Abstract
Investigate intraocular fluid testing’s role in ARNs management and prognosis. 46 ARNs patients (49 eyes) treated from 2021 to 2023 were divided into precision (n = 22) and conventional (n = 24) groups based on intraocular fluid testing. Precision group received intravitreal ganciclovir (20 mg/ml [viral copies < 5 × 106] or 40 mg/ml [> 5 × 106]) twice weekly; conventional group received fixed 20 mg/ml. BCVA, viral copies, cytokines (IL-6, IL-8, IL-10), and biomarkers (VCAM, VEGF, BFGF) were analyzed. No baseline differences in BCVA, IOP, or clinical features between groups (P > 0.05). Precision group showed fewer injections, higher final BCVA, and lower retinal detachment rates (P < 0.05). Viral copies negatively correlated with final BCVA (P < 0.05). In precision group: viral copies positively correlated with injection frequency, IL-6, IL-8, VCAM, BFGF; retinal necrosis extent positively linked to initial/final BCVA but inversely with IL-6, IL-8, and viral load. Conventional group showed retinal necrosis involvement positively correlated with detachment risk (P < 0.05). Intraocular fluid testing optimizes ARNs treatment personalization, improves outcomes, and enhances prognostic evaluation.
Similar content being viewed by others
Background
Acute retinal necrosis syndrome (ARNs) is an infrequently encountered yet profoundly severe ocular disorder, distinguished by the presence of vitreous opacities, arteritic inflammation of the retina, and focal areas of retinal necrosis. The syndrome is marked by its acute onset and rapid progression, which, coupled with the complexity of therapeutic intervention and the unpredictability of its outcome, presents formidable challenges to both the clinical ophthalmologist and the affected patient population1. Systemic and topical antiviral medications currently represent the primary therapeutic approach for ARNs. However, a unified standard for antiviral therapy has yet to be established2. Tailoring individualized treatment regimens, disease monitoring, and prognostic assessments for patients remains a pivotal area of interest in the management of ARNs.
In recent years, intraocular fluid analysis has emerged as a novel diagnostic technique garnering significant attention. By conducting laboratory examinations of aqueous humor and vitreous fluid components, this method provides crucial reference value for the diagnosis, treatment, and disease monitoring of uveitis3,4. Intraocular fluid testing enables quantitative analysis of intraocular pathogens and inflammatory mediators such as cytokines, thus holding substantial significance in ocular infectious diseases5,6. Studies by Song et al.7 have shown that intraocular fluid analysis has certain value in the etiological diagnosis of infectious uveitis and also offers reference value for the etiological diagnosis of non-infectious uveitis. Recent studies have shown that intraocular fluid detection plays an important role in the ARNs prognostic assessment4. Furthermore, studies have indicated that interferon-gamma (IFN-γ) in aqueous humor or vitreous may serve as a specific marker for the inflammatory activity of ARNs, and the ratio of IFN-γ to interleukin-10 (IL-10) could potentially be an important indicator for early prediction of disease prognosis and outcome8. In summary, this study explores the role of intraocular fluid analysis in the treatment and prognostic assessment of ARNs, offering new perspectives and methodologies for the personalized treatment and prognostic evaluation of ARN syndrome.
Methods
Study design
This study adheres to the principles of the Declaration of Helsinki and has been approved by the Ethics Committee of the Affiliated Eye Hospital of Nanchang University (Ethics No.: YLS202101220).
A retrospective analysis. 46 cases of ARNs patients (49 eyes) who visited the Affiliated Eye Hospital of Nanchang University from January 2021 to January 2023 and were included in the study.
Inclusion criteria: (1) Patients diagnosed with ARNs for the first time according to the diagnostic criteria established by the Uveitis Working Group in 20219. (2) All patients enrolled in this study received intravitreal antiviral injection and systemic antiviral therapy.
Exclusion criteria: (1) Patients with concurrent other ocular diseases (such as diabetic retinopathy, age-related macular degeneration, etc.) that may affect the results; (2) Patients with a history of vitrectomy in the affected eye; (3) Patients who could not complete the follow-up, with a follow-up time of less than 12 months. Best-corrected visual acuity (BCVA) was assessed using the international standard visual acuity chart, and the results were converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analysis.
Data collection for the study
Basic medical history, past medical history, disease progression history, relevant treatment history, surgical history, and systemic medical history of the included subjects were collected and recorded. Additionally, ocular examinations including intraocular pressure, BCVA, slit-lamp examination, and ophthalmoscopy were collected before treatment and at 3, 6, and 12 months post-treatment. For patients who underwent intraocular fluid testing upon admission, the viral load and cytokine results were also collected. According to the results of ultra-widefield ophthalmoscopy photographs, vitreous opacities were classified as follows: (1) Mild vitreous opacities: where second-order retinal vessels are visible, or second-order vessels are visible but with indistinct boundaries; (2) Severe vitreous opacities: where second-order retinal vessels are not visible, but some retinal vessels are visible, or no retinal vessels are visible10. The entire retina was divided into three zones: Zone I, centered on the foveal center with a 2DD diameter area; Zone II, extending from Zone I to the equator, with the anterior boundary being the vortex vein; Zone III, extending from Zone II to the ora serrata (Fig. 1). Furthermore, the retina was divided into four quadrants: superior nasal, inferior nasal, superior temporal, and inferior temporal, by the foveal center.
Grouping of study subjects
Subjects were categorized based on whether they underwent intraocular fluid examination. Patients included in the study were divided into an intraocular fluid examination-guided treatment group (hereinafter referred to as the precision treatment group) and a conventional treatment group. Patients in the precision treatment group received personalized intravitreal injections of ganciclovir based on the viral nucleic acid copy number detected in the intraocular fluid. For patients with intraocular viral nucleic acid copy numbers less than 5 × 106, they were treated with intravitreal injections of ganciclovir (20 mg/ml, 0.1 ml) twice a week. For those with copy numbers greater than 5 × 106, they received intravitreal injections of ganciclovir (40 mg/ml, 0.1 ml) twice a week. The conventional treatment group was uniformly treated with intravitreal injections of ganciclovir (20 mg/ml, 0.1 ml) twice a week. Both groups continued the intravitreal injections of ganciclovir until the active necrotic retinal lesions subsided, but not beyond 4 weeks after diagnosis (with a maximum of 8 intravitreal injections of antiviral medication).
Comprehensive treatment
Upon definitive diagnosis at admission, all patients were immediately administered systemic acyclovir at a dosage of 10 mg/kg, three times daily. The precision treatment group adjusted the systemic medication type based on the results of intraocular fluid viral nucleic acid testing (if the intraocular fluid indicated Varicella-zoster virus (VZV), they were treated with systemic ganciclovir at 5 mg/kg, twice daily; if the results suggested Herpes simplex virus (HSV), they continued with acyclovir at 10 mg/kg, three times daily). Systemic antiviral treatment was continued for a total of 14 days, after which patients were switched to oral valacyclovir at 1000 mg, three times daily, for a duration of 3–4 months.
Sample collection
All patients requiring intraocular fluid collection had not received antiviral treatment prior to the first sampling of vitreous or aqueous humor to ensure that the initial sampling could accurately reflect the level of intraocular viral nucleic acid. Before sample collection, all patients signed an informed consent form and were administered ofloxacin eye drops to the affected eye frequently to prevent infection (every 5 min for 5 times). After topical anesthesia, the eye was disinfected and povidone-iodine was used for conjunctival sac irrigation. Under the slit lamp, 0.1 ml of aqueous humor was aspirated from the anterior chamber through the inferotemporal limbus using a 1 ml syringe. Patients undergoing vitrectomy had 0.1 ml of undiluted vitreous fluid aspirated under the surgical microscope. The collected samples were sent to Beijing Zhide Laboratory for quantitative PCR to detect viral nucleic acid load and levels of interleukin-6 (IL-6), IL-8, IL-10, vascular endothelial growth factor (VEGF), vascular cell adhesion molecule (VCAM), and basic fibroblast growth factor (BFGF).
PCR detection of viral nucleic acid load technology
The reagent kit utilizes a constant temperature oscillating metal bath to extract viral DNA from intraocular fluid as a template for the polymerase chain reaction (PCR), which involves high-temperature denaturation, low-temperature annealing, and medium-temperature extension. This process is combined with Taqman probe technology, where the probe has a fluorescent dye at the 5’ end and a quencher group at the 3’ end. When the intact probe pairs with the target sequence, the fluorescence emitted by the fluorescent dye is quenched due to its proximity to the 3’ end quencher group. However, during the extension phase, the 5’ exonuclease activity of the polymerase cleaves the probe, separating the fluorescent dye from the quencher group, thereby emitting light. As the PCR reaction progresses, the reaction products accumulate continuously, and the fluorescence signal increases proportionally. A fluorescence signal is collected after each cycle, resulting in a fluorescence amplification curve. The relationship between the fluorescence signal and the cycle threshold (Ct value) can be used to calculate the concentration of the product.
Data analysis
The experimental data were analyzed using SPSS 24.0. Quantitative data were expressed as mean ± standard deviation (x̄±s). For quantitative data that were normally distributed and had homogeneity of variance, comparisons between two groups were made using the independent samples t-test. Categorical data comparisons were performed using the chi-square test. Correlation analysis for quantitative data was conducted using Pearson’s linear correlation analysis, while ordinal variables were analyzed using Spearman’s correlation analysis. The effect of viral copy number on the prognosis of the disease was analyzed by regression analysis. A difference was considered statistically significant at P < 0.05.
Results
General information
A total of 46 cases involving 49 eyes were included in this study, with 22 cases 22 eyes in the precision treatment group, including 13 males and 9 females, with 13 cases involving the left eye and 9 cases involving the right eye. The conventional treatment group comprised 24 cases 27 eyes, with 16 males and 8 females, 14 cases involving the left eye, 7 cases involving the right eye, and 3 cases with bilateral involvement. During the follow-up, no contralateral eye involvement was observed in the precision treatment group, whereas 2 cases of contralateral eye involvement were noted in the conventional treatment group. The comparison of baseline BCVA, intraocular pressure, disease onset time, age, retinal necrosis lesion zone, extent of necrosis lesion involvement, and vitreous opacity between the two groups showed no statistically significant differences (P > 0.05) (see Table 1). However, the number of intravitreal injections in the precision treatment group was significantly lower than that in the conventional group (P = 0.002). The intraocular fluid results in the precision treatment group showed HSV infection in 2 eyes, VZV/Epstein-Barr Virus (EBV) infection in 2 eyes, HSV/EBV co-infection in 1 eye, and VZV infection in 17 eyes. The viral copy number was 7.59 × 106 ± 1.44 × 107 copies/ml, and the levels of IL-6, IL-8, IL-10, VCAM, BFGF and VEGF were 12079 ± 18727 pg/ml, 10747.06 ± 35609.23) pg/ml, 201.57 ± 411.62 pg/ml, 22000.62 ± 14217 pg/ml, 55.62 ± 95.25 pg/ml, and 142.03 ± 119.88 pg/ml, respectively.
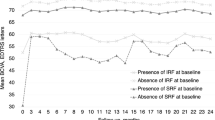
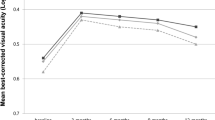
At the final follow-up, the precision treatment group’s BCVA was 4.09 ± 0.46LogMAR, and the conventional treatment group’s was 3.67 ± 0.83LogMAR. The final BCVA of the precision treatment group was significantly improved compared to the conventional treatment group, with a statistically significant difference (t = 2.11, P = 0.04). In the precision treatment group, 6 eyes (27.3%) experienced retinal detachment, while in the conventional treatment group, 16 eyes (59.3%) experienced retinal detachment, with one case occurring 3 months after antiviral treatment. The difference in the incidence of retinal detachment between the two groups was statistically significant (χ2 = 5.01, P = 0.03). In the precision treatment group, 8 eyes (36.36%) underwent vitrectomy, and in the conventional treatment group, 16 eyes (59.26%) underwent vitrectomy. The difference was not statistically significant (χ2 = 2.54, P = 0.11).
Correlation analysis
The results of Pearson’s correlation analysis indicated that in the precision treatment group, there was a positive correlation between baseline BCVA and final BCVA, and a negative correlation between viral copy number and final BCVA (r = 0.96, − 0.50, P < 0.001, 0.02). This implies that the better the baseline BCVA, the better the final BCVA, and the higher the viral copy number, the worse the final BCVA. There was a negative correlation between viral copy number and baseline BCVA (r = − 0.52, P = 0.01), indicating that the higher the viral copy number, the poorer the baseline BCVA. The viral copy number showed a positive correlation with the number of intravitreal injections, IL-6, IL-8, VCAM, and BFGF (r = 0.58, 0.57, 0.78, 0.52, 0.72, P = 0.005, 0.007, < 0.001, 0.01, < 0.001), suggesting that the higher the viral copy number, the more intravitreal injections, IL-6, IL-8, VCAM, and BFGF levels are required. The disease onset time was positively correlated with IL-10 (r = 0.82, P < 0.001), the number of intravitreal injections was positively correlated with IL-8 levels (r = 0.45, P = 0.04), and IL-8 was positively correlated with VCAM and BFGF levels (r = 0.48, 0.86, P = 0.02, < 0.001). Additionally, VCAM was positively correlated with BFGF (r = 0.49, P = 0.02). However, intraocular pressure, age, and disease onset time were not correlated with final BCVA, viral copy number, or the number of intravitreal injections (see Table 2). In the conventional treatment group, the correlation analysis results showed a positive correlation between baseline BCVA and final BCVA (r = 0.79, P < 0.001), indicating that the better the baseline BCVA, the better the final BCVA. The number of intravitreal injections was positively correlated with age (r = 0.44, P = 0.02), suggesting that older age is associated with a higher number of intravitreal injections (see Table 3).
Spearman’s correlation analysis indicated a positive correlation between the number of quadrants involved by retinal necrosis lesions in both groups and the occurrence of retinal detachment (rs=0.524, P < 0.001), and a negative correlation with the extent of retinal necrosis lesion zone (rs = − 0.256, P = 0.035). The final BCVA showed a negative correlation with the extent of retinal necrosis lesion zone (rs=0.318, P = 0.008), suggesting that the more the retinal necrosis lesions involved the area close to the foveal center, the worse the final BCVA. There was also a negative correlation between the extent of retinal necrosis lesion zone and the occurrence of retinal detachment (rs = − 0.541, P < 0.001), indicating that the closer the retinal necrosis lesions involved the macular center, the higher the risk of retinal detachment (Table 4). Spearman’s correlation analysis in the precision treatment group revealed a positive correlation between the extent of retinal necrosis lesion zone and both the initial BCVA and the final BCVA (rs=0.567, 0.537, P = 0.006, 0.010), and a negative correlation with IL-6, IL-8, and viral nucleic acid count (rs=− 0.436, − 0.465, − 0.542, P = 0.043, 0.029, 0.009). This implies that the higher the levels of IL-6, IL-8, and viral nucleic acid in the intraocular fluid, the closer the retinal necrosis lesions to the center of the fovea, and the worse the initial and final BCVA.
The regression analysis demonstrated that viral copy number exerted a significant negative influence on final best-corrected visual acuity (BCVA) (β = − 1.582, P = 0.018). Specifically, each 1 copy/mL increase in viral load was associated with a 1.582 LogMAR reduction in final BCVA. Viral copy number accounted for 25% of the variance in final BCVA outcomes (Table 5).
Discussion
Our study demonstrates that varicella-zoster virus (VZV) infection is the predominant etiology in acute retinal necrosis syndrome (ARNs) with herpes simplex virus (HSV) infection ranking as the secondary cause. This finding aligns with prior research corroborating the utility of intraocular fluid analysis in the etiological diagnosis of ARNs which is characterized by high sensitivity and specificity11.Comparative analysis between the precision treatment group and the traditional treatment group revealed no significant statistical disparity in the rates of final vitrectomy and baseline BCVA. However, the precision treatment group exhibited a markedly reduced frequency of intravitreal injections and incidence of retinal detachment, coupled with superior final BCVA. These outcomes underscore the enhanced efficacy of precision antiviral therapy directed by intraocular fluid assessment. The observed discrepancies in treatment outcomes are hypothesized to stem from the administration of high-concentration antiviral agents via intravitreal injections in patients with elevated viral loads which transiently elevates the intraocular drug concentration. Additionally, the systemic antiviral efficacy conferred by intravenous administration of targeted antiviral medications is also a contributing factor. The precision antiviral therapy guided by intraocular fluid analysis facilitates a rapid, high-intensity antiviral response. Thereby it diminished viral burden. This approach simultaneously mitigates the potential retinal toxicities associated with high drug concentrations in patients with lower viral loads. Furthermore, the use of high-concentration antiviral agents minimizes the necessity for multiple intravitreal injections, thereby reducing the risk of intraocular infections and other complications associated with such procedures. This strategy also alleviates the economic burden on patients by decreasing the overall cost of treatment.
Correlation analysis showed that the baseline BCVA was positively correlated with the final BCVA in both groups and the better the baseline BCVA was, the better the final BCVA was, indicating that the baseline BCVA was closely related to the prognosis regardless of the antiviral treatment regimen. Some studies have also shown that there is a linear correlation between baseline BCVA and final BCVA in ARNs which may be a prognostic factor for visual acuity6. This is consistent with our results. Our study showed that a higher viral copy number was associated with worse baseline BCVA, higher number of intravitreal injections required, higher levels of IL-6, IL-8, VCAM, and BFGF, and ultimately worse BCVA. Research has shown that intraocular fluid before the virus in the high copy number and the final BCVA and inflammation of the retina is wider and the development of retinal detachment12, However, the study by von Hofsten et al.13 etc. found that a high viral load was not associated with a poor visual outcome, but only with an earlier sampling time. IL-6 is widely known as proinflammatory cytokines associated with autoimmune diseases, participate in B cell and T cell activation and differentiation. The binding of IL-6 to IL-6 receptor activates a series of inflammatory signaling pathways, which eventually induces the expression of inflammatory factors and promotes the differentiation of naive CD4 + T cells into Th17 cells which plays an important role in uveitis14,15,16. IL-8 is recruit immune cells involved in inflammation and promote inflammatory chemokines. IL-8 promote the accumulation of the neutrophils in the eye is the neutrophil chemotactic factor. It can be quantitative degree intraocular inflammation reaction activity17,18. Studies have shown that the levels of VACM, IL-6, IL-8 and IL-10 in ARNs are significantly higher than those in other infectious and inflammatory uveitis. VCAM mediates the tight adhesion between lymphocytes, monocytes and vascular endothelium, and then migrates into tissues19. Under the condition of the retina virus infection, proinflammatory cytokines IL-6 and chemical attractant IL-8 May rise in the region inflammation. At the same time, the inflammatory cells infiltration by leukocyte adhesion to the vascular endothelial cells, VCAM mediated by receptor and the ligand interactions, occlusive vasculitis20,21. This is consistent with our results that high viral copy number in the eye leads to increased proinflammatory cytokine IL-6, chemical inducer IL-8, increased VCAM-mediated receptor-ligand interactions, increased occurrence of occlusive vasculitis and poor visual prognosis. Studies have shown that IL-10 is an immunosuppressive regulatory cytokine that enhances B lymphocyte survival and antibody production. IL-10 can be produced by T lymphocytes and it has been proposed that IL-10 down-regulates immune responses to suppress T cell-mediated immune responses, thereby controlling the extent of tissue damage22. That are consistent with the results of our study. Our results also suggest that the onset time are positively correlated with IL-10, longer duration of illness, the body produces a dramatic increase in the anti-inflammatory factor IL-10.
Correlational analyses have revealed a significant positive correlation between the baseline BCVA and the final BCVA in cohorts of patients with ARNs. Superior initial BCVA is predictive of enhanced final visual outcomes, underscoring the prognostic relevance of baseline BCVA irrespective of the specific antiviral treatment modalities. This linear relationship between initial and terminal BCVA has been corroborated by previous studies, which have identified it as a pivotal prognostic indicator in ARNs6. Our investigation further delineates a negative association between elevated viral copy numbers and baseline BCVA, with increased requirements for intravitreal injections and heightened levels of IL-6, IL-8, VCAM, and bFGF, culminating in diminished final BCVA. Prior research has similarly implicated high intraocular viral loads with adverse visual outcomes, extensive retinal inflammation, and progression to retinal detachment12. Notably, previous study did not find a direct correlation between high viral loads and poor visual prognosis, instead suggesting earlier sampling times as a confounding factor13. IL-6, a quintessential pro-inflammatory cytokine implicated in autoimmune pathologies, is integral to the activation and differentiation of B and T lymphocytes. Its interaction with the IL-6 receptor initiates inflammatory signaling cascades that precipitate the expression of inflammatory mediators and foster the differentiation of naive CD4 + T cells into Th17 cells exerting significant influence in uveitis14,15,16. IL-8 recognized for its role in chemoattracting immune cells to inflammatory foci facilitates neutrophil aggregation within the ocular milieu and serves as a biomarker of intraocular inflammatory activity17,18. Elevated levels of VCAM, IL-6, IL-8 and IL-10 in ARNs, in contrast to other infectious and inflammatory uveitides, have been reported, with VCAM orchestrating the intimate adhesion of lymphocytes, monocytes, and vascular endothelium, thereby facilitating their transmigration into tissues19. In the context of retinal viral infection, the upregulation of pro-inflammatory cytokines IL-6 and the chemoattractant IL-8 is observed, alongside the leukocyte infiltration mediated by VCAM receptor-ligand interactions potentially leading to occlusive vasculitis20,21. These findings are congruent with our results. It demonstrated that high intraocular viral copy numbers elicit heightened levels of IL-6 and IL-8 intensifying VCAM-mediated interactions and the incidence of occlusive vasculitis, thereby adversely impacting visual prognosis. IL-10 is recognized as an immunosuppressive regulatory cytokine that enhances B lymphocyte survival and antibody production. IL-10 produced by T lymphocytes, has been proposed to downregulate immune responses to suppress T cell-mediated immune reactions, thereby controlling the extent of tissue damage22. This is in line with our findings which also indicate a positive correlation between the onset time of the disease and IL-10 levels, with a significant increase in the production of the anti-inflammatory factor IL-10 as the duration of the disease progresses.
Retinal detachment is the most common and primary cause of irreversible vision loss in patients with acute retinal necrosis (ARN). Our study indicates that the more quadrants involved by retinal necrosis, the higher the risk of retinal detachment. Additionally, the involvement of the central retina by necrotic lesions is associated with an increased risk of retinal detachment. Our findings are consistent with the research conducted by Qian et al.23. Furthermore, our research demonstrates that in the precision treatment group, the higher the levels of IL-6, IL-8, and viral nucleic acids in the intraocular fluid, and the closer the retinal necrotic lesions are to the fovea, the worse the initial and final BCVA. We believe this is due to the high viral load causing a severe intraocular inflammatory response, resulting in poor initial and final BCVA. The progression of ARNs is characterized by a peripheral to central foveal ring-like development; the closer the retinal necrotic lesions are to the fovea, the longer the disease course and the more severe the condition, leading to worse BCVA. Similarly, studies have reported that the initial BCVA is an important factor affecting the visual prognosis of ARNs patients24. Concurrently, research by Bavinja et al.25 has shown that initial BCVA and early inflammation are closely related to the occurrence of retinal detachment, and the occurrence of retinal detachment is closely related to the final BCVA of ARNs patients, further corroborating our research results.
ARNs is a rare infectious panuveitis syndrome characterized by its potential for rapid progression and sight-threatening complications. Timely diagnosis, appropriate treatment, and close follow-up are essential to maximize the preservation of visual function and reduce the incidence in the fellow eye. With the advent of intraocular fluid detection technology, our ability to rapidly and accurately identify the etiology of ARNs and initiate timely antiviral therapy has significantly improved. Viral load testing may aid in determining prognosis and assessing patients’ responses to treatment. Our study suggests that intraocular fluid testing plays a crucial role in the treatment and prognostic assessment of ARNs. However, our study has certain limitations: (1) a small sample size and short follow-up period; (2) as a retrospective study, selection bias cannot be avoided; (3) the first sample was derived partly from the vitreous and partly from the aqueous humor. In the future, we will conduct a prospective study with a large sample size and long follow-up period to evaluate the efficacy of different frequencies of intravitreal antiviral drug therapy in patients with varying viral nucleic acid counts.
Conclusion
Intraocular fluid analysis holds promising potential in the personalized treatment and prognostic assessment of ARNs. It can provide a more precise basis for the formulation of individualized treatment plans, thereby improving therapeutic outcomes and assessing patient prognoses. Our research findings offer new perspectives and possibilities for the treatment strategies of ARNs, providing significant reference for future clinical practice and scientific research.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Usui, Y. & Goto, H. Overview and diagnosis of acute retinal necrosis syndrome. Semin. Ophthalmol. 23(4), 275–283 (2008).
Putera, I. et al. Antiviral treatment for acute retinal necrosis: A systematic review and meta-analysis. Surv. Ophthalmol. 69(1), 67–84 (2024).
Liu, X. & Tao, Y. Use the examination of intraocular fluid well. Chin. J. Ophthalmol. Med. (Electron. Ed.) 8(5), 193–201 (2018).
Tang, L. & Zhang, H. The role of intraocular fluid detection in the prognosis evaluation of acute retinal necrosis. Chin. J. Ocul. Fundus Dis. 39(9), 767–769 (2023).
Ocular Immunology Group, Chinese Ophthalmological Society. Chinese expert consensus on intraocular fluid testing in the diagnosis and treatment of uveitis. Chin. J. Ophthalmol. Med. 56(9), 657–661 (2020).
Chen, Y. & Gao, L. Application of polymerase chain reaction in diagnosis of infectious uveitis. Int. Eye Sci. 12(11), 2113–2115 (2012).
Song, Y. & Bixia, W. The effect of the intraocular fluid detection on the unexplained uveitis. Chin. J. Ophthalmol. Med. (Electron. Ed.) 13(2), 82–87 (2023).
Zhou, M. The etiological and therapeutic study of acute retinal necrosis syndrome. (Fudan University, 2003)
Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for acute retinal necrosis syndrome. Am. J. Ophthalmol. 228, 237–244 (2021).
Lei, B. et al. Ultra-wide-field fundus imaging of acute retinal necrosis: Clinical characteristics and visual significance. Eye 34(5), 864–872 (2020).
Ganatra, J. B., Chandler, D., Santos, C., Kuppermann, B. & Margolis, T. P. Viral causes of the acute retinal necrosis syndrome. Am. J. Ophthalmol. 129(2), 166–172 (2000).
Calvo, C. M., Khan, M. A., Mehta, S., Garg, S. J. & Dunn, J. P. Correlation of clinical outcomes with quantitative polymerase chain reaction DNA copy number in patients with acute retinal necrosis. Ocul. Immunol. Inflamm. 25(2), 246–252 (2017).
von Hofsten, J., Bergstrom, T. & Zetterberg, M. Alpha herpes virus type and viral load in intraocular fluids in patients with acute retinal necrosis. BMJ Open Ophthalmol. 4(1), e000247 (2019).
Jianbing, L. I. & Guoxing, X. U. Relative cytokines in uveitis and experimental uveitis. Int. Eye Sci. 15(7), 1172–1175 (2015).
Balamurugan, S. et al. Interleukins and cytokine biomarkers in uveitis. Indian J. Ophthalmol. 68(9), 1750–1763 (2020).
Wang, Y. et al. Inflammatory cytokine profiles in eyes with primary angle-closure glaucoma. Biosci. Rep. 38(6), BSR20181411 (2018).
Murayama, T., Mukaida, N., Khabar, K. S. & Matsushima, K. Potential involvement of IL-8 in the pathogenesis of human cytomegalovirus infection. J. Leukocyte Biol. 64(1), 62–67 (1998).
Gonzalez-Aparicio, M. & Alfaro, C. Significance of the IL-8 pathway for immunotherapy. Hum. Vacc. Immunother. 16(10), 2312–2317 (2020).
Le, I. P. et al. Aquaporin-2 levels in vitro and in vivo are regulated by VACM-1, a cul 5 gene. Cell Physiol. Biochem. 30(5), 1148–1158 (2012).
van Kooij, B., Rothova, A., Rijkers, G. T. & de Groot-Mijnes, J. D. Distinct cytokine and chemokine profiles in the aqueous of patients with uveitis and cystoid macular edema. Am. J. Ophthalmol. 142(1), 192–194 (2006).
de Visser, L. et al. Cytokines and chemokines involved in acute retinal necrosis. Invest. Ophthalmol. Vis. Sci. 58(4), 2139–2151 (2017).
Saraiva, M., Vieira, P. & O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 217(1), e20190418 (2020).
Qian L. Clinical feature and prognostic factors in acute retinal necrosis syndrome.
Paolo, M. et al. Visual outcome and poor prognostic factors in acute retinal necrosis syndrome. Graef Arch. Clin. Exp. 258(9), 1851–1856 (2020).
Bavinger, J. C. et al. Risk factors for retinal detachment in acute retinal necrosis. Ophthalmol. Retina 6(6), 478–483 (2022).
Funding
National Natural Science Foundation of China (82260212) Central Government Guided Local Science and Technology Development Fund (2022ZDG02012) Jiangxi Province Science and Technology Department Key Research and Development Plan - Major Project (Bidding and Leading) (20223BBH80W01) Jiangxi Province Health Commission Key Scientific and Technological Innovation Project (2023ZD004) Jiangxi Province Natural Science Foundation Key Project (20232ACB206029) Jiangxi Provincial Department of Education Key Project (GJJ210126) Jiangxi Provincial Health Commission Science and Technology Plan Project (202410040).
Author information
Authors and Affiliations
Contributions
Xiao Yu: Paper writing and statistical analysis of data; Yuling Zou, Yao Zhao, Huimin Fan: Data collection; Ziqing Mao, Yiming Lei, Teng Liu, Hua Zou: Data analysis; Zhipeng You: Paper guidance and revision, fnancial support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, X., mao, Z., Zou, Y. et al. Intraocular fluid analysis-guided precision therapy in the treatment of acute retinal necrosis syndrome. Sci Rep 15, 14009 (2025). https://doi.org/10.1038/s41598-025-98624-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98624-6