Abstract
Organic farming could be a promising approach as an agricultural system utilizing available organic materials without retrogradation natural resources. In this study, the effect of four levels of compost (0, 12, 18, and 24 t/ha) on fenugreek plants (Trigoneila foenum-greecum) over two seasons (2021–2022 and 2022–2023) was investigated in combination with different planting timings on a sandy soil. A randomized complete block design with three replications was used for the experiment conducted at the Agricultural Experiment and Research Centre of Minia University, Egypt. The Results showed that fertilizing fenugreek plants with compost significantly increased the vegetative growth traits such as plant height, number of branches/plants and herb biomass. The highest values of soil organic carbon (SOC), 986.3 mg kg-1, dissolved organic carbon (DOC), 21.l6 mg kg-1, dissolved organic nitrogen (DON), 9.43 mg kg-1, microbial biomass-C (59.67 mg kg-1.), -N (24.88 mg kg-1), -P (14.67 mg kg-1), net bacterial [95.3 (×105 cfu g-1)] and fungi [74.32(×104 cfu g-1)] counts were obtained with 24t/ha of compost. Enzyme activities of dehydrogenase (DH), urease and β-glucosidase (βG) increased significantly as the compost rate increased. For planting dates, the beginning of October was the optimal time for fenugreek production in April. For sandy soils with low fertility under arid conditions, organic and inorganic fertilizer use will enhance crop quality and productivity, soil health and soil organic matter build-up.
Similar content being viewed by others
Introduction
Fenugreek (Trigoneila foenum-greecum, L.), is a medicinal plant distributed all over the world as a traditional low-cost condiment and medicine1. It is a member of the Fabaceae family and Papilionaceae subfamily which is categorized as an annual herb that originated in North Africa and Asia2. Both leaves and seeds of Fenugreek are used to medicate diabetes and hypercholesterolemia in India and China3. Fenugreek seeds powder is able to eliminate renal toxicity that is induced by aluminum chloride by power (5%)4. It is commonly used in bakery products, beverages, pickles, species and in frozen dairy products. Moreover, fenugreek shows antiviral, antimicrobial, antidepressant, antitumor and antioxidant activities5. The growth and yield of the fenugreek plant were improved by fertilizing it with organic materials (compost)6,7,8. When crops are exposed to the favorable growth environment conditions, their yield is at its highest9,10,11. As a result, fenugreek grows and yields more when planted at the right time10,12,13,14. Planting time is one of the most important productivity factors which can be manipulated to resist the impacts of the adverse environment9. In most cases, the highest productivity of crops is when it is exposed to the most compatible growth environment season. Planting crops before or after suitable dates produces lower yield.
Because intensive farming methods require high fertilizer inputs to produce high yields, inappropriate agricultural intensification combined with reckless fertilizer use has negatively impacted soil health15. As a result, organic farming is recently used as an agricultural system which utilizes available organic materials without degrading natural resources. There is a high need to reduce environmental pollution and improve nutrient management by maintaining higher soil nutrient concentration. Organic fertilizers have the ability to increase crops productivity16. Inorganic fertilizers are utilized to strengthen crop productivity and soil fertility, but it has negative impacts on soil microorganisms, biogeochemical cycles that lead to leaching of nutrients and causing environmental degradation17. For all the previous harmful impacts of synthetic fertilizers, there is an increasing need for sustainable agricultural practices with ecofriendly management18. Organic amendment is important for reducing natural and environmental pollution and increasing the productivity of crops19.
Organic fertilizers contain micronutrients that enhance minerals content which are essential for nutrition. The integrated nutrient management practices impacts significantly increased micronutrients uptake20. Organic fertilizers like compost include a large amount of labile carbon (C) and nitrogen (N), that encourage ongoing nutrient availability, microbial activity, and growth21. In contrast to the rapid release of nutrients that occurs when inorganic fertilizers are utilized, the gradual breakdown of organic fertilizers in soils results in a continuous release of nutrients such as C, P, S, and N22,23. The main and most labile carbon pools on the earth’s mantle ecosystem are organic carbon and carbon storage in agricultural soils, and CO2 exchange between agricultural soil ecosystems and the atmosphere has a substantial impact on the carbon cycle in soils24. Sensitive indicators for identifying changes in soil health are temporary responses of soil microbiological and biochemical properties to various organic and inorganic fertilization systems25,26.
Organic and inorganic fertilizers are added to soil ecosystems to compensate for or satisfy the critical nutritional requirements for plant growth and health. One of the most crucial elements for producing an abundant yield of great quality is selecting the proper date for planting as well as the right fertilizing at the right time. Therefore, the aim of this research was to identify the effect of planting dates, examine how fenugreek plants responded to varying levels of compost application and planting dates, as well as the effects of their interactions on fenugreek productivity and growth. Also, this field experiment was conducted to study effects of different compost application rates on sandy soil biochemical and biological properties as sensitive indicators of soil health after cultivation of fenugreek for two seasons.
Results
Soil chemical and biochemical properties
The Q-MIC soil levels ranged from 3.2 to 6.05% across different compost rates, being higher with increasing rate of compost to a peak at 18 ton/ha due to high soil dissolved organic carbon (DOC) content which facilitated more efficient microbial biomass and enzymatic activities, reflecting more nutrients availability for the growth and yield of fenugreek. Generally, ratios of Q-MIC in the soil treated with NPK fertilizers alone were below 3%, indicating that soil microorganisms were under an environmental stress because of labile carbon deficiency.
Table 1 also shows the impact of various compost application rates on soil microbial count of bacteria and fungi. There were significant differences in the microbial counts as well as enzyme activity, with the highest counts at the application rate of 24 tons/ha. Data for enzymatic activities such as dehydrogenase (DH), urease (UR), and β-glucosidase (βG) are shown in Table 1. Dehydrogenase (DH) as an important oxi-reductase enzyme, and hydrolytic enzymes participated in carbon (β-glucosidase, βG), and nitrogen (urease, UR) soil cycles, were activated to different degrees according to compost application rates. Generally, enzyme activities of dehydrogenase (DH), urease (UR) and β-glucosidase (βG) varied significantly with compost application rate in the order 12 < 18 < 24 ton/ha.
Fenugreek vegetative growth
The three compost treatments (12, 18 and 24 t/ha) significantly enhanced plant height, branch count/plant, and herb fresh and dry weights/plant as compared to control over both seasons (Table 2). The highest values were 62.93 and 64.00 cm, 8.98 and 9.31 branches, 87.36 and 110.32 g F.W., 44.85 and 60.75 g D.W, respectively in both seasons for plant height, number of branches/plant and herb fresh and dry weights/plant.
Planting dates had a significant impact on plant height, number of branches per plant, and dried weights of the herbs/plant. The tallest plants (59.0 and 66.40 cm), most branches (8.53 and 8.83), and the largest fresh weight (80.82 and 120.49 g/plant) and dry weight (41.49 and 66.35 g/plant) occurred with the second planting date (5 October).
For plant height, branch count, and herb fresh and dry weights/plant, the interaction between planting date and compost treatments was significant (Table 2). The second planting date (5 October) plus either 24 or 18 t/ha compost was the best interaction treatments.
Yield and yield components
Table 3 shows that all the compost levels (12, 18 and 24 ton/ha) significantly increased the seed yield and yield components. The highest values were obtained at 24 t/ha with 33.33 and 21.03% number of pods/plant, 43.33 and 31.50% number of seeds/pod, 17.36 and 15.92% weight of 1000 seeds, and 121.53 and 79.92% seed yield/plant, over the control.
The second planting date (5th October) considerably outperformed the first and third planting dates in terms of number of pods/plant, number of seeds/pod, weight of 1000 seeds, and seed yield/plant (Table 3). The number of pods/plant, number of seeds/pod, weight of 1000 seeds, and seed yield/plant were all significantly affected by the interaction between the main and sub-plots (A x B) in both seasons, with the maximum values attained by the second planting date (5th October) plus compost at 24 t/ha.
Chemical constituents
Photosynthetic pigments
Photosynthetic pigments [chlorophyll a, b and carotenoids (mg/g F.W.)] were significantly improved with compost application in the order 24t/ha > 18t/ha > 12t/ha) (Table 4).
The highest contents of photosynthetic pigments were obtained from planting dates 5th October, followed by 20th October, and 20th September. Chlorophyll a, b, and carotenoids were significantly affected by the interaction between the compost and planting dates. In general, the interaction treatment of October 5th X 24 ton/ha compost produced the highest values (Table 4).
The content of nitrogen, phosphorus and potassium
The application of compost considerably increased the concentration of NPK in seeds relative to control, especially at 24 ton/ha (Table 5). Between seasons, the concentrations also varied with sowing dates with October 5 sowing having the highest concentrations, especially when combined with compost at 24 ton/ha, followed by 18 ton/ ha.
Fixed and essential oil (% and yield/plant)
All the compost treatments significantly improved the fixed and essential oil contents in fenugreek seeds (Table 6) with the highest contents at 24 ton/ha compost treatment, October 5 sowing date and their combination.
Carbohydrate and protein
Table 7 shows that all the compost treatments significantly enhanced seed carbohydrate (%) and leaf protein (%), especially at 24 ton/ha. Again, the 5th of October sowing date produced the highest percentages in both seasons. The interaction treatment of October 5th X 24 ton/ha compost produced the maximum value.
Discussion
From the findings of this research, combining organic compost with half the recommended NPK artificial fertilizers is preferred because of the beneficial impacts of compost on soil health27. In this experiment, we adopted a two-season which is practically enough to manifest the basic effects of compost on soil physical, chemical, and microbiological parameters under an average annual maximum temperature of 31 °C and an average annual minimum temperature of 17 °C. With soil moisture as no limiting factor, the compost will undergo very dynamic alterations, month by month within one year, by the intensive release of various chemical elements as a result of compost decay and degradation by microbial activities (C/N ratio = 17.5). According to Shen et al.28. , soil biological activity is more exposed to being affected by increased humidity in dry regions than in wet regions. Also, the results showed a considerable level of variability, which might be a result of several environmental factors, like the amount of water that each pot received. In this experiment, all the plant received the same volume of water and the soil used could minimize the evaporation rate as the surface soil drained quickly after irrigation29.
Various studies have established the positive impact of compost on soil biochemical and biological properties25,30,31 ). Compost enriches soil by providing carbon sources via SOC and DOC, this in turn provides energy for microbial biomass carbon and phosphorus which increases soil values of C− MIC, N− MIC and P− MIC reflecting improvements in fenugreek growth and quality. Also, the availability of adjustable C was evaluated by Q− MIC as the percentage of microbial biomass carbon (C− MIC) to soil organic carbon (SOC)30. The obtained results reflected a significant increase in soil microbial biomass carbon (C− MIC), soil microbial biomass nitrogen (N− MIC) and soil microbial biomass phosphorus (P− MIC), implying obvious improvements across compost application rates.
Combination of compost with artificial NPK fertilizers increased the availability of N in the presence of organic carbon which can modify the form and decomposition of soil organic carbon (SOC) and finally soil C turnover due to indispensable spousing of C and N in the soil ecosystem30,32,33. While high compost rates may change the pH and salinity of the soil, which could cause degradation, organic fertilizers can change the way water is distributed in the soil and also have an impact on the transport of salts, which lowers the salinity of the soil34.
This study showed that the use of inorganic NPK fertilizers alone can significantly reduce soil biochemical and microbiological activity. The application of inorganic fertilizers alone reduces the readily metabolizable carbon sources needed by soil microorganisms to activate soil microbial and enzyme activities, unlike organic compost treatments which support microbial and enzyme functions. This was because dissolved organic carbon (DOC) and soil organic carbon (SOC) are both components of soil that are essential for microbial and enzyme activity24,30,35. Inorganic fertilizers may rapidly diffuse and disperse, which result in quick plant uptake, soil particles adsorption and/or leaching into water bodies without significant changes in soil properties, affecting soil microbial and biological properties and crop quality of fenugreek negatively compared to compost applications26,30. The study results imply that the choice of fertilizer type and amount can significantly influence soil microbial biomass which may either be entirely aided or partially prevented after a fertilization system26,30,31.
The utilization of three compost treatments significantly improved plant height, branch count/plant and biomass possibly because of increased nutrient availability for the plant, especially nitrogen, better soil physical conditions, and improved root development through creating an appropriate medium which promotes shoot growth36. These findings align with previous reports37,38,39 on the positive impact of compost on fenugreek plant growth. The various sowing dates also showed a significant impact on plant height, number of branches/plant, and biomass as previously reported for fenugreek plants11,40,41).
The increase in the number of pods/plant, seeds/pod, weight of 1000 seeds, and seed yield/plant in fenugreek plants in our experiment may be contributed to high concentrations of both macronutrients and micronutrients in the compost. The increase in number of pods/plant, and seed yield also can be due to the increase in plant height and the number of branches, beside integrated nutrient management provided basic source for yield attributes which is related to integrated nutrient management practice that enhance soil chemical, physical and biological properties42. Our results are consistent with that of Sharma et al.43, Al-Zyadi and Al-Thahab44, Saxena and Singh45, and Sahu et al.39. Planting dates also significantly influenced plant characteristics similarly to prior research on fenugreek plant by Sowmya et al.11, Bhutia and Sharangi13, Majid et al.14, Kauser et al.41 and Anitha et al.46.
The increase in chlorophyll a, b and carotenoids levels due to compost application at the three used levels and planting dates can be linked to enhanced nitrogen availability leading to a better nutritional environment at the root zone for growth and development of the plant38. Nitrogen is crucial for plant protein formation and chlorophyll synthesis. Compost increases the soil nutrient content, which in turn increases the availability of these nutrients to the plant leading to enhancing chlorophyll content47. This aligns with previous studies on fenugreek6,38,48, ). Our results showed that variations in, planting dates did affect the levels of chlorophyll a, b, and carotenoids, which is similar to earlier reports by Sowmya et al.11 on fenugreek plants and Botros49 on caraway plants.The percentages of N, P, and K increased with compost application, especially at 24 ton/ha as reported in Raghuwanshi et al.50 and Somdutt et al.48 for fenugreek plant. The results on sowing dates are in harmony with those obtained by Obour et al.51 on fenugreek and Botros49 on caraway plants.
The fixed and essential oil in fenugreek seeds were significantly enhanced with all compost treatments. Our results of essential oil are in harmony with those reported by Ahmed et al.52. and Tadayyon et al.38 on fenugreek plant, while those for fixed oil agreed with Mafakheri6, Al-Zyadi and Al-Thahab44 and Abdul-Hafeez53. Planting dates also had a significant impact on the oil % and seed yield/plant during the two growing seasons. Our results are in harmony with those of Ahmed and Aly55 on fenugreek and Botros49 on caraway plants.
The compost treatments significantly enhanced seed carbohydrate and leaf protein which align with the results obtained by Alaghemand et al.55. and Balakrishnan and Arunprasath56. Sowmya et al.11 observed that planting date had a significant impact on carbohydrates percentage. Different compost application ratesshowed temporal improvements in both biochemical and biological characteristics of sandy soil, and these changes ultimately had a positive impact on fenugreek quality and yield. The amount of compost (24 ton/ha) had a significant impact on almost all characteristics of fenugreek, which reflects compost’s beneficial function as an organic fertilizer. It enhances biological, microbial, and microbiological processes in general57. This might be attributed to the ability of organic compost to mitigate and offset the negative effects of inorganic fertilizers on biological properties of sandy soil. This demonstrated that changes in the soil’s dissolved organic substrates and the conditions for soil microbial proliferation were likely responsible for the improvements in the characteristics of sandy soil that led to fenugreek’s high yield and quality. In addition, early October is a preferable period to focus on seed germination, plant development, and ultimately fenugreek production in April44.
Materials and methods
Experimental design
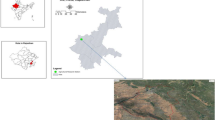
Field experiments were conducted at Agricultural Experiment and Research Centre at Minia University in Shosha (28°18’16’’N latitude and 30°34’38’’E longitude), West Samaloute City, Minia Governorate of Egypt over the course of two consecutive growing seasons (2021–2022 and 2022–2023).
The experiment included two factors, the first one were planting dates (20th September, 5th October and 20th October) and the second factor was four levels of compost (0, 12, 18 and 24 t/ha). The experimental unit (plot) measured 2.4 × 2.2 m and contained eight rows that were 30 cm apart. Seeds were planted in hills that were 10 cm apart on one side of the row, resulting in 160 hills per plot. After two weeks from the planting dates (5 October, 20 October, and 5 November), plants were thinned to two plants per hill. The split-plot design in randomized complete blocks with three replications was used. The main plots (A) included four levels of compost (0, 12, 18 and 24 t/ha), while the sub-plots (B) were three planting dates (20th September, 5th October and 20th October), therefore, the interaction treatments (A x B) were 12 treatments. For the first, second, and third planting dates, the harvesting period fell on April 5, April 20, and May 5, respectively. During the experiment, the warmest month was July with 39 °C and the coolest was January with 21 °C. On the other side, the average annual maximum temperature was 31 °C and the average annual minimum temperature was 17 °C.
Soil and compost
Four levels of compost application were used (0, 12, 18, and 24 t/ha). Nitrate ammonium (33%N), triple super phosphate (15% P2O5), and potassium sulphate (48% K2O) were used for all treatments as sources of NPK fertilizers at rates of 200 N, 60 P, and 150 K kg/ha, which was half of the amount recommended for fenugreek crop by the Egyptian Ministry of Agriculture. Prior to the start of the field trial, the sandy soil was sampled, air dried, sieved to 2.0 mm, and used to determine the basic soil physicochemical properties (Table 8) according to standard procedures58,59,60,61,62. Compost analysis was done before being incorporated into sandy soil and the results are shown in Table 9.
Plant data and analyses
Data recorded were vegetative growth (plant height (cm), number of branches/plant, and fresh and dry herb weights/plant (g), yield (number of pods/plant, number of seeds/pod, yield of seeds/plant, and weight of 1000 seeds/plant (g). Likewise, the chemical characteristics of photosynthetic pigments, NPK%, protein (%) in leaves, fixed and essential oil (% and yield/plant) in seeds, and carbohydrate (%) in seeds were analyzed.
Photosynthetic pigments
The amount of chlorophyll a, b, and carotenoids in fresh leaf samples was measured (mg/g F.W.) using the technique outlined by Moran63.
Nitrogen, phosphors, and potassium percentages
According to Page et al.61, the percentages of N, P, and K in the dried leaves were estimated.
Essential oil (%) determination
The proportion of essential oil in the seed was calculated based on the British Pharmacopoeia64, by distilling 25 g of seeds for three hours, satisfactory results were obtained, and the essential oil yield/plant/ha was determined as follows:
Fixed oil determination
Using a Soxhlet apparatus, the fixed oil was extracted from the samples. For this, 50 g of each sample was removed and put in a thimble (Whatman) after rough grinding, along with 250 ml of the solvent (Hexane), and the device was filled for 4 h. The oil was then separated from the solvent using a rotary evaporator, weighed, and the amount of fixed oil was then calculated65.
Carbohydrates
Carbohydrate (percentage) was determined according to Dubois et al.66 as described by Albalasmeh et al.67. The fundamental idea behind this process is that when carbohydrates are dehydrated by a reaction with strong sulfuric acid, furfural derivatives are created. Detectable color results from further reactivity of phenol and furfural derivatives. This method’s normal operating procedure is as follows: A 2 mL aliquot of a carbohydrate solution is mixed with 1 mL of 5% aqueous solution of phenol in a test tube. Subsequently, 5 mL of concentrated sulfuric acid is added rapidly to the mixture. The test tubes are vortexed for 30 s after standing for 10 min, and then they are placed in a water bath at room temperature for 20 min to allow for color development. Then, light absorption at 490 nm is recorded on a spectrophotometer. Reference solutions are prepared in identical manner as above, except that the 2 mL aliquot of carbohydrate is replaced by DDI water. The phenol used in this procedure was redistilled and 5% phenol in water (w/w) was prepared immediately before the measurements.
Protein
Soil health determination methods
At harvest time, one kilogram of soil from each experimental plot was incubated under controlled conditions to measure some changes in soil biochemical and biological properties as indicators of sandy soil health. All root debris was removed from soil samples before they were transferred to the soil laboratory. The soil was then incubated for 10 days at 300° C under 65% of the soil field capacity.
Analyses of soil chemical properties
After incubation, soil samples were sieved to pass a 0.5 mm mesh to determine the soil biochemical characteristics. Means were then calculated on oven-dried soil bases (105 °C). For determination of N forms, before and after incubation, 10 g soil was extracted with 50 mL of 2 M KCl for 30 min, and by steam distillation using N Analyzer (Kjeltech 2100, Foss), NH+ 4-N and total inorganic N (NH+ 4-N and NO-3-N) were determined68. Soil mineralization capacity was demarcated by differences between values found before and after incubation. The Walkley and Black method was used to determine soil organic C (SOC)69, steam distillation method using N analyzer (Kjeltech 2100, Foss) for mineral N69. Dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) were determined by the method described by Smolander and Kitunen70 using multi-N/C Analyzer (Jena, Germany).
Using the chloroform fumigation-extraction method, the soil’s microbial biomass carbon (C-MIC), microbial biomass nitrogen (N-MIC) and microbial biomass phosphorus (P-MIC) using multi-N/C 2100, analyzer Jena, using KEC of 0.45, KEN of 0.54, and KEP of 0.40, respectively, were measured after aerobic incubation26.
Analysis of soil enzyme activities
Dehydrogenase (DH) activity was determined using 2,3,5-triphenyltetrazolium chloride (TTC) as the substrate, urease (UR) using urea as the substrate71, and β-glucosidase (β G) using p-nitrophenyl-β-d-glucopyranoside as the substrate, as described by Tabatabai72. All the enzyme activities were represented as products per unit of dry soil mass and incubation time, and the amount of p-nitrophenol emitted in each example was calculated spectrophotometrically.
Soil total counts of bacteria and fungi
After two seasons of fenugreek production, total counts of bacteria and fungi were determined in soil samples using the Alef73 plate count technique. Colony forming units (CFU) of all bacteria were measured on nutritional agar, whereas CFU of all fungi were measured on potato dextrose agar media.
Statistical analysis
The experimental data were subjected to analysis of variance using a completely randomized block design (CRBD) with three replicates. According to MSTAT-C74, the data were tabulated and statistically analyzed. Data were subjected to the analysis of variance (ANOVA). At a 5% level of probability (p < 0.05), the significance of the differences was compared using the least significant difference (LSD).
Conclusions
This study on fenugreek, an important crop and medicinal plant, provides convincing evidence that compost application enhances its seed germination, plant growth, and ultimately, production. Planting timings affected its germination and growth and the beginning of October was the optimal time to fenugreek production in April. Also, after cultivation for two seasons, the temporal variations in soil microbial biomass, enzyme activities, and dissolved organic carbon showed that variable compost application rates had important and significant effects. Compared with the conventional application of inorganic fertilizer alone at the recommended levels, the benefits of integrating NPK fertilizers with compost application were generally apparent, even when applied at lower than recommended rates. To create a fertilizer regime that improves soil quality and preserves soil health during fenugreek consecutive cultivation, it is necessary to plant in October and integrate both compost and NPK application at the optimal rates.
Data availability
The datasets used during the current study available from the corresponding author on reasonable request.
References
Acharya, S. N., Thomas, J. E. & Basu, S. K. Fenugreek: An ‘old world’ crop for the ‘new world’. Biodivers 30, 7–27 (2006).
Singh, K. P., et al. Variability in the nutraceutical properties of Fenugreek (Trigonella foenum-graecum L.) seeds. Rev. Colomb Cienc. Hortic. 7(2), 228–239 (2013).
Basch, E., et al. Therapeutic applications of Fenugreek. Altern. Med. Rev. 8, 20–27 (2003).
Nouira, Y. B., et al. Fenugreek seeds, a hepatoprotector forage crop against chronic AlCl3 toxicity. BMC Vet. Res. 9, 1–9 (2013).
Al-Timimi, L. A. N. Antibacterial and anticancer activities of Fenugreek seed extract. Asian Pac. J. Cancer Prev. 20(12), 3771–3776 (2019).
Mafakheri, S. Effect of some organic and chemical fertilizers on morphological and biochemical factors of Fenugreek (Trigonella foenum-graecum L.). Plant production. J. Agric. Sci. 40(3), 27–41 (2017).
Lunagariya, D. D., Zinzala, V. J., Barvaliya, M. M. & Dubey, P. K. Effect of organics on growth, yield, quality and economics of Fenugreek (Trigonella foenum- graecum L.) grown under organic farming system. J. Pharmacogn. Phytochem. 7(3), 2420–2424 (2018).
Ameziane, H., Nounah, A. & Khamar, M. Olive pomace compost use for Fenugreek germination. Agron. Res. 18(3), 1933–1943 (2020).
Farhad, I. M., et al. Effect of variety and planting time on the productivity of Fenugreek in coastal area. World J. Agric. Sci. 11(3), 164–168 (2015).
Bhutia, K. C., Bhutia, S. O., Chatterjee, R. & Chattopadhyay, N. Growth, phenology and yield of Fenugreek (Trigonella foenum-graecum L.) as influenced by date of sowing. Int. J. Curr. Microbiol. Appl. Sci. 6(10), 1810–1817 (2017).
Sowmya, P. T., Naruka, I. S., Shaktawat, R. P. S. & Kushwah, S. S. Effect of sowing dates and stage of pinching on growth, yield and quality of Fenugreek (Trigonella foenum-graecum L). IJBSM 8(1), 91–95 (2017).
Anitha, B., Reddy, M. L. N., Dorajee Rao, A. V. D., Kiran Patro, T. S. K. K. & Salomi Suneetha, D. R. Performance of Fenugreek cultivars for growth and seed yield. Int. J. Pure App Biosci. 6(6), 271–277 (2018).
Bhutia, P. H. & Sharangi, A. B. Effect of dates of sowing and soil moisture level in different growth stages and yield dynamics of Fenugreek (Trigonella foenum-graecum L). Natl. Acad. Sci. Lett. 39(2), 77–80 (2016).
Majid, H. A., Salim, H. A. & Fahmi, A. H. Effect of planting date and spraying of humic acid in the growth traits and active compounds of Fenugreek (Trigonella foenum – graecum L). IOP Conf. Ser. Earth Environ. Sci. 388, 012048 (2019).
Rinot, O., Levy, G. J., Steinberger, Y., Svoray, T. & Eshel, G. Soil health assessment: a critical review of current methodologies and a proposed new approach. Sci. Total Environ. 648, 1484–1491 (2019).
Prasad, H., Sajwan, P. & Kumari, M. Effect of organic manures and biofertilizer on plant growth, yield and quality of horticultural crop: A review. Int. J. Chem. Stud. 5(1), 217–221 (2017).
Ibrahim, H. A. K. Effect of foliar application of compost water extract, humic acid, EDTA and micronutrients on the growth of Fenugreek plants under sandy soil condition. IJEST 16, 7799–7804 (2019).
Esitken, A., et al. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci. Hort. 124, 62–66 (2010).
Adesemoye, A. O., Torbert, H. A. & Kloepper, J. W. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 58, 921–929 (2009).
Ghosh, B. N., Prakash, V. & Singh, R. D. Micronutrient status in soybean (Glycine max)-wheat (Triticum aestivum) cropping system in Kumaon region of Uttaranchal. Indian J. Agric. Sci. 71(2), 149–152 (2001).
Bai, S. H., et al. Combined effects of Biochar and fertilizer applications on yield: A review and meta-analysis. Sci. Total Environ. 808, 152073 (2022).
Abd El-Azeim, M. M., Salah, Z. M. & Hammam, A. A. Assessment of water hyacinth Biochar as a soil amendment for sandy soils. J. Soil. Sci. Agric. Eng. 12(6), 431–444 (2021).
Fischer, D. & Glaser, B. Synergisms between compost and Biochar for sustainable soil amelioration. Waste Manage. 1, 167–198 (2012).
Yang, Y., Dou, Y. & An, S. Testing association between soil bacterial diversity and soil carbon storage on the loess plateau. Sci. Total Environ. 626, 48–58 (2018).
Dinesh, R., et al. Effects of plant growth-promoting rhizobacteria and NPK fertilizers on biochemical and microbial properties of soils under ginger (Zingiber officinale) cultivation. Agric. Res. 2, 346–353 (2013).
Abd El-Azeim, M. M., Sherif, M. A., Hussien, M. S. & Haddad, S. A. Temporal impacts of different fertilization systems on soil health under arid conditions of potato monocropping. J. Soil. Sci. Plant. Nutr. 20, 322–334 (2020).
Burhan, M. G. & Al-Hassan, S. A. Impact of nano NPK fertilizers to correlation between productivity, quality and flag leaf of some bread wheat varieties. Iraqi J. Agric. Sci. 1029, 1–7 (2019).
Shen, X., et al. Global response of soil biodiversity to climate and land use changes. J. Clean. Prod. 471, 143381 (2024).
Yi, J., et al. Assessing soil water balance to optimize irrigation schedules of flood-irrigated maize fields with different cultivation histories in the arid region. Agric. Water Manag. 265, 107543 (2022).
Jian, S., et al. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil. Biol. Biochem. 101, 32–43 (2016).
Song, Y., et al. Short-term response of the soil microbial abundances and enzyme activities to experimental warming in a boreal peatland in Northeast China. Sustain 11(3), 590 (2019).
Abd El-Azeim, M. M., Sherif, M. A., Hussien, M. S., Tantawy, I. A. A. & Bashandy, S. O. Impacts of nano- and non-nanofertilizers on potato quality and productivity. Acta Ecol. Sin. 40, 388–397 (2020).
Galloway, J. N., et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320(5878), 889–892 (2008).
Bi, Y., et al. Effects of Bacillus subtilis on cotton physiology and growth under water and salt stress. Agric. Water Manag. 303, 109038 (2024).
Liu, Y., et al. Functional and structural responses of bacterial and fungal communities from paddy fields following long-term rice cultivation. J. Soils Sediments. 16, 1460–1471 (2016).
Arancon, N., Edwards, C., Bierman, P., Metzger, J. & Lucht, C. Effects of vermicomposts produced from cattle manure, food waste and paper waste on the growth and yield of peppers in the field. Pedobiologia 49, 297–306 (2005)
Al-Jboury& Al-Baddrani, W. Effect of organic matter and phosphate fertilizers on some growth and yield of fenugreek (Trigonella foenum-graecum L.). Mesopotamia J of Agric. 54 (5),115–129 (2017). (2005).
Tadayyon, A., Naeimi, M. M. & Pessarakli, M. Effects of vermicompost and vermiwash biofertilizers on Fenugreek (Trigonella foenum) plant. Commun. Soil. Sci. Plant. Anal. 49(19), 2396–2405 (2015).
Sahu, P. K., Naruka, I. S., Haldar, A., Chundawat, R. S. & Kumar, L. Studies on the effects of integrated nutrient management on Fenugreek (Trigonella foenum- graecum) L. Int. J. Chem. Stud. 8(2), 1082–1089 (2020).
Bieńkowski, T., Żuk-Gołaszewska, K., Kurowski, T. & Gołaszewski, J. Agrotechnical indicators for Trigonella foenum-gracum L. production in the environmental conditions of Northeastern Europe. Turk. J. Field Crops. 21(1), 16–28 (2016).
Kauser, H., Bhoomika, H. R. & Ibaad, M. H. Interaction effects of different sowing dates and stage of pinching on growth, yield and economics of Fenugreek (Trigonella foenum – graecum L). Int. J. Pure Appl. Biosci. 6(2), 167–171 (2018).
Somdutt, B. Nutrient Management in Fenugreek (Trigonella foenum graecum L.), thesis. Bishnoi Swami Keshwanand Rajasthan Agricultural University, Bikaner (2019).
Sharma, S., Patel, R. H., Surve, V. H., Kumawat, S. & Effect of irrigation schedule and organic manures on growth and water use efficiency of Fenugreek (Trigonella foenum-graecum L.) under middle Gujarat conditions. Int. J. Seed Spices. 7(1), 29–34 (2017).
Al-Zyadi, Q. A. S. & Al-Thahab, E. A. M. Effect of iron oxide nanoparticle and humic acid spray on growth and yield of the Fenugreek (Trigonella foenum -graecum L.) and fixed oil content in seeds. Indian J. Ecol. 46(8), 82–84 (2019).
Saxena, A. K. & Singh, S. Growth and yield of Fenugreek (Trigonella foenum-graecum L.) as influenced by liquid and solid biofertilizers (Rhizobium, PSB and KSB). RJCES 7(3), 52–55 (2019).
Anitha, B., Reddy, M. L. N., Dorajee Rao, A. V. D., Kiran Patro, T. S. K. K. & Salomi Suneetha, D. R. Effect of sowing date on yield and quality of Fenugreek. Plant. Arch. 16(1), 479–484 (2016).
Naghavi Marramati, H. The influence of amounts and types of different organic and chemical fertilizers on yield and yield components of different rice cultivars. MSc. Thesis of Agronomy. Faculty of Agriculture Sari Agricultural Sciences and Natural Resources University. Iran (2007).
Somdutt, Dashora, L. N., Mundra, S. L., Choudhary, J. & Choudhary, P. Effect of inorganic and organic sources of fertilization on productivity of Fenugreek (Trigonella foenum-graecum L.) under agroclimatic conditions of Southern Rajasthan. J. Pharmacogn. Phytochem. 8(4), 1886–1888 (2019).
Botros, W. S. E. Physiological studies on caraway plants. M. Sc Thesis, Faculty of Agriculture, Minia University (2013).
Raghuwanshi, O., Jain, P. K., Singh, Y. & Prajapati, S. Response of organic and inorganic source of nutrients on growth, yield and nutrients uptake status of Fenugreek (Trigonella foenum-graecum) CV. RMT–1. HRS 5(1), 34–38 (2016).
Obour, A., Obeng, E. & Holman, J. D. Influence of different seeding dates on Fenugreek (Trigonella foenum-graecum L.) forage yield and nutritive value. Kansas Agricultural Experiment Station Research Reports. Kansas Field Res. 1(2) (2015).
Ahmed, A. G., EL-Housini, E. A., Hassanein, M. S. & Zaki, N. M. Influence of organic and bio-fertilizer on growth and yield of two Fenugreek cultivars grown in sandy soil. Aust J. Basic. Appl. Sci. 6(10), 469–476 (2012).
Abdul-Hafeez, E. Y. Exogenous application of potassium dihydrogen orthophosphate and sowing dates enhance fruit yield and essential oil of Coriandrum sativum, L. Sci. J. Flowers Ornam. Plants. 8(1), 181–194 (2021).
Ahmed, S. & Aly, A. F. Effect of sowing dates and planting method on vegetative growth, yield and active substances of fenugreek plant. In Proceeding of Minia 1st Conference for Agriculture, Environment, Science, Minia, Egypt 393–400 (2002).
Alaghemand, S., et al. Effects of organic fertilizers on growth and biochemical characteristics of Fenugreek. Acta Agric. Slov. 109(2), 197–203 (2017).
Balakrishnan, M. & Arunprasath, A. Impact of plant growth regulators and organic fertilizer on growth and biochemical composition of Trigonella foenum-graceum L. Int. J. Bot. Stud. 3(3), 24–28 (2018).
Godara, A. S., Gupta, U. S., Singh, R. & Mehta, R. S. Effect of different combinations of organic and inorganic nutrient sources on productivity and profitability of Fenugreek (Trigonella foenium-graecum). Int. J. Seed Spices. 2(2), 34–37 (2012).
Jackson, M. L. Soil chemical analysis-advanced course: A manual of methods useful for instruction and research in soil chemistry, physical chemistry of soils, soil fertility, and soil genesis. author. (1973).
Black, C. A. Methods of soil analysis. Sponsored by the American society of agronomy and the American society for testing materials. ASA (1965).
Avery, B. W. & Bascomb, C. L. Soil survey laboratory methods: Soil survey technical monograph No. 6. In Soil survey of England and Wales, Harpendon, Vol. 83 (1982).
Page, A. L., Miller, R. H. & Keeney, D. R. Methods of Soil Analysis 2. Chemical and Microbiological Properties, Vol. 2. Aufl. 1184 S (ASA, Madison, Wisconsin, USA, 1982).
Bao, M. Analysis and Design Principles of MEMS Devices (Elsevier, 2005).
Moran, R. Formula determination of chlorophyllous pigments extracted with N-N-dimethyl-formamide. Plant. Physio. 69, 1376–1381 (1982).
British Pharmacopoeia. Determination of Volatile Oil in Drugs (Pharmaceutical, 1963).
A. O. A. C. Official Methods of Analysis. Association of Official Agricultural Chemists, 13th Ed (Washington, D.C., USA, 1980).
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
Albalasmeh, A. A., Berhe, A. A. & Ghezzehei, T. A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 97, 253–261 (2013).
Mulvaney, R. L. Nitrogen—inorganic forms. In Methods of Soil Analysis, Part 3 Chemical Methods, Vol. 5, 1123–1184 (1996).
Nelson, D. W. & Sommers, L. E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods, Vol. 5, 961–1010 (1996).
Smolander, A. & Kitunen, V. Soil microbial activities and characteristics of dissolved organic C and N in relation to tree species. Soil. Biol. Biochem. 34(5), 651–660 (2002).
Kandeler, E. & Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils. 6, 68–72 (1988).
Tabatabai, M. A. Soil enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties, Vol. 5, 775–833 (1994).
Alef, K. Enrichment, isolation and counting of soil microorganisms. In Methods in Applied Soil Microbiology and Biochemistry 123–191 (Academic Pressm, 1995).
MSTAT–C. A Microcomputer Program for the Design Management and Analysis of Agronomic Research Experiments (version 4.0) (Michigan State Univ., 1986).
Acknowledgements
The authors would like to express their gratitude to the Agricultural Microbiology Department at Minia University for their support in this research and the Researchers Supporting Project Number (RSP2025R293) King Saud University, Riyadh, Saudi Arabia.
Funding
This work was funded by the Researchers Supporting Project Number (RSP2025R293) King Saud University, Riyadh, Saudi Arabia. The publication was financed by the Polish Minister of Science and Higher Education as part of the Strategy of the Poznan University of Life Sciences for 2024–2026 in the field of improving scientific research and development work in priority research areas.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.A.H., M.M.A.E.-A. and S.A.H.; data curation, M.M.A.E.-A., A.S., A.M.M. ; funding acquisition, W.H.A; formal analysis, A.S., A.M.M. ; investigation, A.A.H., . and S.A.H.; methodology, A.A.H., N.S.A., A.M.M., and S.A.H.; resources, J. D., W.H.A. and S.A.H.; software ; supervision, M.M.A.E.-A. and S.A.H.; validation, A.A.H., A.M.M. N.S.A. and J.D.; visualization, J.D., S.A.H.; writing—original draft, M.M.A.E.-A., N.S.A. and S.A.H.; writing—review and editing, J.D., N.S.A.; S.A.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hassan, A.A., Abd El-Azeim, M.M., Menesi, A.M. et al. Enhancing fenugreek productivity under water deficient Egyptian sandy soils: optimizing organic inputs and nutrients efficiencies. Sci Rep 15, 17992 (2025). https://doi.org/10.1038/s41598-025-98314-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98314-3