Abstract
Biodegradable solid polymer electrolytes (BSPEs) have gained significant attention due to their exceptional processability, safety, and flexibility. This work presents the development of sodium ion (Na +) conducting ternary blended (BSPEs) using a standard solution casting technique. Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) validated the complete salt dissociation and demonstrated the formation of polymer-salt complexes. The deconvoluted XRD spectra revealed the degree of crystallinity (\({X}_{c}\)) of electrolytes where the sample incorporated 40 wt% of NaSCN salt content (STC4) was found to be the lowest \({X}_{c}\) value. The deconvoluted FTIR spectra were used to estimate ionic transport parameters of diffusion coefficient (\(D\)), ion mobility (\(\mu\)), and carrier density (\(n\)). Ionic conductivity and electrical properties of electrolyte samples were investigated by electrochemical impedance spectroscopy (EIS). The EIS results were fitted with electrical equivalent circuits to understand the electrical behavior of the films. The highest DC conductivity value (\({\sigma }_{DC}\)) of (2.74 × 10−6 S/cm) was achieved for the STC4 sample, attributed to its highest amorphous region and carrier density. The dielectric studies proved beneficial in distinguishing the areas attributed to molecular polarizations and electrodes. The reduction of relaxation time is indicated by shifting loss tangent peaks (tan δ) toward high frequency ranges. According to dielectric relaxation studies, the appearance of peaks confirmed non-Debye type behavior. Distinct areas attributed to the effects of electrode polarization and (\({\sigma }_{DC}\)) are seen in AC conductivity (\({\sigma }_{AC}\)) spectra.
Similar content being viewed by others
Introduction
An electrolyte is a material that forms a conductive solution upon being dissolved in water. The electrolyte is considered an essential element in electrochemical devices. There is a substantial use of conductive solutions in electrical devices such as supercapacitors and batteries. The ionic conductivity of liquid electrolytes is greater, providing enhanced applicability1. Nonetheless, it also presents various challenges, including leakage, electrode reaction, corrosion, and potential explosions in some cases, which could be dangerous to both human health and the environment. Solid-state polymer electrolytes have been extensively investigated as alternatives to liquid electrolytes due to their potential to mitigate leakage issues. Solid polymer electrolytes (SPEs) have diverse applications, extending from small scale manufacturing of commercial secondary lithium-ion battery, commonly referred to as rechargeable battery, to sophisticated high-energy electrochemical devices, including, fuel cells, sensors, and electrochromic windows2,3. It is crucial to conduct research aimed at achieving outstanding solid polymer electrolytes that exhibit moderate ionic conductivity, high mechanical stability, remarkable electrochemical stability, along with high transport numbers to create the next generation of advanced energy systems. SPE consists of complexes formed by electrostatically interacting between functional groups of polymers with salts, achieved by dissolving inorganic salts into macromolecular chains of polymer containing Lewis’s base, which coordinates the cations4. Numerous advantages are offered by SPEs, such as improved safety, cost-effectiveness, superior thermal stability, and leak-proof characteristics in addition to simplicity in shaping into desired forms and sizes.
Biodegradable host material, whether synthetic or natural, enhances the environmental sustainability of energy storage systems. However, some disadvantages are associated with synthetic polymers, such as elevated costs. Another problem that frequently arises is the technological waste accumulation, which poses significant challenges for the environment, as the materials are not easily biodegradable in natural settings. Therefore, it is crucial to develop novel materials that are non-toxic, safe, and environmentally friendly. Thus, utilizing biodegradable polymer host in solid-state systems can mitigate environmental concerns due to their eco-friendly and short degradation period5,6. Different biodegradable polymers have been employed as the host material for developing solid-state energy systems, including polyvinyl alcohol (PVA)7, chitosan (CS)8, gelatin9, starch10, and cellulose11. These specialized polymers have many advantages, such as a lightweight design, abundant in nature, high-film-forming abilities, and outstanding mechanical characteristics.
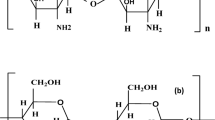
Polyvinyl alcohol (PVA) as a synthetic biodegradable polymer has gained a lot of interest due to its exceptional dielectric strength, sufficient charge storage capacity, and water solubility. It can be derived from hydrolysis of polyvinyl acetate. Its backbone consists of the carbon atom, hydrogen, and hydroxyl attached to second carbon with the chemical formula [CH2 CH (OH)]n. The hydroxyl functional group of PVA may potentially enabling hydrogen bonding and so promoting the formation of polymer electrolytes12,13. Chitosan (CS) is a widely used biopolymer in energy storage systems, environmental practices, and biomedical applications. The cationic properties of CS making it significant in both biomedical and environmental contexts compared to other polysaccharide polymers. Structurally, CS comprises multiple functional groups that incorporate oxygen. This feature renders CS effective in interacting with inorganic salts due to the presence of lone pair electrons on the oxygen atom8,14.
Gelatin is another biopolymer obtained from the hydrolysis of collagen derived from animal bones and skins. It also largely comprises proteins with amino acid sequences linked by peptide bonds such as hydroxyproline, proline, and glycine. It is noteworthy that gelatin displays a high degree of complexity owing to its distinctive tripe-helix chain configuration as a protein compared to synthetic polymer. Furthermore, animal skins and bones are frequently seen as waste from the food industry despite their excellent source of gelatin15. A substantial quantity of collagen is present in fish skin, which is marketed as a food-grade gelatin. Nevertheless, collagen composition derived from cold water fish skin has lower proline and hydroxyproline residue quantities. Also, gelatin derived from marine sources exhibited poor functional qualities, including gel strength and gelling temperature compared to those of mammalian sources16.
Despite its scientific potential, SPEs exhibit several limitations, including a significant propensity to crystallize, considerably decreased ionic conductivity, and a tendency for the ionic guest species to precipitate at elevated salt concentrations9. Polymer blends serve as a successful technique to improve the flexibility and conductivity of polymer electrolytes. This approach offers significant advantages over alternative methods due to its capacity to thoroughly regulate physical parameters influencing the miscibility of matrix composition. It has increasingly emerged as a substantial method to enhance the cost-performance ratio of commercial products. Moreover, the characteristics of the host polymer could be enhanced through blending polymers, leading to better overall performances. Some prevalent interactions like hydrogen bonding, dipole–dipole, and ionic interactions take place during the blending procedure17,18,19. Furthermore, fish skin gelatin (FSG) has a very high-water solubility and comparatively poor mechanical properties, including low tensile strength, which could be enhanced through blending with other biodegradable polymers20.
Among various cations, sodium stands out for its abundance and cost-effectiveness in comparison to lithium. Moreover, the flexibility of sodium metal enhances the stability of the interface between the electrodes and electrolytes in solid-state ionic systems21. Since there is a finite storage of lithium on Earth, scientists are considering sodium as a viable option as a consequence of its cheap production costs and widespread availability22. Due to their numerous benefits, PVA, CS, and FSG are frequently utilized in the development of polymer blend electrolytes separately. The intensive survey of literature reveals that there is no or lack of reports about ternary host polymers for electrolyte preparation. To the best of our knowledge, this work will open a new approach based on ternary polymers for polymer electrolytes. This work presents the design and characterization of biodegradable ternary blended SPEs based on PVA-CS-FSG incorporated in various sodium thiocyanate (NaSCN) concentrations. Further research on this exciting topic is necessary to comprehend the physical properties of SPEs. The distinctive uses of solid polymer electrolytes, such as in batteries and electrical double-layer capacitors, render this topic more appealing to researchers aiming to address fundamental issues related to liquid electrolytes. Alongside polymer electrolytes, glassy systems comprising transition metal oxides (TMOs) were intensively investigated for their potential in electrochemical device applications, but it has some drawbacks as they need high-temperature production, limited flexibility and processability in addition to their toxicity. Moreover, in TMOs doped glassy systems, conduction mainly occurs via small polaron hopping between metal ions, while in SPE systems, ion transport occurs by salt dissociation and segmental polymer motion23,24. This study outlines measures undertaken to accomplish the following objectives:
-
(i)
To design sodium ion conducting ternary blended SPEs by a simple and cost-effective method.
-
(ii)
To characterize a novel SPEs and all factors influencing ion transport characteristics within other physicochemical aspects.
-
(iii)
To determine the most effective composition to be used in electrochemical devices.
-
(iv)
To identify non-Debye type relaxation process as predominant relaxation mechanisms across diverse compositions.
Thereby, this study finding confirmed that selecting an appropriate polymer type for blending with PVA ensures the development of SPE with desired ion transport parameters.
Materials and methods
Materials
The present ternary blended solid polymer electrolytes used polyvinyl alcohol (PVA) with a <Mn> value ranged from 89,000 to 98,000 g/mol (assay 99 + %), chitosan (CS) with a low molecular weight value, and fish skin gelatin (FSG) polymers sourced from Sigma-Aldrich. All the raw materials were used as its form without further purification.
Polymer electrolyte fabrication
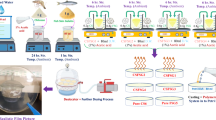
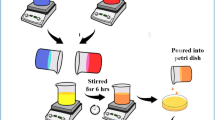
Using solution casting methodology, a ternary polymer blend electrolytes were fabricated in two stages. (i) fabricating ternary polymer blend solutions and (ii) incorporating NaSCN as a salt dopant into a prepared polymer blend solution. At first, for each system, 0.5 g (50%) of polyvinyl alcohol in 30 mL distilled water was dissolved at ~ 90 °C for 2 h. Simultaneously, 0.3 g (30%) of chitosan was dissolved in 18 mL of 1% acetic acid solution at room temperature for around 24 h, and 0.2 g (20%) of fish skin gelatin was dissolved in 12 mL distilled water at room temperature for 1 h. Following that, the separate solutions were mixed and stirred continuously for about 2 h to obtain ternary polymer blend solution. Then, various weight percent ratio of sodium thiocyanate (NaSCN) dopant salt was incorporated in to the blend solutions ranged from 10 to 50 wt% in steps of 10 under continuous stirring for 1 h as portrayed in Fig. 1. The samples were coded as STC1, STC2, STC3, STC4, and STC5 incorporated 10, 20, 30, 40, and 50 wt% of NaSCN doped salt. After casting the solutions onto various labeled plastic Petri dishes, they were allowed to evaporate slowly at RT for a 15 days period. To guarantee complete dryness of the films, they were located a desiccator containing silica gel for a week. Ultimately, fully dry flexible films with a thickness of range of 0.015–0.023 mm were produced, rendering them suitable for characterization.
Characterization techniques
The X-ray diffraction study for the STC samples were conducting via an Angstrom Advance Inc (ADX 2700) diffractometer (Massachusetts, USA) at a wavelength of 1.5406 Å. The 2θ angles were meticulously recorded from 10° to 70°, attaining a precise resolution of 0.02°. The deconvoluted XRD spectra was figured out to evaluate degree of crystallinity (\({X}_{c}\)) of the STC electrolytes using OriginPro 2025 software (Gaussian function).
The FTIR spectra for the STC samples were conducted using the FTIR spectrophotometer (BRUKER-TENSOR-27, Germany). The study was carried out throughout a wavenumber range spanning from 600 to 4000 cm−1, while ensuring a resolution of 1.93 cm−1. Furthermore, the deconvoluted FTIR spectra was determined to estimate ionic transport parameters of diffusion coefficient (\(D\)), ion mobility (\(\mu\)), and carrier density (\(n\)) using OriginPro 2025 software.
The impedance properties of the STC electrolytes were assessed employing an LCR meter (E4980A Precision Keysight, California, USA), measuring real (\({Z}_{r}\)) and imaginary (\({Z}_{i}\)) components of complex impedance (\({Z}^{*}\)) spectroscopy. The analysis was performed at room temperature over a frequency range of (100 Hz to 2 MHz) with an applied small voltage (10 mV). The made-up samples have been sliced into little circular pieces within a diameter of 2 cm. Moreover, sample thickness was assessed at various points across the film, measuring between 0.015 to 0.023 cm with a vernier micrometer. It was then positioned between two stainless-steel (SS) electrodes and forced under a spring pressure to guarantee optimal contact. Following the cell arrangement, the device was connected to a computer to extract the data, then a Nyquist plot of (\({Z}_{r}\) Vs \({Z}_{i}\)) of complex impedance (\({Z}^{*}\)) were obtained, as demonstrated in Fig. 2.
Results and discussion
X-ray diffraction study
The XRD technique has been nominated as a powerful tool in the field of material science and engineering. It relies on the capacity of crystals to diffract X-rays in a distinctive way, enabling an accurate examination of the structure of crystalline phases25. It is considered an effective approach for investigating the structural characteristics and elucidating the overall conductivity trends of solid polymer electrolytes. The XRD pattern for the STC electrolyte samples containing varying salt concentrations is presented in Fig. 3 with 10% smoothing in OriginLab. In essence, a wide peak in an X-ray diffraction pattern indicates an amorphous character. Meanwhile, a sharp peak indicates a crystalline nature of the samples26,27.
PVA is well-known for its semicrystalline structure due to the presence of several crystalline peaks and some broad amorphous peaks. It exhibited a strong crystallographic reflection at 2θ around 23° and a weak peak at approximately 2θ around 43°28.
Two crystalline peaks in pure CS were located close to 2θ ~ 15° and ~ 20°, which were associated with the (110) and (120) planes. Hydrogen bonding between and inside molecules is responsible for these crystalline peaks. Further evidence of the general semicrystalline nature of pure CS was provided by the observation of two amorphous brad peaks in the 2θ = 20° to 36°29.
Two distinct diffraction peaks in the XRD pattern of FSG at 2θ ~ 10° representing gelatin’s crystalline triple helix structure, and 2θ ~ 23.5° indicating an amorphous phase suggesting a semicrystalline nature of FSG20.
Typically, hydrogen bonding takes place when an electron-deficient hydrogen atom bonds with atoms that possess high electronegativity, such as oxygen (O), nitrogen (N), and fluorine (F) atoms. (X–H–Y) serves as a straightforward representation of intermolecular hydrogen bonding where (X and Y) are both highly electronegative elements possessing electron pairs30. It is noteworthy that the cations and the oxygen and nitrogen atoms of polymers engage in indications via dative bonds31. It is interesting to note that a large crystalline hump can be seen with a center 2θ = 21.2° for the STC1, and STC2 samples, denoting a minor spatial arrangement of the chains and robust intermolecular hydrogen bonding. Whereas, the hump broadened and gradually decreased in its intensity up to STC4, signifying the most amorphous structure. Following that, several sharp peaks appeared in the STC5 sample due to the re-association of NaSCN salt at high salt concentrations. The electrostatic interactions between blended polymer functional groups and (Na +) cations from the NaSCN salt notably reduced hydrogen bonding, thereby enhancing amorphous character and boosting effective complexation. However, adding 50 wt% of NaSCN again caused a significant increase in hollow intensity by appearing narrow additional crystalline peaks. This phenomenon is corresponded with the association of free ions, as the host capacity for accommodation is restricted at such elevated salt levels32,33.
In the current study, the percentage of crystallinity of STC samples-based polymer electrolytes was computed to determine the degree of crystallinity, and the results are shown in Table 1. The deconvoluted XRD spectra for the STC samples are presented in Fig. 4. The degree of crystallinity (\({X}_{c}\)) was calculated by isolating the crystalline region (\({A}_{c}\)) and the amorphous region (\({A}_{a}\)) using the following equation34:
It is noticeable that STC4 displayed the lowest degree of crystallinity among the STC electrolyte systems. Including NaSCN salt significantly decreased the degree of crystallinity of host polymers. According to earlier studies, ionic conductivity values follow the general trend of crystallinity. A system with the lowest salt concentration showed the lowest ionic conductivity and the highest degree of crystallinity. On the other hand, a system with 40 wt% salt contents would have the minimum (\({X}_{c}\)) value, which might lead to the highest ionic conductivity15. High DC conductivity is expected from the sustem which it have high content of amorphous phases and thus the system with lowest Xc will exhibit a high ion transport and consequently high DC conductivity.
FTIR study
The results of Fourier transform infrared spectroscopy (FTIR) for the STC based polymer-salt complex systems are illustrated in Fig. 5. Clear alternations are seen in the spectrum characteristics of NaSCN salt systems. A notable broad peak at a wavenumber of ~ 3300 cm−1 is attributed to OH and NH stretching bands. A pronounced peak at a wavenumber of approximately 2930 cm−1 indicates asymmetric stretching of the C-H band in the alkyl groups35. Nonetheless, its intensity changed and slightly shifted as the salt content increased.
Furthermore, a prominent peak at a wavenumber of around 2055 cm−1 is associated with stretching anions (SCN) in the salt. The peaks noted at two close wavenumbers of ~ 1647 and ~ 1531 cm−1 are due to C=O stretching and N–H bending vibrations of polymers, correspondingly36,37. The bands at about 1410 cm−1 and 1300 cm−1 corresponded to CH2 wagging and C–H deformation, respectively. A peak observed at 1070 cm−1 is also linked to the C–O stretching vibration, whereas a peak indicates the C–H rocking at 885 cm−1.
A discernible change in intensity observed in the STC electrolyte systems is ascribed to the interactions among the polar functional groups of polymers based ternary blend and NaSCN salt dopant38.
To evaluate the ion transport characteristics in the synthesized STC samples, the FTIR deconvolution study has been selected alongside with EIS method, which will be discussed in the subsequent section. The FTIR deconvolution technique has several advantages, including the capability to enhance a spectrum by removing extraneous noise and fringes, resolving overlapping absorption bands, and facilitating improved analysis about the correlation among deconvolution parameters. Moreover, the sensitivity of FTIR spectroscopy to minor alternations in structural properties enhances its precision in distinguishing potential peaks along with changes in intensity and their position, leading to the identification of charge carriers in the STC samples39,40. Figure 5 demonstrates the peak overlap in the relevant region attributed to the SCN molecule in the STC samples, which was deconvoluted via OriginPro 2025 software. The overlapping peak is a complex phenomenon that requires the deconvolution technique to separate the peaks. A prominent peak at a wavenumber of 2030 to 2090 cm−1 is observed, which correlates with the SCN molecule stretching mode. The transmitted spectra are transformed into absorbance spectra following baseline correction for from 2030 to 2090 cm−134. Figure 6 presents the results of FTIR deconvolution spectra for the SCN molecule stretching mode in the STC electrolytes. The figure illustrates numerous peaks located at 2041 cm−1, 2042 cm−1, 2043 cm−1, 2058 cm−1, and 2058 cm−1 which are corresponded to the presence of free ions (Na +). Also, several peaks at 2055 cm−1, 2057 cm−1, 2059 cm−1, 2069 cm−1, and 2070 cm−1 are observed, which correlate to contact ions (Na + , SCN −). To be more precise, the sodium salt’s anion (SCN −) serves as the contact ion, while the free ion is (Na +). This seems to be the reason that ion aggregation or re-association in the STC system causes a decrease in conductivity. A similar phenomenon of overcrowding occurs with Na + ions from sodium salts when an excessive quantity of dopant is added31. The free ions, contact ions, and ion aggregates proportions are calculated using the area under each FTIR band via the following equations:
In this context, \({A}_{f}\) stands for free ion peaks, \({A}_{a}\) denotes the ion aggregate peaks, and \({A}_{c}\) stands for the contact ion peaks. The number of moles is represented as M, while \({N}_{A}\) stands for Avogadro’s number and \({V}_{Total}\) is the total volume of polymer electrolyte. Figure 7 displays the percentage of free ions, contact ions, and ion aggregates as a function of salt concentrations. It can be observed that free ions increased in the electrolyte system as the concentration of NaSCN improved. The proportion of free ions in the STC1 is 11.14% and increased to the greatest at 57.35% in the STC5 with a corresponding minimal percentage of contact ions and ion aggregates. Various ion transport parameters of diffusion coefficient \(D\), ion mobility \(\mu\), and carrier density \(n\) for the STC electrolyte samples were determined from the FTIR spectra, as summarized in Table 25,31.
where, \(\sigma\) is the ionic conductivity, \(KT\) stands for the Boltzmann constant, and \(e\) is the electron charge. The dissociation of salts aids in the coordination of cations within the electrolyte system, resulting in improved ionic conductivity. This may further be substantiated by examining the trends of diffusion coefficient \(D\), ion mobility \(\mu\). The chain flexibility and segmental motion of polymer blend hosts are influenced by ionic conductivity trends and transport properties. Nonetheless, an upgrade transport characteristic was seen when salt concentration reached 40 wt%, attributed to an improved number of charge carriers in the electrolyte system41.
Electrochemical impedance spectroscopy (EIS)
Electroanalytical techniques represent a crucial field of analytical chemistry, focusing on the determination of both the quantity of specific analytes and characteristics within electrochemical devices. The assessment of electrochemical properties occurring at the electrode interface indicates the correlation between the concentration of certain chemical species and measured property magnitude. In comparison to other analytical methods, for instance chromatography, electroanalytical techniques are much simpler, suitable for rapid and precise detection, and less expensive. They are classified according to the measured signals as (i) potentiometric analysis, (ii) volumetric analysis, and (iii) coulometric analysis.
EIS is a significant electroanalytical technique that measures circuit impedance in Ohms, the unit of resistance. Compared to other electrochemical methods, EIS has numerous advantages as it can be applied to small signal analysis due to its steady-state nature, and it also has the capability to investigate signal relaxations throughout an extensive frequency spectrum, ranging from below 1 mHz to 2 MHz42. It is a powerful diagnostic tool that offers insights into the ionic conductivity and charge transfer mechanisms within the interfacial region and bulk matrix of polymer-based electrolytes43,44. Typically, the complex impedance spectrum has two distinctly defined regions. Both areas exhibit distinct frequency ranges: one is characterized by low frequency, while the other is defined by high frequency. The blocking electrodes generate a low-frequency line, whereas the bulk effect of electrolytes produces a high-frequency semi-circle. Figure 8 depicts the Nyquist plot (\({Z}_{i}\) vs. \({Z}_{r}\)) for the STC electrolytes at RT.
It can be observed that the arc at high-frequency diminishes with increasing salt concentrations and reaches lowest at STC4, signifying an enhancement in capacitive characteristics45. The bulk resistance (Rb) is ascertained by the real axis intersection with the corresponding spike. It is noteworthy to mention that the Rb decreased with salt concentration increased from 10 to 40 wt% due to the improvement of charge carrier density, resulting in heightening ionic conductivity46. The ionic conductivity (\({\sigma }_{dc}\)) can be determined in all cases, taking into account the electrolyte thickness (t) and surface area (A) via Eq. (8):
It is observed that when the concentration of NaSCN salt increases, the number of charge carrier increases, leading to a drop in (Rb). Table 3 demonstrates how the (\({\sigma }_{dc}\)) is affected by the NaSCN salt concentration. At room temperature, it is evident from the equation below that the total conductivity is influenced by both the quantity of charge carriers and their mobility:
where, \({n}_{i}\), \({q}_{i}\), and \({\mu }_{i}\) denote the charge carrier density, simple charge, and ionic mobility, respectively47,48.
It is recognized that pure polymers like PVA, CS, and FSG exhibited extremely low ionic conductivity at room temperature, attributing to the absence of charge carriers. Meanwhile, with the incorporation of NaSCN salt dopant, there was a progressive improvement in (\({\sigma }_{DC}\)) value as the amount of salt increased, ultimately achieving an optimum conductivity of (2.74 × 10−6 S/cm) at 40 wt% salt concentrations. This is because of the greater availability of free ions in the host medium and the change in the structural characteristics of host blend polymers in transforming the crystalline area into interconnected amorphous regions, thereby enhancing the conduction process.
Nevertheless, the film incorporated 50 wt% NaSCN salt demonstrated diminished in (\({\sigma }_{DC}\)) value as a result of the re-association process among free ions, indicating the polymer host restriction in accepting elevated salt concentrations49,50. This achievement is strongly supported by the FTIR and XRD results.
Modeling of the electric equivalent circuit (EEC) is essential for the fitting procedures, data analysis, and providing a through-electrochemical picture of the system. The impedance spectra could be interpreted by an equivalent circuit which includes Rb response owing to charge carrier mobility and two constant phase elements (CPEs)51. Due to the ion-blocking nature of SS electrodes, a double-layer is generated at the electrode–electrolyte interfaces as ions accumulate alternately at lower frequencies. This appears as a constant phase element (low-frequency inclined spike in the Nyquist plot, CPE2). Furthermore, when the field is applied, there is a resistance to the flow of ions (denoted as Rb) and the realignment of dipoles in the bulk (denoted as another constant phase element, CPE1). Given that these two processes occur at the same time, a parallel configuration of Rb and CPE1 is observed, resulting in a high-frequency depression in the Nyquist plot. In the low-frequency domain, it is simultaneously configured in series with CPE245. The CPEs are defined as follows:
If the impedance plot only shows a semicircle without spike in the high-frequencies, the real (\({Z}_{r}\)) and imaginary (\({Z}_{i}\)) components of complex impedance (\({Z}^{*}\)) spectroscopy are mathematically expressed as:
where, the variable p denotes plot deviation from the real axis, and the following equations characterize an impedance plot featuring a high-frequency semi-circle and low frequency line:
Here, p1 and p2 represent the semicircular deviation from the y-axis and the spike deviation from the x-axis, correspondingly. Also, C1 and C2 represent the capacitance at low and high frequencies, respectively.
Dielectric properties study
Dielectric constant and loss
Investigating a broad spectrum of relaxation frequencies of complex dielectric permittivity (ε*) via spectral measurements serves as a valuable method for analyzing dipole relaxation within the polymer electrolyte system, offering insight into the migration of charged species travestying the medium. The dynamics of ionic and molecular interactions, along with structural characteristics and the roles played by bulk and interfacial regions, can be assessed in this context. The real (ε') and imaginary (ε") parts are components of complex permittivity (ε*) with the former linked to capacitance and reflecting the alignment of dipoles. While the letter pertains to conductance and indicates the energy needed to align the dipoles. The dielectric behavior could be used to infer the polarization process at the electrode–electrolyte interface52,53,54. The values of real (ε') and imaginary (ε") parts can be determined through the application of the following equations15:
Figures 9 and 10 demonstrate the frequency-dependent of (ε') and (ε") components for the STC samples based on solid polymer electrolytes at room temperature. The patterns observed were consistent, showing a significant rise in their value within the low-frequency area, which was then succeeded by a rapid decline in the intermediate frequency area. They subsequently attained a stable condition characterized by nearly constant values in the high-frequency area. The rise in their value within the low-frequency region is mainly due to the electrode polarization (EP) at the electrode/electrolyte terminal. Whereas there is a lag in time between the electric field and dipole frequency, causes a decrease in their value within the high-frequency region55. Moreover, it is worth noting that the ε' value increases with increasing NaSCN concentrations up to 40 wt%. The NaSCN polarization impact the crystallinity of PVA-CS-FSG-NaSCN based ternary polymer blend electrolytes, resulting in the increment of charge carriers55. The high ε′ value is related to high charge carriers (ε′ = Q/V) that conduct electricity and thud high DC conductivity. Thus investigation of dielectric properties is crucial to probe the conductivity behavior of the electrolyte films. In DC measurement and in ε′ analysis it was found that STC4 is the system with highest conductivity.
Loss tangent (tan δ)
Dielectric relaxation process is characterized by the dielectric loss tangent (tan δ), which estimates the energy loss; it can be calculated using the following equation:
To further understand the dipole relaxation process and its correlation with the mobility of ions and ionic conductivity, the loss tangent (tan δ) against log (f) for the whole STC samples is presented in Fig. 11. The plot demonstrates a progressive rise in the tan δ with increasing frequency, showing a peak at a certain frequency, followed by a decline in the high frequency area. In the low-frequency range, the ohmic component predominates over the capacitive component, and the relaxation frequency reaches its maximum value. At elevated frequencies, the decline is associated with the independence of the ohmic component and the increase of the capacitive component56,57. Among the STC samples, the STC4 has the maximum shift towards the high-frequency side. This indicates the minimal relaxation time due to the increased amorphous nature, high dielectric constant of STC4, and optimum DC ionic conductivity. In solid-based electrolytes, dipole relaxation is associated with the emergence of characteristic peaks at a certain frequency. This suggests that the mobile cation (Na +) of the salt, in conjugation with the rapid segmental motion, diminishes the relaxation time and facilitates a more rapid transport procedure, which is expressed mathematically as \(\tau =\frac{1}{2\pi fmax}\), Where, \(\tau\) is relaxation time of ionic charge carriers58. The elevated salt concentration enhanced ionic conductivity by boosting the mobility of ions through the availability of more conduction channels and rising the number of free ions. However, the relaxation time increased in the STC5 sample resulted from reproducing salt crystals, which reduced the amorphous area and lowered free carrier density59.
Electric modulus
The dielectric modulus is a valuable technique for elucidating conductivity relaxation processes since it discloses the bulk dielectric properties. Moreover, the (M*) has advantages over (ε*) since it mitigates electrode polarization at lower frequencies, minimizing erroneous influence on the dielectric constant. The components of (M') real and imaginary (M") of complex modulus formalism (M*) against frequency are shown in Figs. 12 and 13, respectively. The (M') and (M") values for the STC electrolyte samples were determined through applied equations below52,60:
The figures display the rise in the (M') and (M") values at high-frequencies due to the bulk influence of resistance. Nonetheless, clearly marked dispersion peaks were observed in the specified frequency range of (M"). Also, the extended tails in the low-frequency side suggested significant capacitance resulting from ion accumulation, which caused electrode polarization and proved to be of a non-Debye type nature61. The observed dispersion at high-frequencies mostly originated from conductivity relaxation dispersed over the range of frequencies, which indicates the presence of relaxation time. This phenomenon ought to have been represented by a loss peak in the M" spectra versus log (f). Typically, the M" spectrum is divided into two separate areas: a high frequency dispersion showcases the relaxation processes, and a low frequency tail arises from the ionic conduction mechanism. The peaks of conduction relaxation appear in the M" spectra, while no peaks observed in the ε" spectra. This indicates a significant relationship between ions and the movement of polymer segments15,62. In both M’ and M’’ plot the STC4 system appeared at the bottom of other systems, which related to high conductivity of that electrolyte. It is well reported that M* is reciprocal of ε* and thus the systems which exhibit high value of dielectric constant values should exhibit lowest value in M* case parameters.
AC conductivity
AC electrical conductivity (\({\sigma }_{AC}\)) against log (f) at RT for the STC samples was investigated in order to further comprehend the mobility of ions and their transport mechanism. This straightforward method may effectively describe the interfacial (low-frequency) and bulk functions (high-frequency) areas. The subsequent equation is applied to determine the (\({\sigma }_{AC}\)) values for the STC samples2:
The (\({\sigma }_{AC}\)) values against frequency for the STC samples are shown in Fig. 14. Overall, the (\({\sigma }_{AC}\)) spectra for all doped samples displayed a plateau at low-frequency, followed by a dispersion region at high frequency. It is evident that elevating salt concentration into the ternary polymer blend systems led to an improvement in the (\({\sigma }_{AC}\)) value. This enhancement results from the addition of NaSCN salt, which allowed for a greater number of charge carriers up to 40 wt%. However, further improvement in the NaSCN content caused reduced (\({\sigma }_{AC}\)) value due to ion overcrowding within the PVA-CS-FSG blend network63,64. The observation revealed that the (\({\sigma }_{AC}\)) value at lower frequencies stayed consistent with respect to frequency, enabling the determination of (\({\sigma }_{DC}\)) ionic conductivity. It shows the polarization of charges at the contact between electrodes and the STC sample. The high-frequency dispersion area could be linked to the short-range hopping of charges across energy barriers of different heights as they transfer from an already-occupied point to the next free adjustment point in the matrix. The (\({\sigma }_{AC}\)) spectra and relaxation methods were also used to investigate the electrical properties of glassy systems, where conduction is mostly influenced by polaron hopping and correlated barrier hopping models. In contrast with SPEs, glassy systems have distinct polarization behavior, in which grain boundaries, localized sites, and defect-induced hopping processes govern the charge transport mechanism. Meanwhile, in polymer-based electrolytes, salt dissociation and segmental polymer motion facilitate charge transport processes65,66. Earlier research has shown that the exodus of conductivity spectra from the (\({\sigma }_{DC}\)) value, especially in the plateau area, could be explained by the impacts of electrode polarization67. The (\({\sigma }_{DC}\)) values from EIS measurement are supported by the (\({\sigma }_{AC}\)) findings shown in Fig. 14, which also demonstrate that the salt concentration dramatically increases (\({\sigma }_{AC}\)). Thus, its essential to validate the (\({\sigma }_{DC}\)) from impedance spectroscopy by the (\({\sigma }_{AC}\)) pattern and expansion of the plateau area to the Y-axis15,68. It can be notice that STC4 system shows the high AC conductivity compared to other system. Thus dielectric constant, AC behavior and electric modulus representation supported the results of impedance plots and XRD results.
Conclusion
A biodegradable ternary polymer blend electrolyte based on PVA-CS-FSG, as hosts doped with various NaSCN concentrations, was fabricated and studied for its structural, ionic transport, and electrical parameters. XRD study revealed that the degree of crystallinity decreased from 36 to 15% by including NaSCN salt from 10 to 40 wt%. The FTIR analysis confirms the interaction between the electrolyte elements. The electrostatic interactions between (Na +) of the salt and functional groups of polymers were pivotal in decreasing the (\({X}_{c}\)) of the electrolyte systems, thereby promoting greater complexation. The deconvoluted FTIR spectra were used to assess the ionic transport parameters where the (\(n\)) value increased with adding more salts. Meanwhile, the highest (\(\mu\)) was recorded for the STC4 sample, while a further increase in salt content decreased (\(\mu\)) value due to the rise in (\(n\)), leading to ion overcrowding and hindrance to ionic movement. The increment of the NaSCN salt plays a crucial role in improving ionic conductivity by enhancing the quantity of mobile ions and creating more conducting pathways. Enhancement of the ionic conductivity up to (2.74 × 10−6 S/cm) at room temperature has been obtained with the addition of 40 wt% of salt. The non-Debye behavior observed in the spectra of (tan δ) and electric modulus signified the viscoelastic relaxation characteristics of the synthesized STC samples, whereby the ionic mobility of the salt is significantly connected with the segmental motion of the hosts.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Please contact (shujahadeenaziz@gmail.com) for access to the data.
References
Ramesh, S., Liew, C.-W. & Arof, A. K. Ion conducting corn starch biopolymer electrolytes doped with ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J. Non Cryst. Solids 357, 3654–3660 (2011).
Hadi, J. M. et al. Electrochemical impedance study of proton conducting polymer electrolytes based on PVC doped with thiocyanate and plasticized with glycerol. Int. J. Electrochem. Sci. 15, 4671–4683 (2020).
Liew, C.-W., Ramesh, S. & Arof, A. K. Characterization of ionic liquid added poly(vinyl alcohol)-based proton conducting polymer electrolytes and electrochemical studies on the supercapacitors. Int. J. Hydrogen Energy 40, 852–862 (2015).
Woo, H. J., Liew, C.-W., Majid, S. R. & Arof, A. K. Poly(ε-caprolactone)-based polymer electrolyte for electrical double-layer capacitors. High Perform. Polym. 26, 637–640 (2014).
Hadi, J. M. et al. Design of plasticized proton conducting Chitosan: Dextran based biopolymer blend electrolytes for EDLC application: Structural, impedance and electrochemical studies. Arab. J. Chem. 14, 103394 (2021).
Ahmad, N. H. & Isa, M. I. N. Structural and ionic conductivity studies of CMC based polymerelectrolyte doped with NH4Cl. Adv. Mat. Res. 1107, 247–252 (2015).
Polu, A. R. & Kumar, R. Preparation and characterization of pva based solid polymer electrolytes for electrochemical cell applications. Chin. J. Polym. Sci. 31, 641–648 (2013).
Aziz, S. B. et al. Impedance, FTIR and transport properties of plasticized proton conducting biopolymer electrolyte based on chitosan for electrochemical device application. Res. Phys 29, 104770 (2021).
Ramadan, R., Kamal, H., Hashem, H. M. & Abdel-Hady, K. Gelatin-based solid electrolyte releasing Li+ for smart window applications. Sol. Energy Mater. Sol. Cells 127, 147–156 (2014).
Awang, F. F., Hassan, M. F. & Kamarudin, K. H. Study on ionic conductivity, dielectric and electrochemical properties of solid polymer electrolyte for battery applications. Ionics (Kiel) 28, 1249–1263 (2022).
Wang, D. et al. Low‐cost, high‐strength cellulose‐based quasi‐solid polymer electrolyte for solid‐state lithium‐metal batteries. Angewandte Chemie International Edition 62, (2023).
Hirankumar, G., Selvasekarapandian, S., Kuwata, N., Kawamura, J. & Hattori, T. Thermal, electrical and optical studies on the poly(vinyl alcohol) based polymer electrolytes. J. Power Sources 144, 262–267 (2005).
Ben Halima, N. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 6, 39823–39832 (2016).
Thakur, V. K. & Thakur, M. K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustain. Chem. Eng. 2, 2637–2652 (2014).
Hadi, J. M. Influence of plasticizer on structural, electrical, and electrochemical properties of polyvinyl alcohol-chicken gelatin solid polymer electrolytes. J. Solid State Electrochem. https://doi.org/10.1007/s10008-024-06164-2 (2024).
Kadir, M. F. Z. Non-Faradaic-based supercapacitor fabricated with fish skin gelatin biopolymer electrolyte. Ionics (Kiel) 27, 2219–2229 (2021).
Pritam, Arya, A. & Sharma, A. L. Dielectric relaxations and transport properties parameter analysis of novel blended solid polymer electrolyte for sodium-ion rechargeable batteries. J. Mater. Sci. 54, 7131–7155 (2019).
Premalatha, M., Vijaya, N., Selvasekarapandian, S. & Selvalakshmi, S. Characterization of blend polymer PVA-PVP complexed with ammonium thiocyanate. Ionics (Kiel) 22, 1299–1310 (2016).
Chen, C.-H., Wang, F.-Y., Mao, C.-F., Liao, W.-T. & Hsieh, C.-D. Studies of chitosan: II. Preparation and characterization of chitosan/poly(vinyl alcohol)/gelatin ternary blend films. Int. J. Biol. Macromol. 43, 37–42 (2008).
Ghaderi, J., Hosseini, S. F., Keyvani, N. & Gómez-Guillén, M. C. Polymer blending effects on the physicochemical and structural features of the chitosan/poly(vinyl alcohol)/fish gelatin ternary biodegradable films. Food Hydrocoll. 95, 122–132 (2019).
Rani, N. S., Sannappa, J., Demappa, T. & Mahadevaiah. Structural, thermal, and electrical studies of sodium iodide (NaI)-doped hydroxypropyl methylcellulose (HPMC) polymer electrolyte films. Ionics (Kiel) 20, 201–207 (2014).
Ram, R. & Bhattacharya, S. Mixed ionic-electronic transport in Na2O doped glassy electrolytes: Promising candidate for new generation sodium ion battery electrolytes. J Appl Phys 133, (2023).
Bar, A. K., Bhattacharya, K., Kundu, R., Roy, D. & Bhattacharya, S. Anomalous electrical conductivity in selenite glassy nanocomposites. Mater. Chem. Phys. 199, 322–328 (2017).
Ghosh, J. et al. Single polaron hopping in Fe doped glassy semiconductors: Structure–electrical transport relationship. J. Appl. Phys. 132, (2022).
Epp, J. X-ray diffraction (XRD) techniques for materials characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods 81–124 (Elsevier, 2016). https://doi.org/10.1016/B978-0-08-100040-3.00004-3.
Salleh, N. S., Aziz, S. B., Aspanut, Z. & Kadir, M. F. Z. Electrical impedance and conduction mechanism analysis of biopolymer electrolytes based on methyl cellulose doped with ammonium iodide. Ionics (Kiel) 22, 2157–2167 (2016).
Sravanthi, K., Sundari, G. S. & Erothu, H. Development of bio-degradable based polymer electrolytes for EDLC application. Optik (Stuttg) 241, 166229 (2021).
Bhavani, S. et al. Studies on structural, electrical and dielectric properties of nickel ion conducting polyvinyl alcohol based polymer electrolyte films. J. Mater. Sci.: Mater. Electron. 28, 13344–13349 (2017).
Azahar, S. S., Hamidon, T. S., Latip, A. F. A. & Hussin, M. H. Physicochemical and conductivity studies of chitosan-tapioca flour-LiBF4 gel polymer electrolytes. Chem. Phys. Impact 3, 100055 (2021).
He, Y., Zhu, B. & Inoue, Y. Hydrogen bonds in polymer blends. Prog. Polym. Sci. 29, 1021–1051 (2004).
Abdalrahman, A. A., Aziz, S. B. & Karim, W. O. EIS and FTIR approaches to study the ion transport parameters and relaxation dynamics of Na+1 ion in SPE based on MC polymer inserted with sodium salt. Res. Phys. 36, 105439 (2022).
Abdulwahid, R. T., B. Aziz, S. & Kadir, M. F. Z. Design of proton conducting solid biopolymer blend electrolytes based on chitosan‐potato starch biopolymers: Deep approaches to structural and ion relaxation dynamics of H + ion. J. Appl. Polym. Sci. 139, (2022).
Anandha Jothi, M., Vanitha, D., Nallamuthu, N., Manikandan, A. & Asath Bahadur, S. Investigations of lithium ion conducting polymer blend electrolytes using biodegradable cornstarch and PVP. Phys. B Condens. Matter 580, 411940 (2020).
Mazuki, N. F., Abdul Majeed, A. P. P., Nagao, Y. & Samsudin, A. S. Studies on ionics conduction properties of modification CMC-PVA based polymer blend electrolytes via impedance approach. Polym. Test 81, 106234 (2020).
Cholant, C. M. et al. Study of the conductivity of solid polymeric electrolyte based on PVA/GA blend with addition of acetic acid. J. Solid State Electrochem. 24, 1867–1875 (2020).
Choo, K., Ching, Y., Chuah, C., Julai, S. & Liou, N.-S. Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 9, 644 (2016).
Hemalatha, R., Alagar, M., Selvasekarapandian, S., Sundaresan, B. & Moniha, V. Studies of proton conducting polymer electrolyte based on PVA, amino acid proline and NH4SCN. J. Sci. Adv. Mater. Dev. 4, 101–110 (2019).
Badi, N. et al. Fabrication and characterization of flexible solid polymers electrolytes for supercapacitor application. Polymers (Basel) 14, 3837 (2022).
Singh, R., Singh, P. K., Singh, V. & Bhattacharya, B. Quantitative analysis of ion transport mechanism in biopolymer electrolyte. Opt. Laser Technol. 113, 303–309 (2019).
Pistorius, A. M. A. & DeGrip, W. J. Deconvolution as a tool to remove fringes from an FT-IR spectrum. Vib. Spectrosc. 36, 89–95 (2004).
Zainuddin, N. K., Rasali, N. M. J. & Samsudin, A. S. Study on the effect of PEG in ionic transport for CMC-NH4Br-based solid polymer electrolyte. Ionics (Kiel) 24, 3039–3052 (2018).
Magar, H. S., Hassan, R. Y. A. & Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 21, 6578 (2021).
Siracusano, S., Trocino, S., Briguglio, N., Baglio, V. & Aricò, A. S. Electrochemical impedance spectroscopy as a diagnostic tool in polymer electrolyte membrane electrolysis. Materials 11, 1368 (2018).
Brza, M. A., Aziz, S. B., Anuar, H. & Ali, F. Structural, ion transport parameter and electrochemical properties of plasticized polymer composite electrolyte based on PVA: A novel approach to fabricate high performance EDLC devices. Polym. Test 91, 106813 (2020).
Cyriac, V. et al. Ionic conductivity enhancement of PVA: carboxymethyl cellulose poly-blend electrolyte films through the doping of NaI salt. Cellulose 29, 3271–3291 (2022).
Selvasekarapandian, S., Baskaran, R. & Hema, M. Complex AC impedance, transference number and vibrational spectroscopy studies of proton conducting PVAc–NH4SCN polymer electrolytes. Phys. B Condens. Matter 357, 412–419 (2005).
Hadi, J. M. et al. Investigation of ion transport parameters and electrochemical performance of plasticized biocompatible chitosan-based proton conducting polymer composite electrolytes. Membranes (Basel) 10, 363 (2020).
Abdullah, A. M., Aziz, S. B. & Saeed, S. R. Structural and electrical properties of polyvinyl alcohol (PVA): Methyl cellulose (MC) based solid polymer blend electrolytes inserted with sodium iodide (NaI) salt. Arab. J. Chem. 14, 103388 (2021).
Premalatha, M., Mathavan, T., Selvasekarapandian, S., Selvalakshmi, S. & Monisha, S. Incorporation of NH4Br in Tamarind Seed Polysaccharide biopolymer and its potential use in electrochemical energy storage devices. Org. Electron. 50, 418–425 (2017).
Ramesh, S. & Ang, G. P. Impedance and FTIR studies on plasticized PMMA–LiN(CF3SO2)2 nanocomposite polymer electrolytes. Ionics (Kiel) 16, 465–473 (2010).
Pradhan, D. K., Choudhary, R. N. P., Samantaray, B. K., Karan, N. K. & Katiyar, R. S. Effect of plasticizer on structural and electrical properties of polymer nanocompsoite electrolytes. Int. J. Electrochem. Sci. 2, 861–871 (2007).
Dennis, J. O. et al. Substantial proton ion conduction in methylcellulose/pectin/ammonium chloride based solid nanocomposite polymer electrolytes: Effect of ZnO nanofiller. Membranes (Basel) 12, 706 (2022).
Pradhan, D. K., Choudhary, R. N. P. & Samantaray, B. K. Studies of dielectric relaxation and AC conductivity behavior of plasticized polymer nanocomposite electrolytes. Int. J. Electrochem. Sci. 3, 597–608 (2008).
Asnawi, A. S. F. M., Hamsan, M. H., Kadir, M. F. Z., Aziz, S. B. & Yusof, Y. M. Investigation on electrochemical characteristics of maltodextrin—methyl cellulose electrolytes. Mol. Cryst. Liq. Cryst. 708, 63–91 (2020).
Abbas Adam, A. et al. Structural behavior and ion dynamics of methylcellulose/tri-potassium phosphate-based solid biopolymeric electrolytes. Mol. Cryst. Liquid Crystals 759, 29–43 (2023).
Hadi, J. M. et al. Role of nano-capacitor on dielectric constant enhancement in PEO:NH4SCN:xCeO2 polymer nano-composites: Electrical and electrochemical properties. J. Market. Res. 9, 9283–9294 (2020).
Nithya, S., Selvasekarapandian, S. & Premalatha, M. Synthesis and characterization of proton-conducting polymer electrolyte based on polyacrylonitrile (PAN). Ionics (Kiel) 23, 2767–2774 (2017).
Nofal, M. M. et al. A study of methylcellulose based polymer electrolyte impregnated with potassium ion conducting carrier: Impedance, EEC modeling, FTIR, dielectric, and device characteristics. Materials 14, 4859 (2021).
Pradhan, D. K., Choudhary, R. N. P. & Samantaray, B. K. Studies of dielectric and electrical properties of plasticized polymer nanocomposite electrolytes. Mater Chem. Phys. 115, 557–561 (2009).
Hadi, J. M. et al. Electrical, dielectric property and electrochemical performances of plasticized silver ion-conducting chitosan-based polymer nanocomposites. Membranes (Basel) 10, 1–22 (2020).
Hema, M., Selvasekerapandian, S., Sakunthala, A., Arunkumar, D. & Nithya, H. Structural, vibrational and electrical characterization of PVA–NH4Br polymer electrolyte system. Phys. B Condens. Matter 403, 2740–2747 (2008).
Aziz, S. B. & Abidin, Z. H. Z. Ion‐transport study in nanocomposite solid polymer electrolytes based on chitosan: Electrical and dielectric analysis. J. Appl. Polym. Sci. 132, (2015).
Sangeetha, M. et al. Dielectric studies and AC conductivity of PVDF-HFP: LiBF4: EC plasticized polymer electrolytes. Mater Today Proc 44, 2168–2172 (2021).
Basha, S. K. S., Sundari, G. S., Kumar, K. V. & Rao, M. C. Preparation and dielectric properties of PVP-based polymer electrolyte films for solid-state battery application. Polym. Bull. 75, 925–945 (2018).
Chamuah, A., Ojha, S., Bhattacharya, K., Ghosh, C. K. & Bhattacharya, S. AC conductivity and electrical relaxation of a promising Ag2S-Ge-Te-Se chalcogenide glassy system. J. Phys. Chem. Solids 166, 110695 (2022).
Ojha, S., Ali, M. S., Roy, M. & Bhattacharya, S. Hopping frequency and conductivity relaxation of promising chalcogenides: AC conductivity and dielectric relaxation approaches. Mater. Res. Express 8, 085203 (2021).
Christopher Selvin, P. et al. Study of proton-conducting polymer electrolyte based on K-carrageenan and NH4SCN for electrochemical devices. Ionics (Kiel) 24, 3535–3542 (2018).
Hamsan, M. H. et al. Tailoring polymer electrolytes with PVA for improved performance in electrical double layer capacitors. J. Ind. Eng. Chem. https://doi.org/10.1016/j.jiec.2024.11.035 (2024).
Acknowledgements
The authors gratefully acknowledge the financial support, facility and infrastructures provided for this study from the University of Sulaimani and the University of Charmo.
Author information
Authors and Affiliations
Contributions
JMH: Methodology, Investigation, Analysis, Validation, Writing – original draft. BKA: Validation, Supervision, Project administration, Writing – review & editing. SBA: Conceptualization, Writing – review & editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hadi, J.M., Aziz, B.K. & Aziz, S.B. Structural and electrochemical properties of ternary solid polymer electrolytes based on PVA:CS:FSG doped with sodium thiocyanate. Sci Rep 15, 14379 (2025). https://doi.org/10.1038/s41598-025-99489-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99489-5
Keywords
This article is cited by
-
Structural and electrical properties of biopolymer blend electrolytes based on chitosan: dextran doped with lithium nitrate (LiNO3) salt
Scientific Reports (2026)
-
Boosting the performance of PVA/LiClO4 based biopolymer electrolyte using Coffea Arabica waste in Li-ion conducting battery system
Emergent Materials (2025)
-
Molecular-level insights into glycerol-assisted ion transport and dielectric behavior of CS–PVA–NaSCN–Al2O3 polymer electrolytes
Journal of Materials Science (2025)