 cells were plated at 3 × 105 cells/well in a 6-well plate and incubated for 24 h. After incubation, the cells were treated with different concentrations of GNPs, RVT, GRs, and lipopolysaccharide (LPS; Sigma-Aldrich) for 48 h and, subsequently, the supernatant was separated. The supernatant was treated with Griess reagent, and the absorbance was measured at 540 nm to detect NO formation.
Lactate dehydrogenase (LDH) leakage assay
RAW 264.7 cells at a density of 3 × 105 cells were seeded into 6-well plates. After 24 h, the cells were treated with GNPs, RVT, or GRs at various concentrations for 48 h. The supernatant was obtained by centrifugation at 1500 × g for 15 min at 5 °C. The LDH concentration in the samples was analyzed for cytotoxicity using the LDH Cytotoxicity Detection kit (Takara Bio; Shiga, Japan) according to the manufacturer’s protocol15.
Cytokine secretion analysis
LPS was used to stimulate the production of cytokines in RAW 264.7 cells. After LPS treatment, RAW 264.7 cells were treated with GNPs, RVT, and GRs at various concentrations for 48 h, respectively. Then, the media were harvested to examine cytokine concentrations using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems; Minneapolis, MN, USA) according to the manufacturer’s protocol17.
Apoptosis assay
AsPC-1 cells at a density of 5 × 105 cells were seeded into 6-well plates and treated after attachment with each sample. After 72 h, the cells were harvested by trypsinizing and washing with phosphate-buffered saline (PBS) at 670 × g for 5 min. The cells were resuspended in 100 µL binding buffer. Then, the cells were stained with 5 µL propidium iodide (PI; Sigma-Aldrich) and 5 µl Annexin-V in FITC for 10 min in the dark. The apoptotic cell population was analyzed using a FACSCalibur instrument (BD Biosciences; Piscataway, NJ, USA)5.
Quantitative analysis of mRNA expression
Total RNA was extracted using TRIzol reagent (Invitrogen; Carlsbad, CA, USA) and the first strand cDNA was synthesized by RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific; Waltham, MA, USA) according to the manufacturer’s instructions18. Real-time PCR was conducted using TaqMan Gene expression assay (Thermo Fisher Scientific) and TaqMan Gene expression master mix (Thermo Fisher Scientific). The real-time PCR was performed using a CFX-96 real-time PCR machine (Bio-Rad; Hercules, CA, USA) to quantify the relative mRNA expression levels of selected genes. The conditions of the PCR cycle were 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Quantification was normalized in comparison with the GAPDH expression. Results were calculated using an equation of 2−ΔΔCT19.
Quantitative analysis of protein expression
Total cell lysates were extracted from AsPC-1 cells using lysis buffer (M-per buffer, 100 × Protease Inhibitor, 0.1 M phenylmethylsulfonyl fluoride, 200 mM sodium orthovanadate, 1 M sodium fluoride). All reagents were purchased from Thermo Fisher Scientific. The cell lysate was centrifuged at 13,000 × g for 10 min at 4 °C. The supernatants were treated with bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific) according to the manufacturer’s protocol. Aliquot amount of protein samples was separated by 8 ~ 14% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred into polyvinylidene fluoride (PVDF) membrane (0.45 μm, Millipore, Burlington, MA, USA). The membranes were blocked with 5% skim milk or 5% bovine serum albumin (BSA) and incubated with primary antibodies at 4 °C overnight and exposed to horse-radish peroxidase (HRP)-conjugated secondary antibody at room temperature for 2 h. The antigen–antibody complexes were visualized by the enhanced chemiluminescence (ECL) Western blotting detection reagents (Thermo Fisher Scientific). Luminescent images were analyzed using a ChemiDoc XBS (Bio-Rad) and Image Lab software version 4.1 (Bio-Rad)15. Primary antibodies used in this study included rabbit anti-β-actin (Cat. #4970), PARP (Cat. #9532), cleaved PARP (Cat. #5625), BAX (Cat. #5023), Bcl-2(Cat. #4223), Cyclin D1 (Cat. #2978), BAK (Cat. #3814). All the antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). The HRP-conjugated secondary antibody of goat-rabbit (Cat. #7074; Cell Signaling Technology) was applied.
Statistical analysis
Statistical analyses were performed using Student’s t-test using SPSS 27 (IBM, Armonk, NY, USA). Data were labeled with *p < 0.05, **p < 0.01, or ***p < 0.001 to indicate statistical significance.
Results
TEM images and characterizations of GNPs and GRs
The TEM photograph demonstrated that GNPs (Fig. 1A and B) and GRs (Fig. 1C and D) were approximately spherical (Fig. 1). The average size of GNPs and GRs was 23.8 ± 0.09 nm and 26.3 ± 0.28 nm, respectively (Table 1). This indicates that the GRs size became bigger upon the conjugation of RVT on GNPs via PVP. For more detailed information, the polydispersity index (PDI) of GNPs and GRs was calculated for characterization. PDI is a representation of the distribution of size populations within a given sample20. The PDI values of GNPs and GRs were shown to be 0.358 ± 0.006 and 0.347 ± 0.002, respectively (Table 1), meaning that GNPs and GRs were moderately uniform. The magnitude of the zeta potential indicates the degree of electrostatic repulsion between adjacent, similarly charged particles in dispersion21. Zeta potential values of GNPs and GRs were 27.8 ± 1.21 mV and 26.2 ± 2.22 mV, respectively (Table 1). This suggests that the nanoparticles were considered strongly cationic.
Nitric oxide production with the treatment of GNPs, RVT, and GRs on RAW 264.7 cells
The extent of NO production in LPS-treated RAW 264.7 cells was examined. The NO production increased considerably with the treatment of LPS (negative control vs. positive control) (Fig. 2). With the treatment of GNPs and RVT, the extent of NO production slightly increased for 2.5 μM and continued to decrease up to 10.0 μM of GNPs (Fig. 2). The difference in the extent of NO production was not significant with treatments of GNPs and RVT. However, the decrease in the extent of NO production was significant with GRs treatment compared to RVT treatment (Fig. 2). Thus, NO production was much less with GRs treatment, meaning that less inflammatory response occurred with GRs treatment.
Nitric oxide production in RAW 264.7 cells after exposure to LPS, GNPs, RVT, and GRs for 24 h. Negative control (Con(-)) received no treatment; positive control (Con ( +)) received 1 μg/mL LPS. Various concentrations of GNPs, RVT, and GRs were added to the positivel control containing 1 μg/mL LPS. The data were measured in 3 independent experiments. The values were statistically different in the level of ***p < 0.001.
LDH leakage with the treatment of GNPs, RVT, and GRs on RAW 264.7 cells
LDH assay was conducted with RAW 264.7 cells to confirm the cytotoxicity of GNPs, RVT, and GRs. The supernatant of cell culture was collected for lactate dehydrogenase (LDH) assay. Lactic acid dehydrogenase exists in mitochondria in healthy cells but leaks into the cytoplasm when the cells are damaged22. The extent of LDH leakage gradually decreased as the concentration of GNPs increased to 10.0 μM (Fig. 3). The extent of LDH leakage increased with increasing concentrations of RVT and GRs. However, the extent of LDH leakage was much less for GRs treatment than for RVT treatment with all concentrations examined. Up to 5 μM, the extent of LDH leakage was lower or like that of the positive control (LPS-treated group). In addition, the GRs treatment had a lower extent of LDH leakage at all concentrations than the RVT group (Fig. 3). This suggests that the toxicity of resveratrol can be alleviated by conjugation with gold nanoparticles.
Lactate dehydrogenase leakage level was conducted after exposure to LPS, GNPs, RVT, and GRs for 24 h. Negative control (Con(-)) received no treatment; positive control (Con ( +)) received 1 μg/mL LPS. Various concentrations of GNPs, RVT, and GRs were added to the positive control containing 1 μg/mL LPS. Data were expressed in 3 independent experiments. The values were statistically different in the level of ***p < 0.001.
Effect of GNPs, RVT, and GRs on cytokine concentration of RAW 264.7 cells
It was known that high levels of inflammatory cytokines were expressed in the upper fluid of LPS-stimulated RAW 264.7 cells23. The expression extents of positive control were 2611.1 ± 62.2 pg/mL and 19,513.7 ± 2178.3 pg/mL, respectively, for interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). After treatment with increasing concentrations of GNPs, RVT, or GRs, the expression extents of TNF-α (Fig. 4A) and IL-6 (Fig. 4B) gradually decreased differentially. The expression extents were lowest at 5.0 μM of GNPs, RVT, and GRs. The expression extents at 5.0 μM were in sequence of GNPs > RVT > GRs for IL-6. The expression extents at 5.0 μM were in sequence of GNPs ≈ RVT > GRs for TNF-α. This result suggests that the inflammatory cytokine level was significantly reduced with GRs treatment compared to RVT treatment, indicating that GRs treatment increased the anti-inflammatory effect.
Raw 264.7 cells were treated with LPS to see which expressed more cytokines. Variation of expression levels of interleukin-6 (IL-6) (A) and tumor necrosis factor- α (TNF-α) (B) with treatments of various concentrations of GNPs, RVT, GRs to 1 μg/mL LPS-treated Raw 264.7 cells for 48 h. Negative control (Con(-)) received no treatment; positive control (Con ( +)) received 1 μg/mL LPS. The values were statistically different in the level of ***p < 0.001.
Cell viability of AsPC-1 cells with treatment of GNPs, RVT, and GRs in comparison to the control
The extent of AsPC-1 cell viability was observed with GNPs, RVT, and GRs after 72 h treatment. GNPs were treated in the range of 0.625–10.0 μM (Fig. 5). It was confirmed that the cell viability did not change with the increasing concentration of GNPs. RVT and GRs were treated in the range of 1.25–20.0 μM and 0.625–10.0 μM, respectively (Fig. 5). Cell viability of AsPC-1 cells decreased with the increasing concentration of RVT and GRs. The cell viability for RVT treatment was about 96.8, 89.8, 77.4, 68.5, and 40.9% of the control group, respectively, for 1.25, 2.5, 5.0, 10.0, and 20.0 µM of RVT. The cell viability for GRs treatment was about 91.1, 84.0, 67.8, 50.7, and 16.2% of the control group, respectively, for 0.625 1.25, 2.5, 5.0, and 10.0 µM of GRs. The IC50 with treatment of RVT and GRs was about 15 µM and 5 µM, respectively. Especially, compared to treatment with RVT, the cell viability was further decreased with the treatment of GRs. Thus, GRs had a greater effect on cell viability than RVT.
Effect of RVT and GRs on the apoptosis of AsPC-1 cells
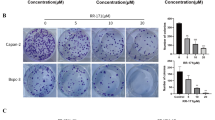
To determine the induction of apoptosis, AsPC-1 cells were treated with GNPs, RVT, and GRs for 72 h. The apoptotic cell death was measured according to the extent of early apoptotic cells and late apoptotic cells. The extent of early apoptosis with the treatment of RVT (4.08 ± 1.47%) was almost the same as the extent of the control (3.80 ± 0.53%) and GNPs (4.07 ± 1.1%) (Fig. 6A). However, the extents of early apoptosis with GRs were greater than with the extent of RVT. The levels of early apoptosis with the treatment of GRs were 10.86 ± 2.4%. The extents of late apoptosis with the treatment of GNPs, RVT, and GRs were 5.41 ± 0.4%, 4.63 ± 1.6%, and 17.51 ± 3.5%, respectively, while that of control was 3.87 ± 0.9%. Thus, the extents of early and late apoptosis with the treatment of GNPs, RVT, and GRs were about 9.48%, 8.71%, and 28.3%, respectively, compared to the extent of control for 7.47%. Therefore, the extent of early and late apoptosis was much bigger with GRs than with control, GNPs, and RVT (Fig. 6B). All these results suggested that the apoptosis of GRs was induced effectively due to the characteristics of GRs.
The percentages of apoptosis in AsPC-1 cells were shown for control (no treatment), GNPs, RVT, and GRs for 72 h. AsPC-1 cells were stained with Annexin V/PI (A) to measure the percentage of apoptotic cell death (B). The apoptotic cell death populations included early apoptotic cells (Annexin V + /PI-) and late apoptotic cells (Annexin V + /PI +). The values were statistically different in the level of *p < 0.05, ***p < 0.001 compared to the control (no treatment).
Effect of RVT and GRs on the mRNA expression of apoptosis-related genes for AsPC-1 cells
The mRNA expression extents of apoptosis-related genes were examined for caspase-3, caspase-7, caspase-9, BAK, and Bcl-224. After treatment of GNPs, RVT, and GRs, the mRNA expression extents of the apoptosis-related genes were measured by RT-qPCR. As shown in Fig. 7A, the extent of caspase-3 gene expression was increased by 1.13, 1.87, and 3.50 times for GNPs, RVT, and GRs, respectively, compared to the control. The extent of caspase-7 gene expression was increased by 1.6, 2.18, and 3.94 times for GNPs, RVT, and GRs, respectively (Fig. 7B). In the case of caspase-9, the extent of gene expression was increased by 1.18, 1.87, and 4.42 times for GNPs, RVT, and GRs, respectively (Fig. 7C). These results indicate that the treatment with RVT and GRs effectively increased the mRNA expression extent of pro-apoptotic 3 caspases. Thus, the mRNA expression extents with GRs treatment were greater than those with the treatment of GNPs and RVT. The extents of pro-apoptotic BAK gene expression were increased by 1.56 and 2.21 times, respectively, for RVT and GRs compared to the control. In contrast, the gene expression extent with the treatment of GNPs was decreased by 0.81 times of the control (Fig. 7D). Compared to the control, the extents of anti-apoptotic Bcl-2 gene expression decreased to 0.86, 0.74, 0.22 times, respectively, for the treatment of GNPs, RVT, and GRs (Fig. 7E). All the results shown above suggest that, compared to GNPs and RVT, GRs strongly induced the extents of apoptosis-related genes in the mRNA level.
AsPC-1 cells were treated for 72 h with GNPs, RVT, and GRs. mRNA expression levels of caspase-3 (A), caspase-7 (B), caspase-9 (C), BAK (D), and Bcl-2 (E) were determined by RT-qPCR. The values were obtained by normalization to the value of GAPDH for internal control (no treatment). Data are expressed as the mean ± SE (n = 3). The values were statistically different in the level of *p < 0.05, ***p < 0.001 compared to the internal control (no treatment).
Effect of RVT and GRs on the expression of protein related to apoptosis and S phase arrest for AsPC-1 cells
Western blot analysis was performed to confirm the mechanism of cell cycle arrest and apoptosis of AsPC-1 cells with the treatment of GNPs, RVT, and GRs (Fig. 8A). The expression extents of cleaved PARP, BAX, and BAK proteins were greater with the treatment of RVT and GRs than with the control and the GNPs treatment. The pro-apoptotic protein expression extents of cleaved PARP, BAK, and BAX were increased by about 1.2–1.6 fold and 1.5–3.4-fold, respectively, for the treatment of RVT and GRs, compared to the control and GNPs (Fig. 8B, C, D). On the contrary, the protein expression extents of cyclin-D1 and Bcl-2 were decreased by 0.65 ~ 0.7-fold and 0.2 ~ 0.46-fold with the treatment of RVT and GRs, respectively, compared to the control (Fig. 8E, F). The protein expression extents of cell cycle arrest-related cyclin-D1 and apoptosis-related Bcl-2 were not much affected by the treatment of GNPs. These Western blot results suggest that GRs strongly inhibited the growth of AsPC-1 cancer cells through cell cycle arrest and apoptotic pathways, compared to the RVT.
The treatment effects of GNPs, RVT, and GRs were analyzed by Western blotting in (A). Levels of n-fold expression of proteins in AsPC-1 cells were obtained by normalization to the value of β-actin for internal control and were presented in cleaved PARP (B), BAK (C), BAX (D), Cyclin D1 (E), Bcl-2 (F). Data are expressed as the mean ± SE (n = 3). The values were statistically different in the level of *p < 0.05, ***p < 0.001 compared to the internal control (no treatment).
Discussion
Pancreatic cancer, one of the most malignant cancers, is known for a challenging treatment. Effective chemotherapy become more important for patients with pancreatic cancer3. In contrast to conventional approaches, gold nanoparticles (GNPs) were shown to be an efficient drug delivery system with nearly minimal cytotoxicity in human cells8,9. To support additive effects, GNPs have been extensively conjugated with antiangiogenic medicines and commonly with other anticancer medications25.
Previous studies have shown that polyphenol resveratrol (RVT), which can be extracted from more than 70 plants, has a characteristic of antioxidant and anti-inflammatory properties. Additionally, RVT is known to have some anticancer effects on pancreatic cancer cells without cytotoxicity26. For this present study, GNPs were synthesized using the seed-growth method14,15,16,27. The seed-growth method was suggested as an effective method to adjust particle size and remove cytotoxic CTAB28. Using polyvinylpyrrolidone (PVP) as a cross-linker, GNPs were shown to be conjugated with RVT. Nanoparticles can be passively and selectively concentrated in tumors due to the enhanced permeability and retention (EPR) effect. They can actively target particular cancer cell receptors by altering their surface affinity ligands29. Therefore, the EPR effect provided information about nanoparticle-based anti-cancer drug delivery strategies30.
WST-1 analysis is the most common method to evaluate cell viability. The IC50 value was calculated using the WST-1 analysis. According to calculations, the IC50 for GRs is about 5 µM, the value of which was substantially less than about 15 µM for RVT (Fig. 2). This result suggests that GRs can be used as an efficient anticancer drug to decrease the proliferation of AsPC-1 cells. The anti-inflammatory efficacy of GNPs, RVT, and GRs was compared by measuring the extent of NO production (Fig. 3). It was known that NO is crucial for maintaining regular physiological processes such as immunological response, vasodilation, and neurotransmission18,31,32. However, a surplus of NO could become a pro-inflammatory mediator that damages tissue and is connected to several inflammatory illnesses33. For the present study, GNPs, RVT, and GRs were demonstrated to suppress LPS-induced NO generation in RAW 264.7 cells. GRs drastically reduced the degree of NO generation in RAW 264.7 cells. The cytotoxicity of GNPs, RVT, and GRs to RAW 264.7 cells was confirmed using a lactate dehydrogenase (LDH) release assay (Fig. 4). When the plasma membrane was damaged, LDH was immediately released into the cell culture supernatant17. Thus, the degree of LDH leakage is regarded as confirmation of cytotoxicity. The result in Fig. 4 showed that the extent of LDH leakage decreased with increasing concentration of GNPs. On the other hand, the extent of LDH leakage increased with increasing concentration of RVT, suggesting that RVT has an extent of cytotoxicity. Interestingly, the extent of LDH leakage with GRs was much lower than that with RVT throughout the concentration tested. These results suggest that the cytotoxicity of RVT was reduced on the conjugation of RVT to GNPs in the form of GRs.
When macrophages were activated in an inflammatory reaction, several cytokines were released to enhance immune defenses. These include the production of pro-inflammatory cytokines like TNF-α and IL-634. These cytokines were increasingly expressed in LPS-stimulated macrophages to initiate and improve the inflammatory response35. LPS activated M1 macrophages and accelerated the inflammatory response by activating or upregulating the expression of other pro-inflammatory mediators and cytokines36. TNF-α is a pro-inflammatory cytokine that controls immune cells and cell death by generating IL-6, thus further promoting the inflammatory cascade37. IL-6 induced B and T cell differentiation to regulate immunity and inflammation and its expression was shown to be regulated by LPS38. In the present study, the highest expression extents of the two cytokines were observed with positive controls of LPS-treated RAW 264.7 cells as expected (Fig. 5). Then, the extents gradually decreased in sequence of GNPs > RVT > GRs with the treatment of increasing concentration. Especially, the group with GRs had the lowest extent of cytokine expression, showing that the GRs may have enhanced anti-inflammatory functionality.
AsPC-1 cells were treated with GNPs, RVT, and GRs to measure the apoptosis of early and late apoptosis with a flow cytometer (Fig. 6). The extent of apoptosis was not that different between the GNPs treatment and the control. However, the extent of apoptosis increased with the treatment of RVT and GRs compared to the control. In particular, the treatment of GRs had about a three-fold increase in the extent of apoptosis compared to the treatment of RVT. All these results indicated that GRs can be a potential treatment for pancreatic cancer. Then, the mRNA expression extents of apoptosis-related and anti-apoptosis-related genes were investigated (Fig. 7). Caspase-3, -7, and -9 are known to be associated with intrinsic apoptosis pathways39. The mRNA expression extents of caspase-3, -7, and -9 were increased with the treatment of RVT and GRs compared to the control. The mRNA expression extents of three caspase genes increased considerably when treated with GRs compared to RVT, indicating that GRs efficiently induced apoptosis. The mRNA expression of BAK is related to the mitochondria’s capacity to release proteins that activate the processes involved in apoptosis40. The mRNA expression level of the BAK gene was greater with GRs treatment than with RVT treatment. Bcl-2 is an anti-apoptotic gene that prevents apoptosis by sequestering death-driving cysteine proteases41. In the present study, the treatment of GRs significantly reduced the mRNA expression extent of Bcl-2, compared to the treatment of RVT. These results indicate that GRs treatment could suppress the pancreatic tumor progression by inducing apoptosis, compared to the RVT treatment.
The expression of apoptosis-related genes and tumor suppressor genes was analyzed at the protein level to demonstrate the induction of cell apoptosis by treatment of RVT and GRs (Fig. 8). Cleaved PARP was shown to play an important role in many processes, including DNA repair and cell death42. Also, BAX and BAK are essential regulators of the intrinsic pathways of apoptosis43. At first, the protein expression levels of cleaved PARP, BAX, and BAK were up-regulated after treatment of RVT and GRs. Especially, the protein expression levels were significantly increased for groups treated with GRs. The protein expression level of cyclin D1 was shown to have a considerable effect on the cell cycle progression and proliferation44. The protein expression extent of the cyclin D1 gene was decreased with the treatment of RVT and GRs. The decrease in the protein expression extent of the cyclin D1 gene was greater with GRs than with RVT. These results suggest that the RVT and GRs are involved in G1-phase cell cycle arrest45,46. The protein expression extent of Bcl-2, an anti-apoptosis-related gene, decreased after treatment of RVT and GRs. The decrease in the protein expression extent of the Bcl-2 gene was greater with GRs than with RVT. According to these results, when compared to RVT, the GRs had a major impact on the protein expression levels for the apoptotic effect.
Gold nanoparticles conjugated with nutraceuticals attracted attention for cancer treatment, especially with resveratrol as a bioactive compound. For example, gold nanoparticles conjugated with resveratrol were investigated for their potential anti-invasive properties in human breast cancer cells MCF-7 to find about 30% less invasion47. Gold nanoparticles conjugated with resveratrol was shown to increase the in vivo anti-tumor effcacy of free resveratrol by activating the intrinsic apoptotic pathway in pancreatic cancer cells PANC-115. In another study, the anticancer effectiveness of gold nanoparticles conjugated with resveratrol to PANC-1 cells was concentration-dependent. This means that the effectiveness of treatment depended on the cargo size of reseveratrol upon gold nanoparicles10. In our previous study, we have experienced that sufficient quantities could be obtained at the lab scale for animal study so that it might be budget frieldly15. However, large-scale production for human application need to be researched further.
Conclusion
In the present study, the results suggest the benefits of GNPs as the carrier of resveratrol to treat pancreatic cancer. GNPs were shown to improve the bioavailability and delivery of resveratrol to cancer cells, making GNPs useful for applications in cancer therapy. Because of the natural anti-angiogenesis properties of GNPs, the entry of GRs into cancer cells might provide synergistic anti-cancer effects. All the results suggest that GRs effectively suppress the cell viability of AsPC-1 cells, a pancreatic cancer cell line. Thus, the current study demonstrated that the anticancer effect of GRs, which are RVT conjugated with GNPs, was greater than that of RVT. GRs induced apoptosis in AsPC-1 cells via G1-phase cell cycle arrest. Although the current study have shown that GRs had a better extent of anticancer effect than RVT to a human pancreatic cancer cell line AsPC-1, further study including in vivo experiment is needed to render GRs as prospective anti-cancer agent to treat pancreatic cancer.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aizikovich, A. Anticancer effect of new cannabinoids derived from tetrahydrocannabinolic acid on PANC-1 and AsPC-1 human pancreas tumor cells. J. Pancreat. Cancer 6(1), 40–44. https://doi.org/10.1089/pancan.2020.0003 (2020).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics. CA. Cancer J. Clin. 73(1), 17–48. https://doi.org/10.3322/caac.21763 (2023).
Springfeld, C. et al. Chemotherapy for pancreatic cancer. Presse Med. 48, e159–e174. https://doi.org/10.1016/j.lpm.2019.02.025 (2019).
Mandal, M. K. et al. A short review on anticancer phytochemicals. Pharmacogn. Rev. 17(33), 11–23. https://doi.org/10.5530/097627870236 (2023).
Yeom, J. H. et al. Anticancer activity of peptide W-0803 derived from Anoplophoa glabripennis. Appl. Biol. Chem. 67, 53. https://doi.org/10.1186/s13765-024-00908-4 (2024).
Annaji, M. et al. Resveratrol-loaded nanomedicines for cancer applications. Cancer Rep. (Hoboken) 4(3), e1353. https://doi.org/10.1002/cnr2.1353 (2021).
Wakaskar, R. R. Promising effects of nanomedicine in cancer drug delivery. J. Drug Target. 26(4), 319–324. https://doi.org/10.1080/1061186X.2017.1377207 (2018).
Zhang, D. et al. Nano-gold loaded with resveratrol enhance the anti-hepatoma effect of resveratrol in vitro and in vivo. J. Biomed. Nanotechnol. 15(2), 288–300. https://doi.org/10.1166/jbn.2019.2682 (2019).
Carabineiro, S. A. C. Applications of gold nanoparticles in nanomedicine: Recent advances in vaccines. Molecules 22(5), 857. https://doi.org/10.3390/molecules22050857 (2017).
Thipe, V. C. et al. Development of resveratrol-conjugated gold nanoparticles: Interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int. J. Nanomed. 14, 4413–4428. https://doi.org/10.2147/IJN.S204443 (2019).
Kinugasa, H. et al. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer 121(13), 2271–2280. https://doi.org/10.1002/cncr.29364 (2015).
Asimgil, H. et al. Targeting the undruggable oncogenic KRAS: The dawn of hope. JCI Insight 7(1), e153688 (2022).
Shai, A. et al. Inhibiting mutant KRAS G12D gene expression using novel peptide nucleic acid-based antisense: A potential new drug candidate for pancreatic cancer. Oncol. Lett. 23(4), 130. https://doi.org/10.3892/ol.2022.13250 (2022).
Lee, D. G. et al. Gold nanoparticles conjugated with resveratrol induce cell cycle arrest in MCF-7 cell lines. App. Biol. Chem. 62, 3. https://doi.org/10.1186/s13765-019-0440-6 (2019).
Lee, D. G. et al. Resveratrol-loaded gold nanoparticles enhance caspase-mediated apoptosis in PANC-1 pancreatic cells via mitochondrial intrinsic apoptotic pathway. Cancer Nanotechnol. 13, 34. https://doi.org/10.1186/s12645-022-00143-w (2022).
Nikhil, R., Jana, L. G. & Murphy, C. J. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir 17, 6782–6786. https://doi.org/10.1021/la0104323 (2001).
Zhang, Z., Hu, X., Lin, L., Ding, G. & Yu, F. Immunomodulatory activity of low molecular-weight peptides from Nibea japonica in RAW264.7 cells via NF-kappaB pathway. Mar. Drugs 17(7), 404. https://doi.org/10.3390/md17070404 (2019).
Cuong, D. M. et al. Evaluation of antioxidant and anti-inflammatory activity and identification of bioactive compound from the marine diatom, Odontella aurita extract. Appl. Biol. Chem. 67, 46. https://doi.org/10.1186/s13765-024-00898-3 (2024).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4), 402–428. https://doi.org/10.1006/meth.2001.1262 (2001).
Danaei, M. et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10, 57. https://doi.org/10.3390/pharmaceutics10020057 (2018).
Tahar, I. B. et al. Green pyomelanin-mediated synthesis of gold nanoparticles: Modelling and design, physico-chemical and biological characteristics. Microb. Cell Fact. 18, 210. https://doi.org/10.1186/s12934-019-1254-2 (2019).
Forkasiewicz, A. et al. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell. Mol. Biol. Lett. 25, 35. https://doi.org/10.1186/s11658-020-00228-7 (2020).
Shahidullah, A. et al. Anti-inflammatory effects of diospyrin on lipopolysaccharide-induced inflammation using Raw264.7 mouse macrophages. Biomedicines 8, 11. https://doi.org/10.3390/biomedicines8010011 (2020).
Yang, B. et al. Characterization of caspase8 and its role in the regulation of apoptosis-related genes in large yellow croaker (Larimichthys crocea). Aquaculture 539, 736595. https://doi.org/10.1016/j.aquaculture.2021.736595 (2021).
Zamborlin, A. & Voliani, V. Gold nanoparticles as antiangiogenic and antimetastatic agents. Drug Discov. Today 28(2), 103438. https://doi.org/10.1016/j.drudis.2022 (2023).
Meng, T. et al. Anti-inflammatory action and mechanisms of resveratrol. Molecules 26(1), 229. https://doi.org/10.3390/molecules26010229 (2021).
Jana, N. R., Gearheart, L. & Murphy, C. J. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir 17(22), 6782–6786. https://doi.org/10.1021/la0104323 (2001).
Kuttner, C. et al. Seeded growth synthesis of gold nanotriangles: Size control, SAXS analysis, and SERS performance. ACS Appl. Mater. Interfaces 10(13), 11152–11163. https://doi.org/10.1021/acsami.7b19081 (2018).
Danhier, F., Feron, O. & Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 148(2), 135–146. https://doi.org/10.1016/j.jconrel.2010.08.027 (2010).
Kumar, D., Saini, N., Jain, N., Sareen, R. & Pandit, V. Gold nanoparticles: An era in bionanotechnology. Expert Opin. Drug Deliv. 10(3), 397–409. https://doi.org/10.1517/17425247.2013.749854 (2013).
Lundberg, J. O. & Weitzberg, E. Nitric oxide signaling in health and disease. Cell 185(16), 2853–2878. https://doi.org/10.1016/j.cell.2022.06.010 (2022).
Jin, H.-G. et al. Anti-inflammatory components isolated from Atractylodes macrocephala in LPS-induced RAW264.7 macrophages and BV2 microglial cells. Appl. Biol. Chem. 65, 11. https://doi.org/10.1186/s13765-022-00673-2 (2022).
Ahmad, N., Ansari, M. Y. & Haqqi, T. M. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell. Physiol. 235(10), 6366–6376. https://doi.org/10.1002/jcp.29607 (2020).
Shapouri-Moghaddam, A. et al. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 233(9), 6425–6440. https://doi.org/10.1002/jcp.26429 (2018).
Aleem, D. & Tohid, H. Pro-inflammatory cytokines, biomarkers, genetics and the immune system: a mechanistic approach of depression and psoriasis. Rev. Colomb. Psiquiatr. (Engl. Ed.) 47(3), 177–186. https://doi.org/10.1016/j.rcp.2017.03.002 (2018).
Zhang, H., Cai, D. & Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 28(5), 555–561. https://doi.org/10.1016/j.joca.2020.01.007 (2020).
Al-Roub, A. et al. Short chain fatty acid acetate increases TNF alpha-induced MCP-1 production in monocytic cells via ACSL1/MAPK/NF-kappaB axis. Int. J. Mol. Sci. 22(14), 7683. https://doi.org/10.3390/ijms22147683 (2021).
Dimou, P. et al. The human glomerular endothelial cells are potent pro-inflammatory contributors in an in vitro model of lupus nephritis. Sci. Rep. 9(1), 8348. https://doi.org/10.1038/s41598-019-44868-y (2019).
Brentnall, M., Rodriguez-Menocal, L., De Guevara, R. L., Cepero, E. & Boise, L. H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 14, 32. https://doi.org/10.1186/1471-2121-14-32 (2013).
Jiang, X., Jiang, H., Shen, Z. & Wang, X. Activation of mitochondrial protease OMA1 by Bax and Bak promotes cytochrome c release during apoptosis. Proc. Natl. Acad. Sci. U.S.A. 111(41), 14782–14787. https://doi.org/10.1073/pnas.1417253111 (2014).
Johnson, C. et al. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl. Gastrointest. Cancer 1(1), 58–70. https://doi.org/10.3978/j.issn.2224-4778.2011.11.02 (2012).
Chaitanya, G. V., Steven, A. J. & Babu, P. P. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal 8, 31. https://doi.org/10.1186/1478-811X-8-31 (2010).
Pena-Blanco, A. & Garcia-Saez, A. J. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 285(3), 416–431. https://doi.org/10.1111/febs.14186 (2018).
Qie, S. & Diehl, J. A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. (Berl.) 94(12), 1313–1326. https://doi.org/10.1007/s00109-016-1475-3 (2016).
Masamha, C. P. & Benbrook, D. M. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 69(16), 6565–6572. https://doi.org/10.1158/0008-5472.CAN-09-0913 (2009).
Rezaei, P. F., Fouladdel, S., Ghaffari, S. M., Amin, G. & Rezaei, E. Z. Induction of G1 cell cycle arrest and cyclin D1 down-regulation in response to pericarp extract of Baneh in human breast cancer T47D cells. DARU J. Pharm. Sci. 20, 101. https://doi.org/10.1186/2008-2231-20-101 (2012).
Park, S. Y. et al. Gold-conjugated resveratrol nanoparticles attenuate the invasion and MMP-9 and COX-2 expression in breast cancer cells. Oncol. Rep. 35, 3248–3256. https://doi.org/10.3892/or.2016.4716 (2016).
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (No. NRF-2021R1F1A106110013). This work was also supported partly by a grant funded by Korea University (K2406241).
Author information
Authors and Affiliations
Contributions
SMH & DJL: Data curation, Formal analysis, Investigation, Writing– Original Draft. JHY & DGL: Resources, Software, Validation. JWL & NC; Conceptualization, Supervision, Writing – Review & Editing. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hong, S.M., Lee, D.J., Lee, D.G. et al. Gold nanoparticle resveratrol complex increases apoptosis in KRAS mutant pancreatic cancer cells. Sci Rep 15, 13760 (2025). https://doi.org/10.1038/s41598-025-98124-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98124-7