Abstract
Nickel is a hormetic micronutrient for plants. This study investigates the comparative impact of seed priming with nickel in the form of bulk particles, and green-synthesized nanoparticles fabricated using leaf extract of Berberis baluchistanica, as reducing and capping agent, on germination, phenotypic characteristics, antioxidants, stomata, and photosynthetic activity of two wheat cultivars in a laboratory pot experiment. The synthesis of NiO-NPs was confirmed by using UV-spectrophotometer (peak at 203 nm) and their size observed was 22 nm. Furthermore, SEM, XRD, EDX, FTIR and evaluation of zeta potential were performed to study the physiochemical characteristics of NiO-NPs. Maximum root-to-shoot Ni translocation was observed under NiO-NPs, while bulk NiNO3 accumulated maximum Ni in root. Both nano and bulk form of Ni had stimulatory effects on urease activity, ammonia, and nitrate content. NiO-NPs increased the root length, shoot length and biomass, which were downregulated under bulk NiNO3 treatment. Bulk NiNO3 exacerbated oxidative stress in the form of MDA, H2O2 and membrane damage, which in turn elicited enzymatic and non-enzymatic antioxidants. This study suggests that green-synthesized NiO-NPs at low concentration are non-toxic and effective in improvement of plant growth and photosynthetic activity.
Similar content being viewed by others
Introduction
Nickel (Ni) at low concentration is crucial for plant growth. However, beyond the optimum range, it induces toxicity and reduces crop growth and productivity. Ni is the central element of urease enzyme, which hydrolyzes urea and helps in nitrogen (N) uptake1. The foliar applied urea with adequate supply of Ni is efficiently utilized in crops2, while in absence of Ni, urea accumulates in plant tissues causing necrosis3. Urea application through foliar spray is beneficial as it enhances urease activity and nitrogen availability to plants, reducing soil-related issues like slow nutrient uptake and fertilizer loss through leaching2. At low concentration, Ni has positive effects on N metabolism, chlorophyll synthesis, iron storage, senescence, plant growth, and crop yield4,5,6. Decrease in plant growth, iron deficiency, early senescence, and decline in grain yield have been observed in wheat, maize, rice, and other cereal crops under Ni deficiency5,7,8,9. The high concentration of Ni, on the other hand, induces the generation of reactive oxygen species (ROS), declines plant growth, and causes chlorosis and necrosis by reducing iron uptake and metabolism in plants2,10. Ni is a redox-inactive metal and is not directly involved in ROS production, but it affects normal functioning of the enzymatic antioxidants4. Also, high Ni concentration causes damage to DNA, proteins and cell membranes causing lipid peroxidation, which increases the MDA content in Ni sensitive plants11,12.
Ni absorbed by the plant roots is translocated to the upper parts via xylem tissues and is a highly mobile heavy metal within plants13. However, Ni in the soil has low mobility and less phytoavailability. Plants use the cation transport system to uptake Ni from soil. Other cations such as Zn2+ and Cu2+ also use the same pathway and show inhibitory effects on Ni2+ uptake and translocation from plant roots to shoots14. Furthermore, Ni in the bulk form binds with Mn, Mg, Ca, Cu, Fe, and Zn in soil and inhibits their uptake leading to their deficiency in plants15,16. Triticum aestivum L. is the second most widely cultivated cereal crop. It is consumed as the staple food by almost 40% of the world’s population17. However, nutrient deficiency and HM stress are declining overall wheat productivity.
To overcome the problems related to reduced phytoavailability of Ni in soil and its effects on crop production, it is crucial for plant researchers to focus on development of alternate approaches. Nanotechnology is increasingly making its mark in agriculture, offering innovative solutions to various challenges. Various studies indicated that seed priming with nanoparticles enhances the nutrient uptake and improves the antioxidant defense system in plants6,18. Moreover, Ni in the form of nanoparticles (NPs) possesses various different characteristics and releases more Ni ions as compared with its bulk form due to its small size and high mobility6,19. Green synthesized NiO nanoparticles have gained much attention in recent years. In the present study, the leaf extract of Berberis baluchistanica was used for synthesis of NiO NPs. B. baluchistanica belongs to the family Berberidaceae and is a potential source of bioactive components, nutrients, and antioxidants20. Phytoconstituents present in Berberis plant extract act as reducing and stabilizing agents which make NPs synthesis efficient, economic, and safe20,21.
Building on previous research from our lab9,22 that focused on understanding the impact of bulk Ni on different wheat varieties, the present study aims to expand our knowledge by investigating the combined impact of nano and bulk Ni seed priming on growth, physiology and urease activity of two high-yielding wheat cultivars. To the best of our knowledge, this is the first comparative study of bulk and nano Ni effects on wheat growth and physiology. The uptake, distribution, and accumulation of Ni in root and shoot, and the antioxidative response of wheat elicited by two different concentrations of bulk and nano NiO will be explored. This study will offer valuable intuition into development of efficient and economical approaches to enhance the utilization of green synthesized NPs for agricultural sustainability.
Material and methods
Collection of plant material
The plant material was collected by Dr. Siraj Uddin20 from the mountainous regions of Quetta, Baluchistan (Latitude: 30.1798° N; Longitude: 66.9750° E), Pakistan. The plant sample was identified by Dr. Amir Sultan in National Herbarium, Pakistan by comparing it with already submitted herbarium specimens and flora of Pakistan. A voucher specimen (RAW100267) was deposited at the National Herbarium, Islamabad, Pakistan for future reference.
Green-synthesis of NiO nanoparticles
Preparation of nanoparticles was carried out following previously described method21. Purified 50 mL of Berberis baluchistanica leaf (BBL) extract was added to the 0.3 M NiNO3 and the mixture was vigorously stirred at 60 °C temp and 500 rpm on a magnetic stirrer for 3 h. Afterwards, the precipitate was centrifuged, and pellet was collected and washed with distilled water. Collected pellet was then dried at 100 °C using hot air oven. Subsequently, dried BBL-NiO-NPs powder was calcinated using air furnace (KSL-1100X, MTI Corporation, China) at 400 °C for 3 h. The change in color confirmed the bio-reduction of NiNO3 to NiO-NPs. Finally, nanoparticles were characterized using different techniques.
Physio-chemical characterization of NiO-NPs
Optical absorbance of biogenic NiO-NPs was performed in the range of 200–800 nm using UV-400 UV–Vis scanning spectrophotometer (Germany). The elemental composition and morphological properties of NPs were analyzed using SEM (NOVA FEISEM-450 fortified with EDX apparatus). Particle size was detected using X-ray diffraction (XRD), while FTIR (Alpha, Bruker, Germany) in scanning range of 400–4000 cm−1 was used to verify the capping and stabilizing properties of functional groups associated with BBL-NiONPs. Additionally, zeta potential was determined to assess the stability of NPs.
Planting material, seed priming and treatments
Healthy seeds of two Wheat (Triticum aestivum L.) cultivars collected from NARC Pakistan were selected based on their high yield and tolerance properties viz., Sarsabz (SSB) and Bars-2009 (BARS). The seeds were surface sterilized with 10% sodium hypochlorite solution for 10 min and then washed with distilled water. The synthetic suspensions of 20 and 40 mg L−1 concentrations of NiNO3 and NiO-NPs were made by dissolving each in deionized water followed by ultrasonication for 30 min. Seed priming treatments included priming with; distilled water (hydropriming; HP), Nickel oxide nanoparticles (NiO-NP; T1: 20 mg L−1, T2: 40 mg L−1), and bulk NiNO3 (NiNO3; T1: 20 mg L−1, T2: 40 mg L−1). Non-primed seeds of both cultivars were used as control. For priming, seeds were placed in dark at room temperature for 24 h. The ratio of seed weight to priming solution volume (w/v) was 1:4. After 24 h., the primed seeds were surface dried by blotting paper and then left at room temperature for 20–24 h to restore the original moisture content of seeds23. For germination percentage determination, ten healthy seeds from each treatment were germinated on two layers of filter paper in Petri plates with equal volume of distilled water applied to maintain moisture content. Seed germination was recorded on a daily basis and germination percentage was calculated as the ratio of number of seeds germinated after seven days and total number of seeds sown24.
Experimentation
The experiment was performed in a growth chamber under controlled conditions. The conditions of growth chamber during experiment were set as; light period: 12 h, temperature (day/night): 28/25 °C and humidity: 50–60%. After nano-priming, 5 healthy seeds of each treatment were separately sown in plastic pot (4.5″) with equal volume of soil: sand: peat moss (1:1:1 v/v/v). All the experiments were performed in a factorial arrangement in completely randomized design with three replications of each treatment. After twenty-one days of sowing, 2% Urea solution was applied through foliar spray.
Phenotypic evaluation
Twenty-eight days old plant samples were harvested for further analysis. The growth and morphological traits i.e., shoot length (SL), root length (RL), plant height (PH), leaf area (LA), fresh weight (FW) and dry weight (DW) of root and shoot samples, and RWC (relative water content) of each replicate were evaluated. Length was recorded using measuring tape, while digital electric balance was used for recording weight of samples. Roots and shoots were separated, and fresh weight was measured immediately after harvesting. For turgid weight, five replicates of each plant sample were immersed in distilled water for 72 h. After that, turgid weight was recorded, and samples were placed in oven for 72 h at 70 °C to measure dry weight. Leaf relative water content (RWC) was measured following method described by Anas, Saeed9 in both cultivars. For measurement of yield stability index17, root dry weight stability index (RDSI) and shoot dry weight stability index (SDSI) were calculated using following formula.
Evaluation of nickel accumulation in leaf and root
For estimation of Ni uptake, wet digestion method was followed4. After acid digestion, samples were subjected to Flame Atomic Absorption Spectrometer (FAAS-AA7000 Shimadzu) for determination of Ni. Merk certified reference material of 1000 ppm was used to prepare standards. Ni concentration below 0.2 mg Kg−1 was considered as not detected.
Analysis of photosynthesis
Total chlorophyll and carotenoid content
Total chlorophyll and carotenoid content were evaluated by following Acetone method for extraction23. For chlorophyll estimation, absorbance was measured at 645 nm and 663 nm, while for carotenoids, the absorbance was measured at 480 nm and 510 nm, respectively.
Estimation of leaf ammonia content, nitrate content and urease activity
For estimation of leaf urease activity, Siqueira Freitas, Wurr Rodak25 method was followed with few modifications. Extraction of fresh plant sample (0.1 g) was performed in 4.0 mL of potassium phosphate buffer (pH 7.4) at 30 °C for 1 h. Afterwards, 1 ml of 10% urea solution and 3 mL of reagent mixture (1.5 mL of reagent 1: 170 µM of sodium nitroprusside; 0.1 M phenol and 1.5 mL of reagent 2: 0.15 M dibasic sodium phosphate; 0.125 M sodium hydroxide; 3% sodium hypochlorite with 3% Cl2) were added to 0.5 mL of supernatant followed by incubation at 37 °C for 30 min. Absorbance was measured at 625 nm using spectrophotometer.
Ammonia concentration was determined according to method described by Siqueira Freitas, Wurr Rodak25. Fresh plant sample (0.1 g) was homogenized in 3 mL mixture of methanol: chloroform (3: 1 v/v) and centrifuged. The supernatant collected was then mixed with 1 mL of reagent mixture (0.5 mL of phenol reagent 1: 170 µM of sodium nitroprusside; 0.1 M phenol and 0.5 mL of phosphate reagent 2: 0.12 M monobasic sodium phosphate; 0.125 M sodium hydroxide; 5% sodium hypochlorite) and incubated at 37 °C for 1 h. Absorbance was measured at wavelength 630 nm.
For estimation of Nitrate content, the method of Cataldo, Maroon26 was followed. The reaction mixture contained 0.2 mL of 5% salicylic acid in concentrated H2SO4 and 0.1 mL of plant extract. After incubation at room temperature for 15–20 min, 1 mL of 4 M NaOH was added, and absorbance was measured at 410 nm. Nitrate content was expressed in μM NO3− g−1 FW.
Measurement of stomatal density and size
Samples for stomatal measurements were observed using light microscope equipped with digital camera, at 40X for stomatal density and 10X for stomata and aperture size measurements. Digital images of both abaxial and adaxial surface of 3 leaf samples per treatment were taken. Stomatal density and size were measured using TCapture software program.
Oxidative stress analysis
Quantification of H2O2 and MDA content
For hydrogen peroxide (H2O2) quantification12, fresh leaf and root tissues were homogenized with 0.1% (w/v) TCA and centrifuged at 12,000 g for 15 min. The supernatant was then added to an equal amount of 10 mM potassium phosphate buffer (pH 7.0) and 1 mL of 1 M potassium iodide. The absorbance was read at 390 nm.
Heath and Packer27 method was followed for estimation of lipid peroxidation by measuring MDA content in leaf and root samples. Each fresh sample (0.05 g) was homogenized with 0.1% trichloroacetic acid (TCA) and then centrifuged (13,000 g; 15 min). The reaction mixture 0.5% TBA: 20% TCA (3: 4 v/v) was added to the supernatant and boiled at 95 °C for 30 min. After centrifugation, absorbance was measured at 450, 532 and 600 nm, respectively.
Assessment of membrane permeability
Damage to membrane integrity was evaluated according to the method described by Leopold, Musgrave28. The extent of solutes leakage due to membrane damage was measured by recording absorbance at 273 nm using a spectrophotometer.
Estimation of total sugar and proline content
Total sugar content was evaluated by Anthrone method29. Absorbance was measured at 630 nm using a spectrophotometer. For proline content analysis, the method explained by Jahan, Guo4 was followed. Toluene was used as standard and optical density was measured at 520 nm using spectrophotometer.
Estimation of enzymatic antioxidants
Fresh leaf and root samples were homogenized and crushed with 0.2 M potassium phosphate buffer (pH 7.8) containing 0.05 mM Ethylenediaminetetraacetic acid (EDTA) and 1% polyvinylpyrrolidone (PVP). The extract was then passed through cheesecloth filter paper and centrifuged (15,000 g; 20 min; 4 °C). The supernatant was collected and stored at 4 °C to measure protein and other enzymatic antioxidants activity. Superoxide dismutase (SOD, EC 1.15.1.1) activity was analyzed by measuring the rate of inhibition of nitroblue tetrazolium (NBT). The assay mixture containing 50 mM phosphate buffer (pH 7.8), 2 mM EDTA, 9.9 mM L‐methionine, 0.025% Triton‐X100, and 55 μM NBT was added to the plant extract (30μL). Subsequently, after adding 1 mM riboflavin, reaction was started by placing sample tubes under fluorescent light (15W) for 15 min and the absorbance was measured at 560 nm using spectrophotometer30. The activity of peroxidase (POD, EC 1.11.1.7) was analyzed by following the method of Chance, Maehly31. The enzyme extract was added to the assay mixture containing 10 mM potassium phosphate buffer (pH 7.0) and 8 mM guaiacol. Finally, 2.75 mM H2O2 was added to initiate the reaction and the change in absorbance was recorded at 510 nm. For catalase (CAT, EC 1.11.1.6) estimation, Aebi32 method was followed. The assay mixture containing 0.1 M potassium phosphate buffer (pH 7.0), 75 mM H2O2 and plant extract were mixed thoroughly. The decrease in absorbance was measured at 240 nm for 1 min. For ascorbate peroxidase (APX, EC 1.11.1.11) analysis, the method described by Nakano and Asada33 was followed. Assay mixture containing 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbate and 0.5 mM H2O2 was mixed with plant extract and absorbance was measured at 290 nm. Ascorbate oxidase (AO, EC 1.10.3.3) activity was analyzed according to Diallinas, Pateraki34 method. The reaction was initiated by adding 25µL enzyme extract to the reaction mixture containing 20 mM potassium phosphate buffer (pH 7.0) and 2.5 mM ascorbic acid. The decrement in absorbance was measured at 265 nm. For glutathione peroxidase (GPX, EC 1.11.1.7) estimation, method described by Anas, Bibi24 was followed and absorbance was measured at 340 nm. Lowry’s method was followed for total protein estimation35. Bovine Serum Albumin (BSA) was used as standard, and absorbance was read at 660 nm.
Estimation of non-enzymatic antioxidants
Determination of ascorbic acid and glutathione
For estimation of Ascorbic acid, method described by Fadhel36 was followed. Absorbance was measured at 350 nm and results were expressed as mg of ascorbic acid equivalent (AAE) g−1 of dry wt. of sample. Glutathione (GSH), glutathione disulfide (GSSG) and total glutathione (GSH+GSSG) were determined by following Anderson37 method. Absorbance was measured at 412 nm using a spectrophotometer and final concentration was expressed as nmol g−1 FW of plant sample.
Determination of total phenolics, total flavonoid and total anthocyanin content
To make extracts, hot 80% methanol was added to dried powder of leaf and root samples and left over night with intermittent shaking for complete extraction. After this, extracts were filtered using Whatman filter paper and the extraction was repeated twice with fresh alcohol. After centrifugation, supernatant was collected, and solvent was evaporated over water bath at 60 °C20. Crude extracts obtained were stored at 4 °C in a refrigerator for further analysis. Aqueous-alcoholic extracts were used for evaluation of phenolics, flavonoids and antioxidant activity.
Folin-Ciocalteu’s Reagent (FCR) method was followed for determination of total phenolics content20. Absorbance was measured at 650 nm, and results were expressed as mg of gallic acid equivalent (GAE) g−1 of dry wt. of extract. For estimation of total flavonoid content, Aluminium Chloride Colorimetric method was followed with some modifications20. Absorbance was measured at 510 nm and final concentration was expressed as mg of Quercetin Equivalent (QE) g−1 of dry wt. of extract. Total anthocyanin content was measured following method reported by Jahan, Guo4 and results were expressed as mg g−1 DW.
Measurement of antioxidant activity
Antioxidant activity was determined by total antioxidant capacity (TAC), total reducing power (TRP), and Diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging analysis. Phosphomolybdenum method was followed for TAC estimation. Reagent mixture containing 4 mM ammonium molybdate, 28 mM sodium phosphate and 0.6 M H2SO4 was mixed with plant extract and left for incubation at 95 °C for 90 min. After cooling, absorbance was measured at 695 nm38. For TRP estimation, ferrous reducing/antioxidant power (FRAP) assay was followed according to method described by Uddin, Bin Safdar20. Ascorbic acid was used as standard reference, and results were expressed as µg of AAE mg−1 of dry wt. of extract for TAC and TRP calculation. For DPPH free radical scavenging activity of plants, the method described by Javed, Usman38 was followed. Plant extract was reacted with 1 mL of a freshly prepared DPPH (2,2-diphenyl-1-picryl hydrazyl) methanol solution and left at room temperature in the dark for 30 min. Methanol was used as a blank while control sample was prepared containing the same amount of DPPH and methanol without plant extract. Absorbance was determined by using a spectrophotometer at 517 nm.
Statistical analysis
Analysis of variance (ANOVA) was performed using XLStat 2021. Differences among the treatments were determined by Tukey’s HSD test (p ≤ 0.05) and results were expressed as mean value ± SD. Principal component analysis (PCA) and cluster heatmaps were constructed using SRplot bioinformatics tool.
Results
Physiochemical characteristics of NiO-NPs
UV–Vis spectra and zeta potential
The successful synthesis of NiO-NPs was confirmed using UV–Vis spectroscopy, which exhibited a characteristic absorption peak at 203 nm (Fig. 1C). This peak indicates the presence of nickel oxide nanoparticles, verifying their nanoscale nature.
The conductivity of suspension was 0.0624 mS/cm that implies a moderate ionic strength (Fig. 1A). Zeta potential of the nanoparticles was recorded as − 19.3 mV, indicating a moderately stable colloidal dispersion with an anionic surface charge. The zeta potential within the range of − 10– − 20 mV suggests that the nanoparticles exhibit some degree of electrostatic repulsion, which contributes to their dispersion stability. Dynamic light scattering (DLS) analysis revealed a polydispersity index (PDI) of 1.00, suggesting a highly polydisperse system rather than a uniform monodisperse substance. Additionally, the Z-average hydrodynamic diameter of the nanoparticles obtained from DLS was 4307 nm (Fig. 1B). These values represent the broad particle size distribution and potential aggregation and agglomeration of nanoparticles in an aqueous medium.
XRD and FTIR
The X-ray diffraction planes showed agreement with JCPDS 00-004-0835 with 4 peak values at 32.0°, 36.1°, 52.1°, and 63.5°, coordinating excellently with the planes 111, 200, 220, and 311. The size of nanoparticles calculated via Scherrer formula was found to be around 22 nm (Fig. 2B). The presence of functional groups was confirmed via FTIR, at various peaks including 1018.90, 1449.37, 1741.54, 2833.47, and 2947.31 representing fluoro compound, alkane, esters, carboxylic acid, and amine salt respectively (Fig. 2A; Table 1).
SEM and EDS
The NiO-NPs powder observed under the energy-dispersive X-ray spectroscopy (EDS) showed 80.19% of Ni and 15.50% of O element in the powder. In terms of atomic percentage, Ni was 63.66% while O accounted for 29.34%. Whereas the shape of NiO-NPs was confirmed to be crystalline using scanning electron microscopy (SEM) (Fig. 3).
Nickel uptake and accumulation in leaf and root
To investigate the accumulation of Ni under NiO-NPs and bulk NiNO3 treatment, Ni content was recorded in shoot and root of both wheat cultivars. Results showed that linear increase in Ni concentration increased the root-to-shoot Ni translocation (Fig. 4B). Translocation factor (TF) showed an increasing trend under NiO-NPs treatment as compared to bulk NiNO3 in both cultivars with maximum values in BARS i.e., 96.8% (NiNP_20) and 97.1% (NiNP_40) with respect to NiNO3 applied at similar concentrations. Highest value of Ni was recorded in shoot of NiO-NPs treated plants at 20 mg/L (39.78 mg Kg−1 DW) and 40 mg/L (88.31 mg Kg−1 DW) in BARS. On contrary, SSB reduced the Ni uptake by 54% and 57% under 20 mg/L and 40 mg/L NiO-NPs as compared to BARS cultivar (Fig. 4A). However, roots of both cultivars accumulated more Ni under NiNO3 treatment than that of the NiO-NPs applied at similar concentration. Besides, maximum Ni accumulation in roots was observed in SSB as compared to BARS cultivar (Fig. 4B).
Concentration of Ni (mg Kg−1) in leaf and root of BARS and SSB cultivars under different treatments (A). Cluster heatmap showing Ni uptake and translocation factor (TF) in root and shoot (B). The blue color in scale (0-(-1)) represents low to medium while red color (0–1) indicates medium to high. NiNP_20 (NiO-NPs at 20 mg/L), NiNP_40 (NiO-NPs at 40 mg/L), NiNO3_20 (NiNO3 at 20 mg/L) and NiNO3_40 (NiNO3 at 40 mg/L) treatments. Lower case letters show statistically significant results among treatments within a cultivar at p < 0.05 according to Fisher’s least square difference (LSD) test. Treatments with similar letters indicate no significant difference exists between them.
NiO-NPs enhance growth and biomass
The results related to the effects of hydro-priming as well as priming with bulk and nano Ni on germination and growth of both wheat cultivars are presented in Fig. 5. In control plants, there were no apparent symptoms of Ni deficiency. However, Ni application enhanced the growth and improved the physiology and antioxidative response in wheat. Seed priming with NiO-NPs significantly improved the germination and biomass. Maximum germination percentage (GP%) was observed under NiO-NPs (20 mg/L) followed by NiNO3 (20 mg/L) treatment in both wheat cultivars. However, it remained unaffected under 40 mg/L of NiNO3 and NiO-NPs as compared to their respective controls. After germination, bulk NiNO3 at 20 mg/L and 40 mg/L accelerated the seedling growth initially (Fig. 5C), but reduced the overall size of root and shoot after 28 days of experiment.
Seed germination percentage of SSB and BARS cultivars under different treatments. Circular cluster heatmap showing wheat seed GP% after 3, 5, and 7 days of germination (A). Horizontal left right bars showing GP% after 7 days (B). Early growth performance of differently primed seeds after 7 days of germination (C). (HP: Hydro-priming, NiNP_20: NiO-NPs at 20 mg/L, NiNP_40: NiO-NPs at 40 mg/L, NiNO3_20: NiNO3 at 20 mg/L and NiNO3_40: NiNO3 at 40 mg/L).
Significant effects of different priming treatments on growth attributes were recorded in both wheat cultivars (Table 2). A remarkable impact (p < 0.01) of all the priming treatments was recorded on phenotypic traits of wheat. Results showed that shoot length (SL), root length (RL), leaf area (LA) as well as fresh and dry biomass were increased under NPs treatment more than that observed in bulk NiNO3 treated plants (Fig. 6). Seed priming with NiO-NPs enhanced the SL, RL, and LA, in both cultivars with maximum values in BARS i.e., 6.59%, 8.18%, and 28.9%, at 40 mg/L NiO-NPs respectively, as compared to control. Cultivar variations regarding fresh and dry biomass were also apparent concerning their response to different priming treatments. As compared with other treatments, hydropriming considerably (p < 0.05) increased the fresh and dry weight of root and shoot while reduced the RL and SL in both wheat cultivars. However, NiNO3 applied at 40 mg/L had negative effects on plant growth and biomass in both cultivars. Decline in SFW by 3.1 and 1.9% and SDW by 15.8 and 1.9% under NiNO3 treatment applied at 20 and 40 mg/L relative to control was observed in SSB cultivar. Similarly, RFW and RDW showed downregulation in plants treated with bulk NiNO3 in both wheat cultivars. Furthermore, RWC showed an upward trend in both wheat cultivars (Fig. 7D). Plants treated with NiO-NPs were more effective in upregulation of RWC as compared to NiNO3 treatment i.e., 7.2%, 8.1%, 5.5%, and 4.5% in SSB and 4.2%, 2.4%, 1.4%, and 0.5% in BARS under NiNP_20, NiNP_40, NiNO3_20, NiNO3_40 treatment with respect to control. Similarly, hydropriming remarkably increased RWC in both cultivars (Table 3). These results confirmed that Ni applied in the form of nanoparticles at both concentrations (20 and 40 mg/L) upregulated growth and improved the germination percentage in both cultivars with maximum values in BARS which is a high yielding cultivar under non-stressed conditions.
Bar graph representation of growth parameters leaf area (A), leaf length (B), shoot length (C), root length (D), shoot fresh wt. (E), shoot dry wt. (F), root fresh wt. (G), root dry wt. (H) of BARS (Bars-09) and SSB (Sarsabz) cultivars of wheat under different treatments (HP: Hydro-priming, NiNP_20: NiO-NPs at 20 mg/L, NiNP_40: NiO-NPs at 40 mg/L, NiNO3_20: NiNO3 at 20 mg/L and NiNO3_40: NiNO3 at 40 mg/L). Lower case letters show statistically significant results among treatments within a cultivar at p < 0.05 according to Fisher’s least square difference (LSD) test. Treatments with similar letters show that no significant difference exists between them.
Bar graph representation of urease (A), ammonia content (B), nitrate content (C), RWC (relative water content) (D), chlorophyll a (E), chlorophyll b (F), total chlorophyll (G) and chlorophyll a/b ratio (H) of BARS (Bars-09) and SSB (Sarsabz) cultivars of wheat under different treatments (HP: Hydro-priming, NiNP_20: NiO-NPs at 20 mg/L, NiNP_40: NiO-NPs at 40 mg/L, NiNO3_20: NiNO3 at 20 mg/L and NiNO3_40: NiNO3 at 40 mg/L). Lower case letters show statistically significant results among treatments within a cultivar at p < 0.05 according to Fisher’s least square difference (LSD) test. Treatments with similar letters show that no significant difference exists between them.
Photosynthetic activity
To study the effects of Ni supplication on photosynthetic activity, we analyzed the activity of urease enzyme, chlorophyll content, ammonia concentration and nitrate content in leaf of both wheat cultivars (Sarsabz; SSB and Bars-09; BARS). Results are presented in Table 3.
Chlorophyll content
Highest chlorophyll a, b and total chlorophyll content was recorded in control plants of SSB cultivar which was slightly reduced following Ni application (Fig. 7). On contrary, a significant increase (p < 0.05) in chlorophyll content with increasing concentration of bulk and nano Ni supply was observed in BARS i.e., two- to three-fold increase as compared to those in control and hydro-primed plants. Additionally, data associated with chlorophyll a/b ratio showed significant (p < 0.01) differences in both wheat cultivars. In BARS, Chl a/b ratio increased significantly under Ni treatment i.e., 2.98 times with NiO-NPs application, while 1.85 times increase under NiNO3 treatment, respectively. However, only NiO-NP at 20 mg/L increased (15.9%) chlorophyll a/b ratio in SSB cultivar (Fig. 7H).
Leaf urease activity
Urease enzyme catalyzes the hydrolysis of urea into ammonia and carbon dioxide. After foliar application of urea, slight necrosis occurred at leaf tips of hydro-primed, control and NiNO3 (40 mg/L) treated plants. However, seed priming with NiO-NPs positively affected the leaf urease activity. Although bulk NiNO3 exhibited reduced activity of urease enzyme in both cultivars as compared to NiO-NPs, nevertheless, their values were greater than those observed in their respective control plants (Table 4). In particular, 5 and 151% increase in activity of this enzyme was observed in BARS, while in SSB cultivar, 88.26% and 126.68% increase was recorded under 20 mg/L and 40 mg/L NiO-NP treatment with respect to their controls. Similarly, NiNO3 treatment enhanced the activity of this enzyme in both cultivars relative to hydropriming and control plants (Fig. 7A). However, increase in activity of urease under NiNO3 treatment was 38.78 and 25.24% less as compared to NiO-NPs treated plants in SSB. It is confirmed from the results that urease activity enhanced with increasing conc. of Ni as compared to untreated plants.
Leaf ammonia concentration
Ammonia is a product of urea hydrolysis by urease enzyme. Ni supply in both bulk and nano form enhanced leaf ammonia concentration as compared to hydro-primed and control plants (Table 3). Significant increase (p < 0.05) in ammonium concentration was observed with increasing concentration of Ni in both cultivars with maximum increment in BARS under NiO-NPs treatment i.e., 6% (20 mg/L) and 10% (40 mg/L), respectively. However, NiNO3 showed 5.5% and 10% less ammonia conc. as compared to NiO-NPs at 20 and 40 mg/L. Similarly, Ni application enhanced the ammonia conc. in SSB cultivar relative to control plants but less than that of BARS cultivar (Fig. 7B).
Nitrate concentration in leaf
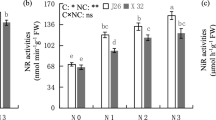
Ni supplementation positively affected the leaf nitrate concentration. Application of NiO-NPs significantly (p < 0.01) increased the nitrate content i.e., 25.6 and 32.7% in BARS, while 83.5 and 125% in SSB under 20 and 40 mg/L concentration as compared to control. Similarly, NiNO3 treatment enhanced the nitrate content relative to control plants in both cultivars (Fig. 7C). These results demonstrate that Ni at low concentration improves the activity of urease enzyme and nitrogen assimilation in wheat (Table 3).
Stomata
The results related to stomatal density (SD), stomatal length (SL), aperture length (ApL), stomatal area (SA) and aperture area (ApA) on abaxial and axial surface are presented in Table 4. Results showed an increase in SD on abaxial and adaxial surface under all the treatments with maximum increase recorded in hydro primed and NiO-NP treated plants. Similarly, an increase in SA and ApA was observed on abaxial surface while reduction in SL, ApL, SA, and ApA was recorded on adaxial surface with maximum reduction under bulk NiNO3 treatment in both cultivars. Conversely, NiO-NP treatment increased SL and ApL on abaxial surface in BARS cultivar.
Oxidative stress analysis
H2O2 and MDA content
Results regarding H2O2 and MDA content in root and shoot of both cultivars are presented in Fig. 8. Maximum H2O2 accumulation was recorded in leaf of BARS and root of SSB cultivar under Ni application with respect to their control plants. In SSB, the highest level of H2O2 detected in roots of control plant was 4.67umol/g FW, which proliferated to 16.85 and 23.4 µmol/g FW under NiO-NPs, while 24.76 and 27.62 µmol/g FW under NiNO3 treatment at 20 and 40 mg/L, respectively (Fig. 8B). Similarly, compared with control, H2O2 conc. increased by 77.5 and 86.1% under NiO-NPs and NiNO3 treatment at 40 mg m/L concentration in leaf of BARS cultivar.
Effects of bulk and nano Ni on stress indicators MDA content (A), H2O2 content (B), leaf membrane permeability % (C), proline content (D), total soluble sugar (E) and protein (F) of BARS (Bars-09) and SSB (Sarsabz) cultivars of wheat under different treatments (Ctrl: control, Hydro: Hydro-priming, NiNP_20: NiO-NPs at 20 mg/L, NiNP_40: NiO-NPs at 40 mg/L, NiNO3_20: NiNO3 at 20 mg/L and NiNO3_40: NiNO3 at 40 mg/L). Lower case letters show statistically significant results among treatments within a cultivar at p < 0.05 according to Fisher’s least square difference (LSD) test. Treatments with similar letters show that no significant difference exists between them.
Under stress, membrane lipids undergo peroxidation forming lipid peroxides in the form of thiobarbituric acid reactive substance (TBARS), specifically, malondialdehyde (MDA). In BARS, the MDA content increased with increasing conc. of Ni in leaf i.e., by 8 and 36.7% under NiO-NPs and NiNO3 at 40 mg/L, respectively. However, NiO-NP and NiNO3 at 20 mg/L considerably (p < 0.05) lowered the MDA content by 55 and 16% relative to control (Fig. 8A). Besides, an increase in MDA content was recorded in leaf of SSB cultivar under all the treatments, while only decline was observed under NiNP_20 (7.6%), respectively, over control. Similarly, NiNO3 at 40 mg/L increased the MDA content in roots of both cultivars. Results showed that NiO-NPs lowered the accumulation of MDA content in both cultivars as compared to NiNO3 applied at similar concentration.
Membrane permeability
Compared with control, NiO-NPs remarkably reduced the leaf membrane permeability (MP) in both wheat cultivars. In BARS, MP in control plants was 36.75% which dropped to 21.95% in NiNP_20 and 19.05% in NiNP_40 treated plants. Similarly, 36.4% MP in control plants was reduced to 26.88% and 15% in SSB cultivar at 20 and 40 mg/L NiO-NPs, respectively. In contrast, NiNO3 raised MP at 20 mg/L in BARS (10.24%) and 40 mg/L in SSB cultivar (66.67%) with respect to control (Fig. 8C).
Osmotic regulation substances
Heavy metal stress triggers osmotic imbalance in plants and causes an increase in the substances involved in the osmotic regulation of cells39. An upward trend was observed in total soluble sugar (TSS) content of SSB with maximum increment under NiO-NPs i.e., by 74 and 250% in leaf and 7.87 and 50% in root at 20 and 40 mg/L, respectively, as compared to control. BARS cultivar on the other hand, showed a decline in TSS content in roots under all treatments, however, the only increase was recorded in leaf of NiO-NP (60.91%) and NiNO3 (91.09%) at 40 mg/L, respectively (Fig. 8E). Conversely, Ni application raised the proline (Pr) content by 43.09% (NiNO3_40) in leaf, and 72.72% (NiNP_20), 11.09% (NiNP_40), and 83.32% (NiNO3_20) in roots of BARS with respect to control. However, in SSB cultivar, the only increase in Pr under Ni treatment was observed in leaf (25.74%) and root (19.79%) of NiNO3 at 40 mg/L respectively, over control (Fig. 8D).
Antioxidative response
Activity of enzymatic antioxidants
Antioxidants help to counteract the damage caused by reactive oxygen species formed in plants under stress conditions. To investigate the impact of NiO-NPs and bulk NiNO3 on antioxidative defense system in wheat, we measured the activity of enzymatic antioxidants in control, hydro-priming, NiO-NPs and bulk NiNO3 treated plants. The activity of SOD fluctuated with increasing Ni application (Fig. 9A). Compared with control plants, decrease in the SOD activity was observed in both leaf and root of BARS with maximum decline in leaf by 18.89% (HP), 16.06% (NiNP_20), 29.4% (NiNP_40), 16.5% (NiNO3_20) and 12.8% (NiNO3_40), respectively. A similar trend was observed in leaf of SSB, however, in roots, the activity of this enzyme elevated significantly (p < 0.01) by 122% (HP), 92.5% (NiNP_20), 50.83% (NiNP_40), 129.07% NiNO3_20) and 263.9% (NiNO3_40) relative to control (Fig. 9). Correspondingly, CAT activity was downregulated under Ni stress, however, NiO-NPs at 20 and 40 mg/L upregulated the CAT activity by 14.66 and 7.90% in leaf of BARS, while 12.38 and 76.02% in roots of SSB cultivar with respect to control (Fig. 9B). POD on the other hand showed an increasing trend following stress with maximum increase in leaf (76.3%) of BARS and root (61.11%) of SSB under NiNO3_40 treatment (Fig. 9C). There were significant differences (p < 0.01) among antioxidant activity of both cultivars. Furthermore, maximum activity of these enzymes was recorded in root of SSB while leaf of BARS cultivar under Ni treatment, respectively.
Effects of bulk and nano Ni on activity of SOD (superoxide dismutase) (A), CAT (catalase) (B), and POD (peroxidase) (C), of BARS (Bars-09) and SSB (Sarsabz) cultivars of wheat under different treatments (Ctrl: control, Hydro: Hydro-priming, NiNP_20: NiO-NPs at 20 mg/L, NiNP_40: NiO-NPs at 40 mg/L, NiNO3_20: NiNO3 at 20 mg/L and NiNO3_40: NiNO3 at 40 mg/L). Lower case letters show statistically significant results among treatments within a cultivar at p < 0.05 according to Fisher’s least square difference (LSD) test. Treatments with similar letters show that no significant difference exists between them.
AsA-GSH cycle
APX activity showed decline in both root and shoot of SSB cultivar following Ni application with respect to control (Fig. 10B). On contrary, activity of this enzyme upsurged in BARS with increasing conc. of Ni by 143.5% (NiNP_20), 157.2% (NiNP_40), 172.5% (NiNO3_20), and 172.9% (NiNO3_40) in leaf, while 68.4% (NiNP_40), 158.3% (NiNO3_20), and 194.3% (NiNO3_40) in root with respect to control. Moreover, the activity of ascorbate oxidase (AOX) also enhanced following Ni application with maximum increment in leaf (60.9 and 57.2%) of BARS and root (52.6 and 52.2%) of SSB under NiNP_40 and NiNO3_40 treatments, respectively, over control (Fig. 10C).
Effects of bulk and nano Ni on AsA-GSH cycle; Ascorbic acid (A), APX (ascorbate peroxidase) (B), AOX (ascorbate oxidase) (C), GPX (glutathione peroxidase) (D), GSH (glutathione) (E), GSSG (glutathione disulfide) (F), GSH+GSSG (total glutathione) (G), and GSH/GSSG ratio (H) of BARS (Bars-09) and SSB (Sarsabz) cultivars of wheat under different treatments (Ctrl: control, Hydro: Hydro-priming, NiNP_20: NiO-NPs at 20 mg/L, NiNP_40: NiO-NPs at 40 mg/L, NiNO3_20: NiNO3 at 20 mg/L and NiNO3_40: NiNO3 at 40 mg/L). Lower case letters show statistically significant results among treatments within a cultivar at p < 0.05 according to Fisher’s least square difference (LSD) test. Treatments with similar letters show that no significant difference exists between them.
Ascorbic acid (AsA) and glutathione levels are presented in Fig. 10. Results indicated that all the treatments reduced AsA in both organs of BARS cultivar, however, NiNP_20 treatment raised ASA content by 6.27% (leaf) and 0.61% (root) over control. Similar reduction in AsA was recorded in root of SSB cultivar. By contrast, the leaf of SSB showed an increase in AsA by 8.81% (HP), 8.35% (NiNP_20), 5.56% (NiNP_40), and 4.68% (NiNO3_20). However, only decline was observed in NiNO3_40 (9.92%) as compared to those in control plants (Fig. 10A).
Compared with control, the contents of GSH, GSH+GSSG, enzymatic activity of GPX and GSH/GSSG ratio showed significant increase (p < 0.01) under all the treatments in both cultivars except for root of SSB where all these parameters were downregulated in NiNP_20 and NiNO3 treated plants at 20 and 40 mg/L, respectively (Fig. 10). Regarding GSSG, it was increased significantly (p < 0.001) in leaf of BARS under NiNP_40 (9.7%) and NiNO3_40 (8.9%), and in root of SSB under NiNP_20 (63.9%) and NiNO3_40 (16.8%) over their respective controls (Fig. 10F).
Non-enzymatic antioxidants
Ni in the form of nanoparticles improved the phenolics content as compared to bulk NiNO3 in both cultivars. In comparison to control, Ni application elevated the total phenolics content (TPC) in both organs of SSB with maximum increase recorded in root i.e., 29.29, 39.24, 31.23 and 1.04% in NiNP_20, NiNP_40, NiNO3_20 and NiNO3_40, respectively. However, in case of BARS, an increase in TPC was observed only in leaf by 14.21% (NiNP_20), 23.37% (NiNP_40), 12.29% (NiNO3_20), and 9.78% (NiNO3_40), respectively, over control (Fig. 11A). As a result of Ni accumulation, increase in total flavonoid content (TFC) was observed only in plants exposed to NiNO3_40 treatment by 29.2% (leaf) and 5.78% (root) relative to control in BARS cultivar. Conversely, leaf and root of SSB showed an increase in TFC under all treatments, only decline was observed under NiNO3_40 by 4.39% (leaf) and 1.35% (root) over control (Fig. 11B).
Effects of bulk and nano Ni on total phenol (A), total flavonoid (B), anthocyanin (C) and total carotenoid content (D) of BARS (Bars-09) and SSB (Sarsabz) cultivars of wheat under different treatments (Ctrl: control, Hydro: Hydro-priming, NiNP_20: NiO-NPs at 20 mg/L, NiNP_40: NiO-NPs at 40 mg/L, NiNO3_20: NiNO3 at 20 mg/L and NiNO3_40: NiNO3 at 40 mg/L). Lower case letters show statistically significant results among treatments within a cultivar at p < 0.05 according to Fisher’s least square difference (LSD) test. Treatments with similar letters show that no significant difference exists between them.
The results showed that all the treatments significantly (p > 0.01) increased the anthocyanin content (AC) in leaf of BARS (Fig. 11C). However, a remarkable decrease in the AC was observed in roots under NiNP_20 (49.46%), NiNP_40 (69.35%), NiNO3_20 (24.23%), and NiNO3_40 (28.46%), respectively. Similarly, an increase in AC was recorded in SSB under all treatments. The only decline was observed in leaf of plants treated with NiNO3_20 (0.93%) and NiNO3_40 (8.45%) with respect to control (Fig. 11C). Similar to AC, total carotenoid content (TCrC) showed an increasing trend in leaf of BARS cultivar, however, it showed downregulation in roots following Ni application. Ni in the form of nanoparticles enhanced TCrC to increase stress tolerance in both cultivars with maximum increment in BARS i.e., two-to four-folds maximum TCrC was measured in plants treated with NiO-NPs as compared to those in bulk NiNO3 treated plants (Fig. 11D).
Total antioxidant capacity, total reducing power, and DPPH scavenging activities
To investigate the impact of Ni in bulk as well as nano form on total antioxidant activity of wheat cultivars, we measured the total antioxidant capacity (TAC), total reducing power (TRP), and DPPH scavenging activity in control, hydro-primed and Ni treated plants. Pronounced variations were observed in antioxidant activity of both wheat cultivars under different treatments (Fig. 12). Both cultivars exhibited significant (p < 0.05) increase in total antioxidant capacity (TAC) following Ni application. In SSB, all the treatments upregulated the TAC in both organs (Fig. 12A). However, NiO-NPs lowered TAC in BARS cultivar by 5 and 8.9% in leaf and 2.75 and 1.6% in root at 20 and 40 mg/L, respectively, over control. Similarly, total reducing power (TRP) showed an increasing trend in both cultivars. However, only decline was observed in root of SSB by 16.55% (NiNO3_20) and 58.26% (NiNO3_40) with respect to control (Fig. 12B).
Effects of bulk and nano Ni on antioxidant activity of TAC (total antioxidant capacity) (A), TRP (total reducing power) (B), and DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) (C) of BARS (Bars-09) and SSB (Sarsabz) cultivars of wheat under different treatments (Ctrl: control, Hydro: Hydro-priming, NiNP_20: NiO-NPs at 20 mg/L, NiNP_40: NiO-NPs at 40 mg/L, NiNO3_20: NiNO3 at 20 mg/L and NiNO3_40: NiNO3 at 40 mg/L). Lower case letters show statistically significant results among treatments within a cultivar at p < 0.05 according to Fisher’s least square difference (LSD) test. Treatments with similar letters show that no significant difference exists between them.
DPPH is a free radical widely used to measure the ability of a compound or plant extract to scavenge the free radicals. Compared with control, plants exposed to NiNO3 at 20 and 40 mg/L reduced the DPPH scavenging activity by 9.52 and 9.88% in root of SSB cultivar (Fig. 12C). In contrast, an upward trend of scavenging activity was observed in both organs of BARS cultivar with maximum increment in plants treated with NiO-NPs at 40 mg/L (2.24% in leaf and 10.15% in root) relative to control.
Principal component analysis
Principal component analysis (PCA) was performed using antioxidants, secondary metabolites, and stress indicators to study the correlation between these components in leaf and root and to identify stress tolerant wheat cultivar. First (PC1) and second (PC2) principal components contributed to the total variation of 26.6% (among cultivars) and 25.1% (among parts) and 14.5% (among cultivars) and 13.9% (among parts), respectively (Fig. 13). The PC1 showed strong positive correlation with ascorbic acid, APX, AOX and anthocyanin. GSH, GSH+GSSG, GPX, DPPH%, protein, TAC, TPC, TRP, proline and SOD were the major indices utilized by the plants. However, other antioxidants also contributed significantly. Proline, TAC, TPC, and POD showed positive correlation and displayed maximum concentration in roots. Contrarily, protein, APX, Ascorbic acid, anthocyanin, and TSC were positively correlated and appeared maximum in leaf.
PCA analysis of antioxidants and secondary metabolites. PCA analysis for both cultivars (A), PCA analysis for root and leaf of both cultivars (B). SOD (superoxide dismutase), CAT (catalase), and POD (peroxidase), AscA (Ascorbic acid), APX (ascorbate peroxidase), AOX (ascorbate oxidase), GPX (glutathione peroxidase), GSH (glutathione), GSSG (glutathione disulfide), GSH+GSSG (total glutathione), TPC (total phenol content), TFC (total flavonoid content), Antho (anthocyanin) and TCrC (total carotenoid content), TSC (total soluble sugar content), MDA (malondialdehyde), H2O2, TAC (total antioxidant capacity), TRP (total reducing power), and DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate).
Discussion
Nickel is an essential micronutrient and is beneficial for plants at low concentration. Nonetheless, at high concentration it negatively affects plant growth and physiology10. Ni toxicity in crops has emerged as a worldwide threat to agricultural sustainability. Hence, researchers need to make strategies to improve Ni uptake and utilization in plants. In the present study, Ni was used in bulk as well as nano form to study their comparative impact on two high yielding wheat cultivars. B. baluchistanica leaf extract was used as a capping agent for synthesis of NiO-NPs that belongs to the family Berberidaceae (Fig. 14). It is easily available, economic and safe to use due to its potential bioactive components, antioxidants, and nutritional contents21.
The purity and size of nanoparticles was within the optimum range of similar NiO NPs40. UV–Vis spectra confirmed NiO-NPs synthesis with an absorption peak at 203 nm. The zeta potential of − 19.3 mV indicates moderate colloidal stability, with limited electrostatic repulsion leading to some aggregation. This aggregation of nanoparticles facilitates a gradual and controlled release in plants and soil, enhancing their availability over time while minimizing their toxicity. The PDI of 1.00 and a Z-average of 4307 nm from DLS suggest a highly polydisperse system, primarily due to van der Waals interactions and weak electrostatic stabilization, which is common in green-synthesized metal oxide nanoparticles41. Despite this, SEM and XRD analyses confirmed a primary particle size of 22 nm, highlighting the nanoscale nature of NiO-NPs. The discrepancy between DLS and solid-state imaging is expected, as DLS measures the hydrodynamic radius, including hydration layers and agglomerates, while SEM and XRD provide actual physical size. These findings confirm that while NiO-NPs aggregate in liquid suspensions, their nanoscale structure remains intact, making them suitable for agricultural applications where solid-phase interactions are more relevant than colloidal stability. Current study found that seed priming with Ni nanoparticles improved the growth and physiology of wheat. Results showed an increased uptake of Ni with increasing Ni concentration. NiNO3 exposure increased Ni accumulation in roots of both cultivars, while NiO-NP treatment increased Ni uptake in BARS cultivar, leading to maximum accumulation in shoot. The accumulation of Ni in roots inhibits the uptake of other essential nutrients leading to their deficiency in plants. Compared with bulk NiNO3, NiO-NPs enhanced root-to-shoot translocation of Ni, thus increasing its availability to plants. Previous studies also reported that nano Ni leads to more effective delivery of Ni2+ thus increasing its TF as compared to bulk Ni which causes slowest release of Ni2+ in plants6,18,19. Oukarroum, Barhoumi42 stated that NiO NPs as compared to bulk NiO are more soluble in aqueous solution which alters their physiochemical properties and increases their bioavailability.
In our study, hydropriming, nano and bulk Ni at lower concentration (20 mg/L) increased the germination percentage (GP) relative to control. Conversely, GP under NiNO3 and NiO-NP at 40 mg/L remained unchanged. In comparison with control and NiO-NPs, hydro-priming and NiNO3 accelerated the seedling growth at initial stages after germination but reduced it gradually. This reduction in shoot and root growth under bulk NiNO3 observed at the end of the experiment (after 28 days) can be attributed to negative effects of Ni on various metabolic reactions, reduced cell wall elasticity and increased oxidative stress43,44. Reduction in plant growth and biomass was more pronounced in SSB cultivar. Our results are in correlation with previous studies where high bulk Ni concentration reduced the growth and biomass in wheat9,44,45,46, and other plants47,48,49. Hydro-primed plants showed an increase in fresh and dry biomass as well as RWC but reduced the root and shoot growth relative to other treatments. In contrast, priming with NiO-NPs increased SL, RL, and fresh and dry biomass in both cultivars. All the treatments enhanced RWC over control in both cultivars. Maximum increment was observed in plants treated with NiO-NPs relative to NiNO3 (Fig. 14). Consistent with our results, the increase in plant growth and biomass under NPs has been reported in wheat23, Chinese cabbage seedling50 soyabean6 and Nigella arvensis 51.
Similar to phenotypic traits, photosynthetic parameters of wheat also improved under Ni supplementation. Ni constitutes the active site of urease which is a metalloenzyme directly involved in nitrogen metabolism1. It has been suggested that foliar application of urea with adequate supply of Ni improves N metabolism in crops by increasing the concentration of free amino acids which are helpful in N transport and storage2,52. In the present study, foliar applied urea increased the activity of leaf urease enzyme following an increase in Ni concentration in both cultivars with maximum increment recorded in NiO-NPs treated plants. Interestingly, high NiNO3 concentration did not pose a negative effect on urease enzyme, rather it raised the activity of this enzyme to two-folds than that observed in hydro-primed and control plants. This might be due to the availability of Ni required for urease activity, but stress induced by bulk Ni could be due to other physiological disruptions. Furthermore, concentration of ammonia and nitrates also enhanced after foliar application of urea under Ni supplication in both cultivars. Ammonia formed from hydrolysis of urea degrades into different free amino acids, which in turn increase secondary compound synthesis and positively regulate plant growth25,52. HP also showed an increase in the activity of urease enzyme and ammonia and nitrate content relative to control but less than that observed in Ni treated plants. Regarding chlorophyll content, NiNO3 and NiO-NPs raised the Chl a, b, and total chlorophyll content in BARS cultivar with maximum increment under NiO-NPs treatment. Conversely, only NiNP_40 raised chlorophyll content in SSB cultivar relative to control. Reduction in the chlorophyll pigments is associated with degradation of these pigments and inhibition of enzymes involved in pigment synthesis under heavy metal toxicity53. Previous studies also demonstrated decline in chlorophyll pigments under increased Ni concentration in wheat7,44,45. Moreover, NiO-NPs also upregulated the production of carotenoid pigments in both cultivars. However, plants exposed to NiNO3 treatment showed a decline in carotenoid content with respect to other treatments. Carotenoids are the pigment molecules and antioxidants that contribute to scavenging of oxidative species and protect photosynthetic reaction center from auto-oxidation due to intense light and abiotic stress54. The reduction in photosynthetic pigments under bulk NiNO3 treatment suggests Ni induced toxicity in wheat. In contrast, NiO-NPs presented positive effects on pigment synthesis and photosynthetic activity. Our results are in line with the previously reported studies regarding the effects of metallic nanoparticles on photosynthetic activity in wheat. Hussain, Rizwan23 and Rizwan, Ali53 stated that seed priming with silicon, zinc and iron NPs enhanced chlorophyll and carotenoid content, photosynthetic activity, growth, and biomass in wheat. Similarly, Siqueira Freitas, Wurr Rodak25 and Zhou, Jiang6 also found positive effects of Ni fertilization on photosynthesis and N assimilation in soyabean. Additionally, the present study revealed an increase in stomatal density (SD) (maximum in NiO-NPs treatment) and decrease in stomata size under Ni treatment. Moreover, SD was high on adaxial surface as compared to abaxial surface, while stomatal and aperture area were found maximum on abaxial surface, respectively. This increase in SD can enhance the CO2 influx while reduction in stomata size reduces the transpiration rate and thus avoid excessive water loss. Previously, many studies have shown that heavy metal stress increases SD in plants55,56 which may be a mechanism of plants adaptation to stress conditions.
To investigate the oxidative damage in wheat under bulk and nano Ni treatment, H2O2 and MDA content was measured in shoot and root of both wheat cultivars. Heavy metal toxicity escalates the level of ROS production, resulting in increased lipid peroxidation and formation of MDA content4. Previously, an increase in MDA content has been reported in wheat under Ni stress4,9,12. In the present study, an increase in MDA content was detected in shoot and root of both cultivars. On contrary, H2O2 was raised only in shoot of BARS and root of SBB which can be ascribed to greater accumulation of Ni and enhanced SOD activity in these parts of both cultivars. At lower levels, H2O2 acts as a signaling molecule, while at higher levels it triggers oxidative stress57. Previous studies also reported a rise in H2O2 in wheat under Ni application9,12. Increase in H2O2 and MDA was more pronounced under NiNO3 treatment as compared to NiO-NPs, HP, and control plants. Besides, NiO-NPs reduced the membrane permeability (MP) in both cultivars. Conversely, NiNO3 thrived leaf MP relative to control and hydro-primed plants. These results showed that NPs at lower concentration are more effective in protecting plant membranes. Our results are in line with the findings of Rai-Kalal and Jajoo58 who reported that ZnO NPs lowered the MDA content and membrane damage as compared to bulk ZnSO4 in wheat.
The accumulations of organic osmolytes such as proline and soluble sugars in plants are considered as important biochemical markers for stress evaluation. Proline protects plants from oxidative damage and helps maintain osmotic balance59. In the present study, treatment with bulk NiNO3 enhanced proline content like MDA in root and shoot of both cultivars. However, NiO-NPs exposed plants increased proline only in roots of BARS cultivar. Additionally, sugar content significantly increased in root and shoot under all treatments (maximum in bulk NiNO3 treatment). Similar to our results, an increase in sugar and proline content has been reported in wheat under Ni stress46,60. Moreover, Jahan, Guo4 observed increase in proline under Ni stress in tomato. Zhou, Jiang6 found an increase in proline content under bulk Ni while significant decline was observed in nano Ni treated soyabean. These results suggest that NiO-NPs mitigate stress in plants.
HM binds to the sulfhydryl group in proteins leading to structural damage and inhibition of protein function61. In the present study, all the treatments enhanced protein content in both cultivars except for root of SSB cultivar where bulk Ni reduced protein relative to control. Conversely, NiO-NPs exposed plants showed maximum increase in protein relative to bulk NiNO3. Consistently, Saleh, Kandeel46 reported stimulatory effect of Ni and Cd on protein at low concentration while an inhibitory effect at high concentration in leaf and root of wheat seedlings. Furthermore, Ni treatment triggered the antioxidative response. In our study, all the treatments lowered the activity of SOD enzyme in both cultivars. However, the root of SSB cultivar showed a significant increase in SOD activity under NiNO3 which can be attributed to greater accumulation of H2O2 in these plants. Moreover, an increase in the activity of POD was also observed under NiNO3 supplication. Under stress conditions, POD helps in H2O2 scavenging and increases lignin biosynthesis thus reducing cell wall plasticity62. A similar increase in the activity of SOD and POD has been recorded in wheat44,63 and soyabean6 under bulk Ni. Similar to POD, a remarkable increase in activity of AOX and APX was observed under Ni supplication with maximum increment recorded in NiNO3 treated plants in BARS cultivar. However, SSB cultivar showed a decline in APX with maximum reduction under NiNO3 treatment. Tehseen, Attia12 and Shafiq, Munawar44 found a remarkable increase in APX and POD activity in wheat under Ni treatment. In present study, NiNO3 treatment declined CAT activity, while it was improved under NiO-NPs treatment. Correspondingly, Gajewska and Skłodowska43 also reported reduction in CAT activity under 100uM Ni in wheat. Our results are in agreement with the findings of Wang, Chen64, where an increase in SOD, POD and APX while decline in CAT activity was observed under Ni toxicity in Aegilops tauschii seedlings. Moreover, Shafiq, Munawar44 also reported a similar trend in enzymatic activity under Ni and Cd stress in wheat seedlings. Increase in activity of antioxidants helps to cope with the stress induced by Ni treatment. Upregulation of SOD, POD, AOX and APX while decline in CAT activity was more pronounced under NiNO3 treatment relative to NiO-NPs, respectively, which suggests increased accumulation of ROS in bulk NiNO3 treated plants. Zhou, Jiang6 found that nano Ni can maintain a constant Ni source over a longer period thus reducing Ni toxicity as compared to rapidly available large doses of bulk Ni.
Apart from enzymatic antioxidants, low molecular mass non-enzymatic antioxidants also control the level of ROS in plant tissues. Secondary compounds such as phenols, flavonoids, anthocyanin, ascorbic acid, and glutathione were measured in the root and shoot of both cultivars. AsA-GSH cycle scavenges stress induced ROS in plant cells. GPX is an enzyme that uses GSH as a substrate to reduce H2O2, organic peroxides and lipid peroxides thus alleviating oxidative stress37,65. All the treatments significantly increased the GPX activity, GSH and GSH/GSSG ratio in both cultivars except for SSB root that showed decline in above mentioned parameters while increase in the level of GSSG under NiNO3 treatment. Like GPX, APX activity was also downregulated in SSB cultivar. Likewise, slight reduction in AsA content relative to control plants was observed in both wheat cultivars under Ni treatment. A similar decline in AsA and GSH has been reported in wheat seedling9, mustard plant65 and tomato seedling4 under Ni stress. This reduction in GSH and AsA content might be associated with their rapid consumption due to increased oxidative stress. Maintenance of high AsA and GSH/GSSG ratio is crucial to enhance tolerance and lower oxidative stress in plants65. Decline in these components observed in SSB cultivar suggests least Ni tolerance in this cultivar as compared to BARS cultivar.
POD consumes phenolic compounds as substrate and catalyzes their oxidation to convert H2O2 into H2O and O266. Additionally, phenolic compounds can enhance heavy metal induced stress tolerance in plants by neutralizing ROS67. In the present study, compared with nano Ni, NiNO3 treatment showed less increase in total phenolic compounds (TPC) over control in shoot of BARS and root of SSB. This reduction in TPC in NiNO3 treated plants might be due to enhanced level of POD activity in these treatments. Besides their role as superoxide scavengers, flavonoids improve plant photosynthetic efficiency and are involved in signaling pathways67. Similar to phenols, flavonoid content showed increase under all the treatments (maximum in NiO-NPs relative to NiNO3 and control) in both root and shoot of SSB cultivar. In contrast, only NiNO3 at 40 mg/L raised the flavonoid content in both organs of BARS cultivar. In the present study, phenol and flavonoid content showed maximum concentration in roots as compared to shoot which can be attributed to increased Ni accumulation in roots.
Anthocyanins are the effective secondary metabolites that play role in photoprotection, ion chelation, ROS scavenging and osmoregulation which protect plants under biotic and abiotic stresses68. In our study, bulk NiNO3 raised the anthocyanin content (AC) in both organs relative to control with maximum increase recorded in leaf. Moreover, plants exposed to NiO-NPs further enhanced AC synthesis in both cultivars. Jahan, Guo4 found a similar increase in phenol, flavonoid, and anthocyanin content in tomato seedling under Ni stress. Conversely, Mustafa, Raja54 found reduction in phenol and flavonoid content in wheat under salinity stress. This reduction might be due to enhanced oxidative stress and reduced plant tolerance to that stress.
Analogous to phenols and flavonoids, total antioxidant capacity (TAC) of SSB plants exposed to nano and bulk Ni resulted in a remarkable increase compared with HP and control plants in both organs. Relative to this, only NiNO3 treatment raised TAC in BARS cultivar. Among treatments, NiNO3 exposed plants showed maximum TAC relative to NiO-NP treatment with roots showing maximum values as compared to shoots. Contrary to TAC, DPPH scavenging activity showed upward trend in both root and shoot of BARS cultivar under all the treatments with maximum activity recorded in NiO-NPs treated plants. Conversely, NiNO3 at both concentrations and NiO-NPs at 40 mg/L declined DPPH (relative to control) in SSB cultivar roots. Previous studies have reported a direct correlation between high DPPH scavenging activity and increased stress tolerance in wheat67,69. Our results indicate that BARS cultivar had maximum capacity to combat oxidative stress induced by Ni. Regarding total reducing power (TRP), plants exposed to NiO-NPs and NiNO3 showed similar trend as DPPH free radical scavenging activity exhibited in both cultivars. Lowest TRP was observed in roots of SSB cultivar treated with NiO-NPs at 40 mg/L and NiNO3 at both concentrations which also had lower DPPH relative to control. Consistent with our results, Javed, Usman38 found that ZnO NPs at 10 mg/L concentration raised phenols, flavonoids, TAC, TRP and DPPH. However, their results showed that 1000 mg/L ZnO lowered these parameters in S. rebaudiana leaves. Chahardoli, Karimi51 evaluated the effects of NiO and Al2O3 NPs on TAC, TRP and DPPH activity of N. arvensis. They showed an increase in these parameters of plants exposed to 1000 mg/L of both NPs while reported decline at higher concentrations.
Conclusion
The current study performed to evaluate the comparative impact of bulk and nano Ni seed priming on growth and physiology of two wheat cultivars concluded that Ni at low concentration in the bulk as well as nano form improved germination and physiology of wheat. However, bulk NiNO3 at 40 mg/L presented slight deleterious effects on growth and biomass of both wheat cultivars. Conversely, NiO-NPs at both concentrations upregulated the growth and photosynthetic activity of wheat. Furthermore, hydro-priming also improved the germination rate, plant biomass and photosynthesis but less than that observed in NiO-NP treated plants. Besides, it was also noted that Ni treatment increased the stomatal density to enhance CO2 influx and reduce transpiration. Decline in growth and biomass while increase in H2O2, MDA and stress markers like soluble sugars, proline and antioxidants were pronounced more in SSB cultivars as compared to BARS cultivar. Overall, these findings suggest that seed priming with nano Ni at 20 and 40 mg/L positively regulates the photosynthetic activity, pigment synthesis, and N assimilation by increasing urease enzyme activity and the contents of ammonia and nitrates and alleviates bulk Ni induced toxicity by reducing oxidative stress in wheat. Further study, however, needs to investigate the impact of nano Ni on grain yield and Ni uptake in field experiments.
Data availability
All the raw data in this research can be obtained from the corresponding author javed89qau@gmail.com upon reasonable request.
References
Dixon, N. E. et al. Jack bean urease (EC 3.5. 1.5). Metalloenzyme. Simple biological role for nickel. J. Am. Chem. Soc. 97(14), 4131–4133 (1975).
Kutman, B. Y., Kutman, U. B. & Cakmak, I. Nickel-enriched seed and externally supplied nickel improve growth and alleviate foliar urea damage in soybean. Plant Soil 363, 61–75 (2013).
Arkoun, M. et al. A physiological and molecular study of the effects of nickel deficiency and phenylphosphorodiamidate (PPD) application on urea metabolism in oilseed rape (Brassica napus L.). Plant Soil 362, 79–92 (2013).
Jahan, M. S. et al. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 197, 110593 (2020).
Hussain, S. et al. Metal toxicity and nitrogen metabolism in plants: An overview. Carbon Nitrogen Cycl. Soil 221–248 (2020).
Zhou, P. et al. Nickel oxide nanoparticles improve soybean yield and enhance nitrogen assimilation. Environ. Sci. Technol. 57(19), 7547–7558 (2023).
Khan, M. A. et al. Effect of nickel on the germination and biochemical parameters of two rice varieties. Fresenius Environ. Bull. 29(2), 956–963 (2020).
Amjad, M. et al. Nickel toxicity induced changes in nutrient dynamics and antioxidant profiling in two maize (Zea mays L.) hybrids. Plants 9, 5. https://doi.org/10.3390/plants9010005 (2020).
Anas, M. et al. Histological and ionomics assessment to elucidate tolerance mechanisms of nickel-tolerant and sensitive cultivars of bread wheat (Triticum aestivum L.). Plant Stress 10, 100277 (2023).
Li, J. et al. Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L. Front. Plant Sci. 12, 710093 (2021).
Yılmaz, H. et al. DNA fragmentation, chromosomal aberrations, and multi-toxic effects induced by nickel and the modulation of Ni-induced damage by pomegranate seed extract in Allium cepa L. Environ. Sci. Pollut. Res. 30(51), 110826–110840 (2023).
Tehseen, M. et al. Biochemical, physiological, and nutrient acquisition response of wheat cultivars to nickel and vanadium toxicity. J. Plant Growth Regul. 43(12), 4532–4555 (2024).
Ameen, N. et al. Biogeochemical behavior of nickel under different abiotic stresses: Toxicity and detoxification mechanisms in plants. Environ. Sci. Pollut. Res. 26(11), 10496–10514 (2019).
Yusuf, M. et al. Nickel: An overview of uptake, essentiality and toxicity in plants. Bull. Environ. Contam. Toxicol. 86(1), 1–17 (2011).
Rubio, M. et al. Cadmium and nickel accumulation in rice plants. Effects on mineral nutrition and possible interactions of abscisic and gibberellic acids. Plant Growth Regul. 14(2), 151–157 (1994).
Mustafa, A. et al. Nickel (Ni) phytotoxicity and detoxification mechanisms: A review. Chemosphere 328, 138574 (2023).
Lamba, K. et al. Heat stress tolerance indices for identification of the heat tolerant wheat genotypes. Sci. Rep. 13(1), 10842 (2023).
Rhaman, M. S. et al. Seed priming with nanoparticles: An emerging technique for improving plant growth, development, and abiotic stress tolerance. J. Soil Sci. Plant Nutr. 22, 1–16 (2022).
Bezerra de Oliveira, J. et al. Fate of nickel in soybean seeds dressed with different forms of nickel. Rhizosphere 21, 100464 (2022).
Uddin, S. et al. Bioprospecting roots, stem and leaves extracts of Berberis baluchistanica Ahrendt. (Berberidaceae) as a natural source of biopharmaceuticals. J. Taibah Univ. Sci. 16(1), 954–965 (2022).
Uddin, S. et al. Green synthesis of nickel oxide nanoparticles from Berberis balochistanica stem for investigating bioactivities. Molecules 26(6), 1548 (2021).
Safdar, L. B. et al. Genetic dissection of Ni toxicity in a spring wheat diversity panel by using 90 K SNP array. Curr. Plant Biol. 24, 100175 (2020).
Hussain, A. et al. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 26, 7579–7588 (2019).
Anas, M. et al. Manganese oxide nanoparticles elicit cadmium tolerance in wheat seedlings by ionomic and phenomic adjustment, regulation of AsA–GSH pathway, cellular thickness recovery, and antioxidant modulation. Environ. Sci. Nano 11(11), 4588–4605 (2024).
Siqueira Freitas, D. et al. Hidden nickel deficiency? Nickel fertilization via soil improves nitrogen metabolism and grain yield in soybean genotypes. Front. Plant Sci. 9, 614 (2018).
Cataldo, D. et al. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 6(1), 71–80 (1975).
Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125(1), 189–198 (1968).
Leopold, A. C., Musgrave, M. E. & Williams, K. M. J. P. P. Solute leakage resulting from leaf desiccation. Plant Physiol. 68(6), 1222–1225 (1981).
Hedge, J., Hofreiter, B. & Whistler, R. J. A. P. Carbohydrate Chemistry 17 (Academic Press, 1962).
Dhindsa, R. S., Plumb-Dhindsa, P. & Thorpe, T. A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32(1), 93–101 (1981).
Chance, B. & Maehly, A. J. G. L. Assay of catalases and peroxidases. Methods Enzymol. 2, 764–775 (1955).
Aebi, H. [13] Catalase in vitro. In Methods in Enzymology 121–126 (Elsevier, 1984).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22(5), 867–880 (1981).
Diallinas, G. et al. Melon ascorbate oxidase: Cloning of a multigene family, induction during fruit development and repression by wounding. Plant Mol. Biol. 34, 759–770 (1997).
Peterson, G. L. J. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83(2), 346–356 (1977).
Fadhel, D. H. J. Spectrophotometric determination of ascorbic acid in aqueous solutions. Al-Nahrain J. Sci. 15(3), 88–94 (2012).
Anderson, M. E. [70] Determination of glutathione and glutathione disulfide in biological samples. In Methods in Enzymology 548–555 (Academic Press, 1985).
Javed, R. et al. Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol. Biochem. 110, 94–99 (2017).
Hare, P. D. & Cress, W. A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 21(2), 79–102 (1997).
Kondak, S. et al. Nickel oxide nanoparticles induce cell wall modifications, root anatomical changes, and nitrosative signaling in ecotypes of Ni hyperaccumulator Odontarrhena lesbiaca. Environ. Pollut. 341, 122874 (2024).
Aryani, N. L. D. et al. Development, characterization, molecular docking, and in vivo skin penetration of coenzyme Q10 nanostructured lipid carriers using tristearin and stearyl alcohol for dermal delivery. J. Basic Clin. Physiol. Pharmacol. 32(4), 517–525 (2021).
Oukarroum, A. et al. Toxic effects of nickel oxide bulk and nanoparticles on the aquatic plant Lemna gibba L. BioMed Res. Int. 2015, 501326 (2015).
Gajewska, E. & Skłodowska, M. Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 20(1), 27–36 (2007).
Shafiq, A. et al. Health risk assessment of bread wheat grown under cadmium and nickel stress and impact of silicic acid application on its growth, physiology, and metal uptake. Environ. Sci. Pollut. Res. 31(43), 55535–55548 (2024).
Uruç Parlak, K. Effect of nickel on growth and biochemical characteristics of wheat (Triticum aestivum L.) seedlings. NJAS Wagening. J. Life Sci. 76(1), 1–5 (2016).
Saleh, S. R. et al. Wheat biological responses to stress caused by cadmium, nickel and lead. Sci. Total Environ. 706, 136013 (2020).
Amjad, M. et al. Comparative physiological and biochemical evaluation of salt and nickel tolerance mechanisms in two contrasting tomato genotypes. Physiol. Plant. 168(1), 27–37 (2020).
de Oliveira, J. B. et al. Fate of nickel in soybean seeds dressed with different forms of nickel. Rhizosphere 21, 100464 (2022).
Helaoui, S. et al. Physiological, biochemical and transcriptomic responses of Medicago sativa to nickel exposure. Chemosphere 249, 126121 (2020).
Chung, I.-M., Venkidasamy, B. & Thiruvengadam, M. Nickel oxide nanoparticles cause substantial physiological, phytochemical, and molecular-level changes in Chinese cabbage seedlings. Plant Physiol. Biochem. 139, 92–101 (2019).
Chahardoli, A. et al. Effects of engineered aluminum and nickel oxide nanoparticles on the growth and antioxidant defense systems of Nigella arvensis L. Sci. Rep. 10(1), 3847 (2020).
Witte, C.-P. Urea metabolism in plants. Plant Sci. 180(3), 431–438 (2011).
Rizwan, M. et al. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214, 269–277 (2019).
Mustafa, N. et al. Exogenous application of green titanium dioxide nanoparticles (TiO2 NPs) to improve the germination, physiochemical, and yield parameters of wheat plants under salinity stress. Molecules 27, 4884. https://doi.org/10.3390/molecules27154884 (2022).
Nasirzadeh, L. et al. Foliar-applied selenium nanoparticles can alleviate soil-cadmium stress through physio-chemical and stomatal changes to optimize yield, antioxidant capacity, and fatty acid profile of wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 22(2), 2469–2480 (2022).
Haworth, M. et al. Allocation of the epidermis to stomata relates to stomatal physiological control: Stomatal factors involved in the evolutionary diversification of the angiosperms and development of amphistomaty. Environ. Exp. Bot. 151, 55–63 (2018).
Nazir, F., Fariduddin, Q. & Khan, T. A. J. C. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 252, 126486 (2020).
Rai-Kalal, P. & Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 160, 341–351 (2021).
Bates, L. S. et al. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973).
Gulzar, N. et al. Nickel induced modulations in phosphorus metabolism and other growth parameters of wheat. Int. J. Res. 11(1), 1–4 (2020).
Feng, Z. et al. Recent advances in metabolomics for studying heavy metal stress in plants. TrAC Trends Anal. Chem. 143, 116402 (2021).
Jaleel, C. A. et al. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 31, 427–436 (2009).
Pishchik, V. N. et al. Epiphytic PGPB Bacillus megaterium AFI1 and Paenibacillus nicotianae AFI2 improve wheat growth and antioxidant status under Ni stress. Plants 10, 2334. https://doi.org/10.3390/plants10112334 (2021).
Wang, N., Chen, H. & Tian, Y. Effects of nickel, lead, and copper stress on the growth and biochemical responses of Aegilops tauschii seedlings. Sci. Rep. 14(1), 24832 (2024).
Zaid, A. et al. Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol. Environ. Saf. 180, 575–587 (2019).
Muñoz-Muñoz, J. et al. Enzymatic and chemical oxidation of trihydroxylated phenols. Food Chem. 113(2), 435–444 (2009).
Kiani, R., Arzani, A. & Mirmohammady Maibody, S. A. M. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front. Plant Sci. 12, 646221 (2021).
Tereshchenko, O. Y. et al. Anthocyanin pigmentation in Triticum aestivum L.: Genetic basis and role under abiotic stress conditions. J. Stress Physiol. Biochem. 8(3), S16 (2012).
Sadak, M. S. et al. Exogenous aspartic acid alleviates salt stress-induced decline in growth by enhancing antioxidants and compatible solutes while reducing reactive oxygen species in wheat. Front. Plant Sci. 13, 987641 (2022).
Acknowledgements
The authors extend their appreciation to the Vice Deanship of Scientific Research Chairs, King Saud University, Saudi Arabia for funding this research work; Research Chair of “Kayyali Chair for Pharmaceutical Industries”.
Author information
Authors and Affiliations
Contributions
S.B., performed the experimental work, data analysis and wrote original draft, S.U., helped in nanoparticle synthesis and reviewed the manuscript, M.A., and M.E., M.M., J.I., and S.K., contributed to writing manuscript, performed statistical data analysis and data visualization. U.M.Q., designed and supervised the research work. M.K and F.K.A comprehensively reviewed the manuscript and provided funds. TM provided the resources and supervision. All authors made valuable revisions and edited the manuscript and approved the last version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The experiment was performed keeping in mind the ethical values and guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bibi, S., Uddin, S., Anas, M. et al. Investigating the role of bulk and nano nickel in amelioration of morphophysiology and photosynthetic activity of Triticum aestivum L.. Sci Rep 15, 20412 (2025). https://doi.org/10.1038/s41598-025-98964-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98964-3