Abstract
Burning rice straw contribute to Atmospheric Pollution, which makes it unsustainable in the long-run, but are still opted by farmers due to faster removal of residue. Lignocellulose Degrading Microorganisms, facilitating sustainable management, may accelerate the breakdown of various crop residues. A study comprised of twenty-one treatments including fungal strains, bacterial strains and microbial consortia. In addition, nitrogen supplementation as well as the retention of rice crop residue and incorporation were included in treatments accordingly. The results showed that T12 (Residue Incorporated + Microbial Consortia + Urea) have highest decomposition and enzyme activities. With content of rice residue decreased to cellulose 17%, hemicellulose 4% and lignin 16% from 41%, 9%, and 28% respectively in T12. Enzyme activities being highest on 30th DOA (Day of Application) were; cellulose activity of 0.691 µmole/ml/h, laccase activity of 705.7 µg/µl/h and peroxidase activity of 271.2 µmole/g/h in T12. The CO2 emission by the T12 were also highest on 30th day as 6.16 mg CO2/g/d, which still when compared to the values of open field burning were prominently lower. Therefore, for the purpose of sustainable residue management, we deduce that the employment of microbial consortia in conjunction with residue-incorporated nutrient supplementation has the potential to accelerate the larger-scale decomposition of rice straw.
Similar content being viewed by others
Introduction
Burning crop residue is a popular practice in poor nations since farmers are often unaware of the advantages of using it for other purposes. Because of this, open burning is a tempting alternative for getting rid of crop residue, particularly in Asian countries1. Burning crop residues may be a cheap and convenient choice for farmers, but the emissions from it change the chemical makeup of the atmosphere and large-scale agricultural waste burning significantly affect the carbon cycle2. The primary industry in Pakistan is agriculture, and field burning is a major source of air pollution. It was found that among the 750 Tg (million tonnes) of waste burned in Asia in 2000, 250 Tg consisted of agricultural leftovers. Though Pakistan’s contribution to agricultural residue burning was just about 10 Tg, it is nevertheless significant when compared to other Asian nations3.
Rice straw is the most commonly produced agricultural waste worldwide4. About 650–975 million tons of rice straw is produced globally per year, from which a large part is used as feed for cattle and the rest is rice straw waste5. The majority of farmers decide to burn their fields because it is a quick and easy way to do so instead of cleaning them, and because they lack the technologies needed for in-situ integration of residues and are ignorant of their value. Regrettably, this burning method causes large losses of valuable organic matter and plant nutrients in addition to environmental contaminants, fire risks, etc6. This practice releases large amounts of Particulate Matter (PM10 and PM2.5) and greenhouse gases like CH4, CO2, NO2, and SO2, which have a negative impact on health of humans and cause unfavorable alterations in the environment7.On the other hand, rice straw is resistant to quick breakdown because to the distinctive structural feature generated by the complex interplay between cellulose, hemicellulose, and lignin8. A cost-effective and ecologically responsible way is needed, so to efficiently manage varied crop wastes in order to reduce the harmful impacts of burning crop waste on-site and offer convenience to farmers9. A variety of in-situ rice reside techniques were used, including rotational tillage, deep burying, and crushing10,11. However, when applied alone, these techniques are not very efficient at speeding up the breakdown of straw. The breakdown of lignocellulosic straw in soil is mostly caused by microbes. Numerous natural microbes, including Bacillus, Pseudomonas, Trichoderma, Aspergillus, and others, produce lignin-degrading enzymes, cellulase, and hemicellulase, which can break down lignocellulose8,12. Different hydrolytic exo-enzymes are known to be released by lignocellulose degrading microorganisms (LCDMOs) for the de-polymerization of cellulose, lignin, proteins, lipids, and other complex organic compounds13. For the decomposition of cellulose the primary producers of cellulases, are cellulolytic bacteria, fungi, and protozoa14. Whereas, there are lignin-decomposing microbes which produce a variety of enzymes that break down lignin, such as manganese peroxidase, laccase, and lignin peroxidase, which all exhibit a high degree of lignin breakdown selectivity15. The enzymatic hydrolysis of cellulose can be monitored using CMCase activity (CA), which displays endoglucanase activity16. Peroxidases and laccases are oxidative enzymes which contribute in the decomposition of lignin17,15and their activity can be monitored. The microbes are widely distributed in the native rice-growing fields so their diversity in degrading efficiency and optimal time for rice straw utilization can be investigated. Low decomposition rates, decreased soil microbes and deficiency of ammonium nitrogen in soil, are all significant factors that restrict the rate of breakdown in any approach for managing crop residues18. Because of its efficiency, potential for energy savings, cost-effectiveness, and environmental friendliness, using microbial agents to speed up straw decomposition is a promising approach19,10.
Keeping in view of above literature a study was designed with hypothesis hat the decomposition of rice straw was speed up with LCDMOs by themselves or in combination with N supplementation and providing an eco-friendly substitute for open-field burning and minimizing atmospheric pollution. A novel blend of microbial strains and amendments were implied in this research. In it a distinct combination of bacteria and fungi were introduced i.e., fungal strains (Aspergillus flavus, Aspergillus niger, Penicillium spp., Emmonsia pasteurina) and bacterial strains (Monococcus echinophorus, Bacillus subtilis, Streptococcus sanguis, Pseudomonas fluorescens). The use of grow bags with controlled amounts of soil, straw, nutrients, and microbial inoculants enables precise measurements of decomposition dynamics and could provide valuable data to the science.
The objectives of the current study was to determine (i) use of fungi or bacteria consortium and N supplementation, both separately and together for effective decomposition of rice straw, and (ii) estimate the fungi or bacteria consortium enzymatic assays.
Materials and methods
Selection of microbial strains
The site of ten years long term residue management and conservation tillage plots were selected for isolation of potential lignocellulolytic microorganisms from Rice Research Institute (31°72’ N, 74°28’ E, Slope 1.8%), Kala Shah Kaku (RRI, KSK), Sheikhupura, Punjab, Pakistan20. Soil samples were collected and processed after that, it was put through a series of dilutions and spread-plate plated on modified carboxymethyl cellulose media21. For three days, the plates for bacteria were incubated at 30 °C and for fungus were incubated at 25 °C. Following isolation and purification, morphologically distinct colonies of bacteria and fungus were repeatedly streaked on the appropriate solid media. The cultures that had been cleansed were kept at 4 °C. Sub-culturing was carried out once a month. The potential strains were selected on the basis of primary and secondary screening. The primary screening which was qualitative screening included estimation of cellulolytic activity using CMC (Carboxymethyl cellulose) agar plate method22and oxidative test using Tannic acid agar plate method23,24,25,26,27. The secondary screening was the quantitative estimation of lignocellulolytic enzymes, it included CMC Assay26,28,29,30,31, FPase Assay26,28,29,30,31and Laccase Assay32,33,34. The fungal and bacterial strains were identified morphologically35 and used. The fungal strains used were Aspergillus flavus, Aspergillus niger, Penicillium spp. and Emmonsia pasteurina and the bacterial strains that were used were Monococcus echinophorus, Bacillus subtillus, Streptococcus sanguis and Pseudomonas flourescens. Fungal and bacterial cultures were then prepared for treatment imposition36,37.
Treatment imposition
To estimate the potential of isolated bacterial, fungi strains, their consortia and nitrogen supplementation a pots experiment designed with microbes and residue management practices. The experiment comprised of 21 treatments details in (Table 1) with three replications. The whole setup displayed in Fig. 1. In the present study, we intended to compare crop residue removal (CR), residue retention (CRt) as in Fig. 2a and residue incorporation (CI) as in Fig. 2b with respect to residue management practices.
A grow bag of size (14 × 10 inches) filled with 6 kg of soil. In each pot containing straw, 9 g of straw, 0.03 gram urea, 9 g of cow dung, Fungal Spore solution (\(\:{10}^{8}\) spores \(\:{ml}^{-1}\))38, Bacterial Culture Broth (\(\:{10}^{8}\) viable cells \(\:{ml}^{-1}\))36 and microbial consortia in treatments containing fungi and bacteria was 40 ml in each respected treatment pot.
Sample collection
All samples for assay techniques and CO2 estimation were collected at 15 days’ interval. With samples collected at 15 th, 30 th, 45 th and 60 th day of Application. In case of content measurement, the samples were collected on the 45 th day of application only.
Assay techniques
Cellulase activity
To measure cellulose activity firstly 1 gram of sample was taken in a test tube. In it as a substrate 10 ml of 1%CMC was added. Then 10 ml (0.2 M) Sodium Acetate buffer was added (with pH 5.9). Then each test tube’s mouth was covered with aluminum foil and they were then incubated for 24 h at 30 °C. Then the released sugars were measured by the DNS method to quantitatively measure the cellulose decomposition, by plotting a Glucose Standard Curve39,40. Cellulase Activity was calculated as per formula in Eq. (1)41;
Laccase activity
A 15 ml Falcon tube was filled with 1 g of sample in order to quantify the laccase activity. It was agitated on a shaker for half an hour then after 10 milliliters of distilled water was added in it. The samples were shaken and then run through Whatman No. 51 Filter Paper for filtering. Then the filtrate was centrifuged at 12,000 rpm for 15 min.
We took 25 µl of the generated supernatant. It was then filled with 1.5 milliliters of 0.2% ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)) in a 100 mM sodium acetate (SA) buffer. After that, it remained 30 min being incubated at 30 °C. Soil suspension plus buffer, substrate plus buffer, and buffer alone were the preparations for the controls. After incubation, the absorbance was measured at 436 nm39. Laccase activity was calculated as per formula in Eq. (2)42;
Peroxidase activity
The amount of hydrogen peroxide (H2O2) that was added to the pyrogallol substrate oxidation rate was used to calculate the peroxidase activity. A test tube was filled with 0.1 g of every single soil sample. In it was added 25 ml of 50 mM sodium acetate buffer (pH 5). The suspension was subsequently combined for a minute with a vortex mixer.
500 µl of pyrogallol solution was mixed with 2 milliliters of soil suspension to determine the enzyme activity. In each sample and control, 10 µl of 0.3% hydrogen peroxide was added. Substrate plus buffer, soil suspension plus buffer, and buffer alone were the three sets of controls that were prepared. Then next the samples were incubated for 4 h at 20 °C. Then absorbance was calculated at 460 nm43,17. Peroxidase Activity was calculated as per formula in Eq. (3)44;
Cellulose, hemicellulose and lignin content
For the estimation of lignin, hemicellulose and cellulose, the Ultrasound Assisted Alkaline Extraction Method was used39,45. Samples were collected at 45 th DOA, were oven dried and then were grinded to powder.
20 ml of 2 M NaOH (Sodium Hydroxide) were added to a 50 ml Falcon Centrifuge tube containing one gram of the material, which was then submerged in a water bath (90 °C) for 1 ½ hours and then Ultra-Sonicated for 30 min at a power of 500 W. Then the samples were centrifuged at 3500 rpm for 20 min. After centrifugation the filtrate and residue were separated out using a nylon cloth. After that, distilled water used for washing the residue multiple time until transparent or clear filtrate was reached. The residues were then oven dried at 50–60 °C for collection of cellulose.
The filtrate after collection of cellulose was acidified with HCl (Hydrochloric acid) adjusting pH to 5.5. Then, three liters of a 90% ethanol solution (1:3; sample: ethanol) were used to precipitate it. It was then centrifuged for 20 min at 3500 rpm. The filtrate and residue were separated. Following a 70% ethanol wash, the residue was placed inverted in the tubes for a full day. The residue was collected for hemicellulose content by oven drying it at 50–60 °C.
Following ethanol’s evaporation, the filtrates were acidified (pH 1.5) using 6 M HCl. Then centrifuged at 3500 rpm for 20 min. The residue then washed with HCl and the pH adjusted to 2 and then samples were freeze dried. Following freeze-drying, the contents of tubes were weighed to determine lignin content, with an empty tube serving as a control. Regarding the rice straw, the yields of cellulose, hemicellulose, and lignin were reported on a dry weight basis.
CO2 Estimation
CO2was estimated by acid-base titration46. To do so, 20 ml of NaOH solution was taken in each beaker. The beakers were then placed in each pot, and plastic bottles were placed over them to create a sealed environment. They were then left there for three hours.
To precipitate the absorbed carbon dioxide as barium carbonate, the plastic bottles were removed after three hours and 2 ml of 0.5 M barium chloride solution was added to each beaker. 2–3 drops of phenolphthalein were added, the solution turned pink. The remaining NaOH was titrated against 0.1 M HCL until it went from pink to colourless. The amount of Diluted HCl used was noted. Controls were also prepared and the whole procedure was repeated on them without soil.\(\:\:{\text{C}\text{O}}_{2}\) released was estimated on the basis of difference in values between initial NaOH to final NaOH. The CO2 was estimated as per formula in Eq. (4)46;
Statistical analysis
ANOVA (Analysis of Variance) using Statistics 8.1 statistically analyzed the recorded data. The design used for statistical analysis was factorial design and after the analysis, LSD (Least Significant Difference) was applied to find the significance between the different treatments.
Results
Enzymatic activities
All the enzymatic activities calculated, the highest in the treatments containing microbial inoculation. The treatments containing microbes in microbial consortia showed the highest level of enzymatic activities, among which the treatments having supplementation of Urea as well had greatest values. The presence of microbes increased the rate of decomposition, which was evident from the increase in enzymatic activities by microbes, as these assay techniques demonstrate the Lignocellulosic activities of enzymes.
The highest enzymatic activities were observed in the initial DOA as shown in the heat map of the enzymatic activities (Fig. 3). This was the same for all three enzymatic activities i.e. the cellulase, laccase and peroxidase activities. The red colour intensity shows how greater the activity is and the gradual loss in red colour with passing days indicates towards the decrease of enzymatic activities. Most of the treatments with added microbes i.e. Treatment 7–18 showed increased enzymatic activities on day 15 earlier than the others with microbes removed, which showed the start of enzymatic activities from day 45 th of addition of straw. Also the treatments with just fungi added i.e. T7, T10, T13, and T16 showed higher enzymatic activities rates than the treatments with just bacteria added i.e. T8, T11, T14, and T17. Which showed that fungi have higher microbial decomposition rates than the bacteria. But when compared to the microbial consortia with both fungi and bacteria added i.e. T9, T12, T15 and T19, the enzymatic activities were the highest of microbial consortia treatments than the treatments with microbes separated. Among the microbial consortia treatments, T12 had the highest enzymatic activities, in Fig. 3 the red colour variants from day 15 th to day 60 th showed that the microbial decomposition rate, which were high at all days when, compared with the other treatments.
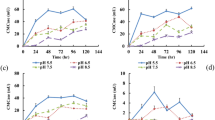
The highest cellulase, laccase and peroxidase activities in all treatments were recorded at 30 th day of application. The activities increased from day 15 th to 30 th and onward a decreasing trend followed. As red colour being the display of activity in our heat map, the lack of red colour in the Control Treatment 1 (straw removed) showed that it had the least microbial activity. When compared to the Control the highest cellulase activity was observed in Treatment 12, which on the 30 th day was 0.691 µmole/ml/h, whereas the average activity of treatment 12 was 0.335 µmole/ml/h. On average as well as on the 30 th day, treatment 15 had the second highest cellulase activity, which was 0.272 µmole/ml/h and 0.632 µmole/ml/h respectively. The highest laccase activity on 30 th day was observed in Treatment 12, which was 705.7 µg/µl/h. Average laccase activity recorded in treatment 12 was 328.5 µg/µl/h, whereas the highest average of laccase activity was recorded in treatment 18, which was 370.6 µg/µl/h. The highest peroxidase activities were also present on the 30 th DOA, and among all the treatment samples, the highest activity was measured in Treatment 12 followed by T9, which were 271.2 µmole/g/h and 262.2 µmole/g/h. The highest average peroxidase activity was found in treatment 12 followed by treatment 13, which were 218.8 µmole/g/h and 185.1 µmole/g/h respectively. These activities values showd that the highest activities wre found to be in T12.
Cellulose, hemicellulose and lignin content
The highest decomposition rates were observed in the treatments containing microbial consortia. The treatments containing nutrient supplementation and in which the residue was incorporated showed the highest values of cellulose, hemicellulose, and lignin decomposition after a time span of 45 days of application. The initial cellulose, hemicellulose and lignin concentration observed were 41%, 9%, and 28% respectively. The highest decomposition rates were observed in treatment T12 (Residue Incorporated + Urea + Microbial Consortia), which were decreased to cellulose 17%, hemicellulose 4% and lignin 16% from 41%, 9%, and 28% respectively.
It can be seen in content graph (Fig. 4) that the least content was obtained from treatment 12 followed by Treatment 18, both of which contained microbial consortia and the residue was incorporated in them. In treatment 18 the cellulose, hemicellulose and lignin content found were 19%, 5% and 18% respectively. The difference between treatment 12 and treatment 18, which happened to be the cause of more decomposition in Treatment 12 was Nutrient supplementation. Treatment 12 was supplemented with Urea, whereas Treatment 18 was without any nutrient supplementation. Therefore, the presence of available nitrogen increased the decomposition of rice straw residue.
CO2 Emission
The emissions of CO2 were seen the highest in treatments, which had microbial inoculation. The presence of microbes increased the emissions of CO2, with nutrient supplementation further increasing the emission values. The highest rate of emission of CO2 was seen on 30 th day of Application, followed by 15 th day of Application, which can be seen in Fig. 5. Also in Fig. 3 Heat map of CO2 the gradual loss of red colour, which indicates the high emission rates, can be seen, this loss of red colour with days shows the decrease in emissions and also indicates towards the decrease in microbial activity rates. These values indicated that the growth of microbes in the initial days led to the increase in CO2 emissions. In addition, the negligible change in emissions in the control T1 (as seen in Fig. 5) that was 2.93 ± 0.07, 2.93 ± 0.07, 3.23 ± 0.08 and 2.93 ± 0.07 mg CO2/g/d on the 15 th, 30 th, 45 th and 60 th day respectively showed that the increase in CO2 emissions was due to the microbial activity. Among all the treatments, the highest amount of mean \(\:{\text{C}\text{O}}_{2}\) emissions were from T12 (Residue Incorporated + Microbial Consortia + Urea) with emission of 6.16 mg CO2/g/d on 30 th DOA, this indicated towards the high microbial decomposition activity in the Treatment 12.
Discussion
In our study, treatments with added microbes i.e. T7-18 showed higher enzymatic activities and CO2 emissions on D15 earlier than ones with microbes removed (D45). Also with just fungi added i.e. T7, T10, T13, and T16 showed higher enzymatic activities rates and CO2 emissions than the treatments with just bacteria added i.e. T8, T11, T14, and T17. Showing that fungi have higher microbial decomposition rates than the bacteria. But in comparison the enzymatic activities and CO2 emissions in microbial consortia i.e. T9, T12, T15 and T19, were the highest. T12 had the highest enzymatic activities and CO2 emissions. Lignocellulosic enzymatic activities were highest on 30 th Day of Application (DOA). As the content measurement was done on the 45 th day, so the peak enzymatic activities show the high decomposition rates of different treatments. Highest average cellulase activity recorded was highest in T12 (0.335 µmole/ml/h) followed by T15 (0.272 µmole/ml/h). Highest average laccase activity recorded was highest in T18 (370.6 µg/µl/h) followed by T12 (328.5 µg/µl/h). Highest average peroxidase activity recorded was highest in T12 (218.8 µmole/g/h followed by T13 (185.1 µmole/g/h). The highest amount of CO2 emission were from treatment T5 followed by T12 which were, 6.45 mg CO2/g/d and 6.16 mg CO2/g/d, respectively. Highest degradation rates were observed in the treatments containing microbial consortia. Treatments containing nutrient supplementation and in which the residue was incorporated showed the highest values. The initial cellulose, hemicellulose and lignin concentration observed were 41%, 9%, and 28% respectively. The highest degradation rates were observed in T12 in which the decrease to cellulose 17%, hemicellulose 4% and lignin 16% was observed. The least content obtained from T12 followed by T18, both of which contained microbial consortia and incorporated residue with only difference being Nutrient supplementation of Urea in T12.
Large amounts of lignocellulosic rice straws are produced worldwide and have the potential to be recycled for the manufacture of bio fuel, biochar, and pulp39. Microbial populations have the ability to produce enzymes that degrade various crop residues47. Various fungal species, such as Fusarium spp., Aspergillus terreus, Paecilomyces fusisporous, Micromonospora, and Coriolus Versicolor, have been shown to have lignocellulolytic activity in numerous investigations48. Numerous bacterial species, including Bacillus, Pseudomonas, including Actino-bacteria, have been found to be capable of breaking down lignin and cellulose materials39. The microbes used in our current study are those that may be separately used in different studies for decomposition of crop residues, but the combination of the microbes was distinct and new. The microbes combination used was fungal strains (Aspergillus flavus, Aspergillus niger, Penicillium spp., Emmonsia pasteurina) and bacterial strains (Monococcus echinophorus, Bacillus subtilis, Streptococcus sanguis, Pseudomonas fluorescens). Multi-strain microbial consortia are frequently said to have strong cellulose-degrading abilities49. A mixture of fungus and cellulase enzyme-secreting strains of Bacillus is necessary for efficient cellulose decomposition50. In our study we used multi-strain microbial consortia, containing 4 fungal and 4 bacterial strains, for attaining highest degree of decomposition. Similarly, in some other study a multi-strain approach in which a five-member microbial consortium created showed noticeably greater effectiveness in destroying bitter Ginseng residues and straws51.
In a research on rice and wheat residues, it was demonstrated that rice and wheat residues underwent significant reductions in cellulose and lignin content following 30 days of in situ decomposition employing a microbial consortium consisting of bacteria, actinomycetes, and fungus. Rice residue with microbial consortia saw a 12.5% greater rate of residue decomposition than rice residue alone52. Our study showed similar results, after 45 days of application the rice crop residue were decreased to cellulose 17%, hemicellulose 4% and lignin 16% from 41%, 9%, and 28% respectively. According to another research the cellulose decrease extent was similar to our cellulose decrease but the hemi-cellulose and lignin decrease in our study was greater due to microbial consortia application, with not only bacteria present in it but also fungi. In that study the main consortia was of bacteria, the initial lignin, cellulose, and hemicellulose contents in rice straw were 39.4, 20.4, and 9.3%, respectively, which significantly decreased at the 28 th DOC (Day of Composting) by 18.4, 17.8, and 7.2%, respectively39. This proves that different strains of microbes showed different extent of residue decomposition, with fungal strains being more efficient in decomposition of more difficult lignin components. Even in fungi, different strains show different decomposing efficiency. One such study found the strains A. flavus RPW 1/3 with 31% residue loss and A. terreus RPW 1/6 with 29% residue loss were the isolates with the largest residue loss. The minimum loss was noted with P. janthinellum RPWM 2/2 (21%), C. cladosporioides MWM 4/14 (2%), Alternaria alternata RZWM 3/2 (26%), and P. oxalicumMWM 4/13 (18%)23. The same outcomes of 21.1% weight decrease in rice residue following 3 weeks of treatment were noted with A. terreus53. Similarly, in our study one such fungal strain that was used was A. flavus with other high degrading efficient strains as they had high decomposing efficiency.
Our study showed that the highest cellulase activity was demonstrated in treatments containing microbial consortia, with highest activity on the 30 th DOA which them declined upto 60 th DOA. Another study conducted showed similar results with treatments having microbial consortia with highest cellulose activity and that all treatments had greater cellulase activity at the 28 th DOC, which then declined until the 42nd DOC39. Another study’s results also showed similarity that the CMCase activity of all extracellular hydrolytic enzymes at 30 DAT (Day After Treatment), showed an overall rise, but around 60 DAT, it started to fall. For this study lignocellulolytic bacteria were used, which were C. cinerea and C. stercoreusthat exhibited CMCase activity within 2.30 and 5.58 µmoles/g/d3. In our study we also used 4 different bacterial strains that exhibited the most lignocellulolytic activities. But the strains used in our study were different from other studies as they were isolated from residue management and conservation tillage plots. When only bacterial strain considered they showed high cellulose activity, but in comparison to the fungal strains and microbial consortia used in the experiment, the activity was lesser in comparison. The highest average of CMCase activity of the microbial consortia was 0.335 µmole/ml/h in our experiment.
Our study showed that the highest peroxidase and laccase activities, which are evident of lignin decomposition, were seen on 30 th DOA. The treatments containing microbes had highest decomposition rates, with nutrient supplementation enhancing the decomposition potential of microbes. The highest average peroxidase and laccase activities were found to be 218.8 µmole/g/h\(\:\:\text{a}\text{n}\text{d}\)370.6 µg/µl/h, respectively. The peroxidase activities were much higher than other studies carried out before, which may be due to the distinct combination of microbial consortia used. Another study of residue decomposition showed that the 28 th Day of Composting had the highest laccase activity, followed by the 21 st Day of Composting. At the 28 th Day of Composting, microbial consortium treatments showed higher laccase activities compared to the control which showed the lowest laccase activity39. Microbial inoculation’s impact on agricultural leftovers were significant for peroxidases and laccases, with significant enhancement on 20 th day of incubation55. Another study demonstrated that residue incorporation along with microbial consortia with added nutrient supplementation had the greatest decomposition efficiency. It showed that because of the increased microbial population and the soil’s active breakdown of residues, the T3 (residue incorporation + microbial consortium + urea) may have higher peroxidase activity. At 30 DAT, the T2 treatment exhibited the highest peroxidase activity (2.42 µmoles/g/h), while the T3 treatment displayed the highest activity54.
In our study the highest decomposition rates and highest activity was found in treatments with urea supplementation. The presence of available N was ensured through urea, which facilitated the decomposition process. A study on the effect of N on the degradation potential showed similar findings. It concluded that the treatment’s inclusion of N encourages the infected fungi’s mycelial growth. This increased lignin-degrading enzyme production increased the activity of peroxidase in the fungal biomass56.
According to our current study, there was a spike in CO2 emissions with the increase in decomposing activities. However when compared to CO2emissions by burning the value is on the lower side, being environmentally sustainable. In another study, it was found that the rise in CO2 emissions was caused by the inclusion of microbial consortia, which doubled the rate of decomposition54. In another study, CO2emissions peaked at the 28 th DOC and were higher initially before declining, which added further evidence to the fact that CO2 emissions increase with the increase in decomposing activities39. The same results were found in our study, where the CO2 emissions peaked on the 30 th DOA and then gradually decreased.
Still, even if the lignocellulolytic activities increase the CO2 emissions, but when the value of these emissions are compared with the emissions by residue burning, they are the least significant. When in various studies the amount of CO2 emissions were estimated of open field burning of rice straw, they were 1663.01 kg CO2eqv/kg-d57, 1177 g/kg58and 1160.9 ± 180.9 g/kg59 which are equivalent to 1663 mg/g, 1177 mg/g and 1160 mg/g of CO2 emission. Whereas, in our study the highest value of CO2 emission that was found was 6.16 mg CO2/g/d, which is clearly lower than any CO2 emission by burning. The CO2 emissions by microbial decomposition of residues in our present study and various different studies were clearly lesser than the emissions by open burning of rice straw, proving use of microbial consortia to be a sustainable alternative to residue burning. Our study used a distinct combination of microbial consortia, with bacterial and fungal strains combined. Which can provide a valuable insight to the scientific research on residue waste management. With further modifications, this method of rice straw decomposition can be used on larger scale in fields.
Conclusion
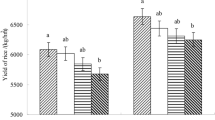
In the rice-wheat agricultural system, rice straw provides a sustained source of nutrients and soil organic matter for improvement of soil health and environment. However, poor degrading methods and a shorter turnaround time between harvests result in burning and air and soil pollution from rice straw management. For the farmers, their rapid in situ management might create a sustainable economy. This study proves the efficacy of lignocellulose-degrading microbial consortia, in conjunction with urea addition and residue introduction, in speeding up rice straw decomposition. The results of study indicated that cellulose, hemicellulose and lignin contents in rice residue decreased 17%, 4% and 16% respectively under treatment T12 (application straw incorporated + Urea + Microbial Consortia). The enzymes with the greatest activity on the 30 th day of application (DOA) were peroxidase (271.2 µmole/g/h), laccase (705.7 µg/µl/h), and cellulose (0.691 µmole/ml/h) in T12. On the 30 th day, the T12’s CO2 emissions peaked at 6.16 mg CO2/g/d, which was still significantly less than the open field burning readings. The treatment T12 had the highest rates of decomposition, with pronounced cellulose, hemicellulose, and lignin depletions and higher enzyme activities, along with regulated CO₂ emissions. These results highlight the promise of microbial consortia as a green substitute for open-field burning, providing a clean solution to management of rice crop residue.The applications of this study reach beyond short-term decomposition gains since enhanced microbial action can increase soil fertility and agricultural sustainability in the long term. Future studies can investigate the feasibility of scaling these techniques in a variety of field conditions, engineer optimal combinations of microbial strains across various soils, and evaluate long-term effects on soil health and crop yields. By optimizing these techniques, microbial residue management may emerge as an effective and usable solution for sustainable agriculture.
Data availability
The datasets analyzed during the current study available from the corresponding author on reasonable request.
References
Permadi, D. A., Kim, N. T. & Oanh Assessment of biomass open burning emissions in Indonesia and potential climate forcing impact. Atmos. Environ. 78, 250–258 (2013).
Wiedinmyer, C. et al. The fire inventory from NCAR (FINN): a high resolution global model to estimate the emissions from open burning. Geosci. Model. Dev. 4 (3), 625–641 (2011).
Kumar, A. et al. Fungal consortium and nitrogen supplementation stimulates soil microbial communities to accelerate in situ degradation of paddy straw. Environ. Sustain. 5 (2), 161–171 (2022).
Jayakumar, M., Thiyagar, T., Abo, L. D., Arumugasamy, S. K. & Jabesa, A. Paddy Straw as a Biomass Feedstock for the Manufacturing of Bioethanol Using Acid Hydrolysis and Parametric Optimization Through Response Surface Methodology and an Artificial Neural Network (Biomass Conversion and Biorefinery, 2024).
Binod, P. et al. Bioethanol production from rice straw: an overview. Bioresour. Technol. 101 (13), 4767–4774 (2010).
Mohanty, M. Rice residue-management Options and Effects on Soil Properties and Crop Productivity (Journal of Food Agriculture and Environment, 2004).
Singh, J. Paddy and wheat stubble blazing in Haryana and Punjab States of India: A menace for environmental health. Environ. Qual. Manage. 28 (2), 47–53 (2018).
Chen, H. Biotechnology of Lignocellulose: Theory and Practice 1-511 (Springer Dordrecht, 2014).
Irfan, M. et al. Estimation Charact. Gaseous Pollutant Emissions Agricultural Crop Residue Combust. Industrial Househ. Sectors Pakistan Atmospheric Environ., 84: 189–197. (2014).
Wang, X. et al. Effects of different returning method combined with decomposer on decomposition of organic components of straw and soil fertility. Sci. Rep. 11 (1), 15495 (2021).
Zhang, G. & Dong, Y. Design and application of an efficient cellulose-degrading microbial consortium and carboxymethyl cellulase production optimization. Front. Microbiol. (13), 957444 (2022).
Song, K. et al. The effects of earthworms on fungal diversity and community structure in farmland soil with returned straw. Front. Microbiol. 11, 594265 (2020).
Huang, S. et al. Effect of environmental C/N ratio on activities of lignin-degrading enzymes produced by phanerochaete Chrysosporium. Pedosphere 30 (2), 285–292 (2020).
Nero, G., Kivirand, K., Ben Othman, S. & Rinken, T. Amperometric method for the determination of cellulase activity and its optimization using response surface method. J. Anal. Sci. Technol. 13 (1), 21 (2022).
Nazar, M. et al. Biological delignification of rice straw using laccase from Bacillus ligniniphilus L1 for bioethanol production: A clean approach for agro-biomass utilization. J. Clean. Prod. 360, 132171 (2022).
Grata, K. Determining cellulolytic activity of microorganisms. Chemistry-Didactics-Ecology-Metrology 25 (1–2), 133–143 (2020).
Micuți, M. M., Bădulescu, L., Burlacu, A. & Israel-Roming, F. Activity of peroxidase and catalase in soils as influenced by some insecticides and fungicides. AgroLife Sci. J. 7 (2), 99–104 (2018).
Liu, W. et al. Improvement of straw decomposition and rice growth through co-application of straw-decomposing inoculants and ammonium nitrogen fertilizer. BMC Plant Biol. 23 (1), 244 (2023).
Han, J., Song, X., Fu, H., Liu, C. & Yang, F. Effects of the Decomposition Agent Application on the Physicochemical Properties and Microbial Community Structure of Wheat straw-returning Soil35p. 103668 (Environmental Technology & Innovation, 2024).
Zahid, A., Ali, S., Ahmed, M. & Iqbal, N. Improvement of soil health through residue management and conservation tillage in Rice-Wheat cropping system of Punjab, Pakistan. Agronomy 10 (12), 1844 (2020).
Sahu, A. et al. Thermophilic ligno-cellulolytic fungi: the future of efficient and rapid bio-waste management. J. Environ. Manage. 244, 144–153 (2019).
Gahfif, O. et al. Isolation and screening of fungal culture isolated from Algerian soil for the production of cellulase and Xylanase. J. Drug Delivery Ther. 10, 108–113 (2020).
Choudhary, M. et al. Crop residue degradation by fungi isolated from conservation agriculture fields under rice–wheat system of North-West India. Int. J. Recycling Org. Waste Agric. 5 (4), 349–360 (2016).
Dabhi, B. K. V.R.a.S.H. Biodegradation of lignin by fungal cultures. J. Pharmacognosy Phytochemistry. 6(4), 840–1842 (2017).
Kausar, H., Sariah, M., Saud, H. M., Alam, M. Z. & Ismail, M. R. Development of compatible lignocellulolytic fungal consortium for rapid composting of rice straw. Int. Biodeterior. Biodegrad. 64, 594–600 (2010).
Shinde, R. Isolation of lignocelluloses degrading microbes from soil and their screening based on qualitative analysis and enzymatic assays. Annals Plant. Soil. Res. 24, 347–354 (2022).
Thormann, M. N., Currah, R. S. & Bayley, S. E. The relative ability of fungi from Sphagnum fuscum to decompose selected carbon substrates. Can. J. Microbiol. 48 (3), 204–211 (2002).
Awadalla, O., Metwally, M., Bedaiwy, M. & Rashad, R. Cellulolytic Activities of some Filamentous fungi from Soil13p. 1 (THE EGYPTIAN JOURNAL OF EXPERIMENTAL BIOLOGY (Botany), 2017).
Choudhary, M., Sharma, P. & Garg, N. Crop residue degradation by autochthonous Fungi isolated from cropping system management scenarios Sharma Prabodh Chander and Garg Neelam. Bioresources 10, 5809–5819 (2015).
Ekundayo, T. & Juwon, A. Isolation and identification of cellulytic Fungi from Agrowastes and sawmill soils. Br. Biotechnol. J. 7, 147–159 (2015).
Sivaramanan, S. & Samaraweera, P. Isolation of cellulolytic fungi and their degradation on cellulosic agricultural wastes. J. Academia Indus. Res. (JAIR) 2, 458–463 (2014).
Mardetko, N. et al. Screening of lignocellulolytic enzyme activities in fungal species and sequential Solid-State and submerged cultivation for the production of enzyme cocktails. Polymers. 13(21), 3736 (2021).
Nayak, B. & Choudhary., R. Lignocellulolytic fungal isolation and screening for their laccase producing ability. Indian J. Sci. Res. 13 (2), 188-191(2017).
Zhang, B. Y., Dou, S., Guan, S., Yang, C. & Wang, Z. Deep straw burial accelerates straw decomposition and improves soil water repellency. Agronomy 13 https://doi.org/10.3390/agronomy13071927 (2023).
Pitt, J.I. and A.D. Hocking, Fungi and food spoilage: J.I. Pitt … and A.D. Hocking. Second edition ed. A Chapman and hall food science book (Gaithersburg: Aspen publication Gaithersburg, 1999).
Tankeshwar, A. Serial dilution method for estimating viable count of bacteria. Available from: https://microbeonline.com/serial-dilution-method/ [cited 2023 23 May]; (2022)
Wet, M. M. M. & Brink, H. G. Fungi in the bioremediation of toxic effluents. 18, 407-431 (Academic Press, 2021).
Avin, F. Easy way to count spores and prepare spore suspension by Hemocytometer. (2019).
Dash, P. K. et al. Efficient lignin decomposing microbial consortium to hasten Rice-Straw composting with moderate GHGs fluxes. Waste Biomass Valoriz. 13 (1), 481–496 (2022).
Miller, G. L. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31 (3), 426–428 (1959).
Baker, B. A. J. Measurement of Cellulase Activities: Laboratory Analytical Procedure (LAP) (National Renewable Energy Laboratory (NREL), 2008).
Baltierra-Trejo, E., Márquez-Benavides, L. & Sánchez-Yáñez, J. M. Inconsistencies and ambiguities in calculating enzyme activity: the case of laccase. J. Microbiol. Methods. 119, 126–131 (2015).
Bach, C. E. et al. Measuring phenol oxidase and peroxidase activities with pyrogallol, l-DOPA, and ABTS: effect of assay conditions and soil type. Soil Biol. Biochem. 67, 183–191 (2013).
German, D. P. et al. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 43 (7), 1387–1397 (2011).
Dinh Vu, N., Thi Tran, H., Bui, N. D., Duc Vu, C. & Viet Nguyen, H. Lignin and cellulose extraction from Vietnam’s rice straw using ultrasound-assisted alkaline treatment method. Int. J. Polym. Sci. 2017, 1063695 (2017).
Franz Schinner, R. Ö., Kandeler, E. & Margesin, R. Methods in Soil Biology. 1 ed. IX, 426 (Springer Berlin, Heidelberg, 2021)
Zhu, L., O’Dwyer, J. P., Chang, V. S., Granda, C. B. & Holtzapple, M. T. Structural features affecting biomass enzymatic digestibility. Bioresour Technol. 99 (9), 3817–3828 (2008).
Sangwan, V. & Deswal, S. In-situ management of paddy stubble through microbial biodegradation. E3S Web of Conferences 241 p. 03001 (2021).
Zhang, E., Wang, M., Pan, X. & Wang, X. Establishment of a Highly Efficient Corn Stock-Degrading Microbial Consortium and its Degradation Effect2022p. 8034553 (Advances in Agriculture, 2022). 1.
Zhang, Z., Shah, A. M., Mohamed, H., Tsiklauri, N. & Song, Y. Isolation and Screening of Microorganisms for the Effective Pretreatment of Lignocellulosic Agricultural Wastes. BioMed Research International, 5514745 (2021).
Yu Hai-Bin, H. L. R., Jun-Shou, F. & Xin, Z. Screening of highly efficient cellulose degradation microbes and construction of composite strains. J. Agricultral Biotechnol. 农业生物技术学报 23 (4), 421–431 (2015).
Bhattacharjya, S. et al. In Situ Decomposition of Crop Residues Using Lignocellulolytic Microbial Consortia: a Viable Alternative To Residue Burning28 (Environmental Science and Pollution Research, 2021).
Safari Sinegani, A. A., Emtiazi, G., Hajrasuliha, S. & Shariatmadari, H. Biodegradation of some agricultural residues by fungi in agitated submerged cultures. Afr. J. Biotechnol. 4, 1058–1061 (2005).
Kumar, A. et al. Fungal consortium and nitrogen supplementation stimulates soil microbial communities to accelerate in situ degradation of paddy straw. Environ. Sustain., (5), 161–1771 (2022).
Sharma, S., Kumawat, K. C. & Kaur, S. Potential of Indigenous ligno-cellulolytic microbial consortium to accelerate degradation of heterogenous crop residues. Environ. Sci. Pollut Res. Int. 29 (58), 88331–88346 (2022).
Huang, X., Li, M., Li, J. & Song, Y. A high-resolution emission inventory of crop burning in fields in China based on MODIS thermal anomalies/fire products. Atmos. Environ. 50, 9–15 (2012).
Mothe, S. et al. Comparison of GHG emissions from open field burning and anaerobic digestion of rice straw. Environ. Technol. 28, 1–11 (2022).
Kumar Sakhiya, A. et al. Sustainable utilization of rice straw to mitigate climate change: A bioenergy approach. Materials Today: Proceedings 46, 5366-5371 (2020).
Pham, C. T. et al. Emission Factors Sel. Air Pollutants Rice Straw Burning Hanoi Vietnam Air Qual. Atmos. Health, 14(11): 1757–1771. (2021).
Acknowledgements
All authors are very thankful to First Fungal Culture Bank and Field Assistants of Faculty of Agricultural Sciences, University of the Punjab, Lahore.
Author information
Authors and Affiliations
Contributions
Ammara Fatima and Adnan Zahid (A.B.): Design the research plan, help in chemical analysis and write up Sajid Ali and Waheed Anwar (C.D.): Support in chemical analysis and write up. Rutaba Zia, Amina Arshad and Sana Imdad: (E.F.G.): Support in collecting the sample and chemical analysis and write up. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fatima, A., Zahid, A., Ali, S. et al. Microbial consortia-mediated rice residue decompositon for eco-friendly management. Sci Rep 15, 32381 (2025). https://doi.org/10.1038/s41598-025-99613-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99613-5