Abstract
This study aims to assess levels of salivary immune markers in women during the 3rd trimester of pregnancy and explores the association between immune markers and dental caries, as well as oral carriage of oral pathogens Candida albicans and Streptococcus mutans. These organisms are known to be associated with oral infectious diseases such as dental caries and oral candida infection. Methods: Pregnant women from underserved background participated in this study (n = 181). Participants received a comprehensive oral examination by trained and calibrated dentists. Levels of 36 immune markers in unstimulated saliva samples were measured using Bio-Plex200 (Luminex). Salivary S. mutans and C. albicans were quantified using viable count (CFU/ml). Quantities of immune markers and colony-forming-units of S. mutans and C. albicans were converted to natural log data for statistical analysis. Latent class analysis was used to assess the clustering effect of immune markers among the participants. Results: The study found high quantities of salivary immune markers in pregnant women with varying detection rates. Univariate analysis found a higher salivary level of IFN-g and TNF-b among women with < 4 decayed teeth, compared to those with ≥ 4 decayed teeth (p < 0.05). Among women with more than 105 CFU/ml S. mutans in saliva, FLT-3 L, IL-17a, TNF-B, and VEGF-a levels were significantly decreased (p < 0.05), while G-CSF levels were significantly increased (p < 0.05). Lower levels of VEGF-a, Eotaxin and IL-9 were found among the individuals with salivary C. albicans (p < 0.05). Two distinct clusters were identified among the 181 participants, low-level and high-level of immune markers. The logistic regression model with Least Absolute Shrinkage and Selection Operator (LASSO) penalty identified several potential variables associated with high-level of salivary immune markers. Debiased results indicated statistically significant association between plaque C. albicans and S. mutans and immune markers (p < 0.05), with higher levels of these variables correlating with lower level of immune markers. In summary, our research underscores the intricate relationship between caries status, microbial populations, and immune markers in pregnant women.
Similar content being viewed by others
Introduction
Pregnancy is a natural physiological process that involves temporary alterations in a woman’s bodily structure, hormonal balance, metabolism, and immune function1. In mothers, inadequate and untreated prenatal oral health care significantly contributes to poor maternal oral health, the potential for negative birth outcomes, and a heightened risk of early childhood caries in their offspring2. Dental caries is the most common and widespread noncommunicable disease impacting people globally today3. In terms of immunological response, both periodontitis and caries exhibit a similar pattern where the nuclear factor kappa B (NF-kB) is activated upon bacterial challenge, which then triggers the release of pro-inflammatory cytokines4. Caries triggers oral immune responses through the release of the cytokines interleukin 6 (IL-6), IL-1, IL-8, as well as tumor necrosis factor (TNF)-α5,6.
In early childhood caries (ECC), a significant global health concern, the roles of Streptococcus mutans and Candida albicans are critically examined, particularly focusing on their synergistic interactions which potentiate cariogenicity7. S. mutans, known for its acidogenic and biofilm-forming capabilities, significantly contributes to tooth demineralization by metabolizing carbohydrates into acids. Similarly, C. albicans enhances the cariogenic environment through acid production and its ability to form complex biofilms on tooth surfaces that can encapsulate S. mutans. Their interaction in the oral cavity reveals cooperative dynamics; synergistically, they enhance each other’s cariogenic effects by metabolic cooperation and increased biofilm viability, resilience, and complexity8. Understanding these microbial interactions provides crucial insights into the pathogenesis of ECC and offers potential therapeutic targets to disrupt these interactions and reduce caries prevalence and severity8.
Previous studies explored the maternal influence of C. albicans and S. mutans transmission in early life, highlighting the importance of women’s oral health during pregnancy9,10. This study aimed to assess levels of salivary immune markers (cytokines, chemokines, and growth factors) in women during the 3rd trimester of pregnancy and explore the association between immune markers and dental caries, as well as oral carriage of C. albicans and S. mutans. Thus, we hypothesize that elevated levels of specific immune markers correlate with varied loads of cariogenic microorganisms during pregnancy.
Materials and methods
Study population
This study utilized data and saliva samples from a cohort study involving 186 pregnant women who were selected from economically disadvantaged demographics at the University of Rochester Medical Center, specifically from Highland Family Medicine (HFM) and Eastman Institute for Oral Health (EIOH) during the years 2017 to 202010,11. Of these women, 181 completed the required saliva sample collections and were included in this study. All participants provided written informed consent. The study’s protocol was approved by the University of Rochester Research Subject Review Board, under approval number #124812. All methods were performed in accordance with the relevant guidelines and regulations. The study cohort consisted of pregnant women who met specific inclusion and exclusion criteria. The inclusion criteria required participants to be over 18 years old, in their third trimester of pregnancy, carrying a single fetus, and eligible for Medicaid insurance, indicating an income below 138% of the Federal Poverty Line. The exclusion criteria ruled out participants with severe systemic diseases, oral cancer, cleft lip or palate, and those who had used antifungal medication in the past three months13. These criteria ensured the selection of a study population suitable for our research objectives.
Data collection and examination methods
The examination and data collection methods used in this study involved comprehensive oral examinations and detailed data/sample collection processes. For instance, oral examinations were conducted during the mother’s third trimester. These examinations assessed caries scores, plaque index, and oral candidiasis, and involved the collection of oral samples such as saliva, plaque, and mucosal swabs, which were performed by 1 of 3 calibrated dentists using standard dental equipment and portable lighting10,11. The supragingival plaque was collected using a sterile scaler and immersed in an Eppendorf tube containing 1 ml of saline solution. A homogeneous mixture was created by sonication and then plated on appropriate agar plates for colony identification. Medical background data, including physician-diagnosed systemic diseases, medication use, and smoking status, were gathered through self-reporting and confirmed using electronic medical records11. Demographic, socioeconomic, and oral hygiene practices data were collected via questionnaires. Saliva samples were collected by instructing participants to abstain from eating, drinking, or brushing their teeth for 2 h prior to collection, ensuring the samples were transported to the lab within 2 h and stored appropriately for further analysis13.
Colonies identification
Salivary and plaque S. mutans and C. albicans were quantified using viable count (CFU/mL)14. The saliva and plaque samples were processed within 2 h after collection by plating them on BBL™ CHROMagar™ Candida (BD, Sparks, MD, USA) using an automated EddyJet Spiral Plater (IUL, SA, Barcelona, Spain) and then incubated at 37 °C for 48 h to isolate C. albicans. This medium allows for the preliminary identification of various significant Candida species, including C. albicans, by observing the color and morphology of the colonies13. The samples were also plated on Mitis Salivarius agar with Bacitracin, incubated at 37 °C for 48 h, to isolate S. mutans, which were identified based on their colony morphology15. C. albicans and S. mutans were further verified using colony polymerase chain reaction method16. Colony-forming unit (CFU) counting methods were employed for the quantification of microbial species.
Immune markers measurement
Thawed unstimulated saliva samples were subjected to high-speed ultracentrifugation to remove large debris17. The profiles of host immunological markers were analyzed using a MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A - Immunology Multiplex Assay, Cat# HCYTA-60 K (Merck Millipore, Darmstadt, Germany)18. A total of 36 analytes were assessed, encompassing a broad spectrum of immune biomarkers found in saliva. These included epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2), C-C motif chemokine ligand 11 (CCL11, formerly eotaxin), transforming growth factor alpha (TGFA), colony stimulating factor 3 (CSF3, formerly granulocyte colony stimulating factor), fms related receptor tyrosine kinase 3 ligand (FLT3LG), vascular endothelial growth factor A (VEGFA), C-X3-C motif chemokine ligand 1 (CX3CL1, formerly fractalkine), C-X-C motif chemokine ligand 1 pseudogene 1 (CXCL1P1, formerly growth-regulated oncogene), and additional markers such as interleukin 1 receptor antagonist (IL-1RN), interleukin 4 (IL-4), interleukin 10 (IL-10), interleukin 8 (IL-8), interleukin 18 (IL-18), interleukin 1 alpha (IL-1a), interleukin 1 beta (IL-1b), interleukin 2 (IL-2), interleukin 6 (IL-6), interleukin 12p40 (IL-12p40), interleukin 12p70 (IL-12p70), interleukin 17a (IL-17a), tumor necrosis factor alpha (TNF-a), tumor necrosis factor beta (TNF-b), monocyte chemoattractant protein-1 (MCP-1), monocyte chemoattractant protein-3 (MCP-3), macrophage-derived chemokine (MDC), macrophage inflammatory protein-1 beta (MIP-1b), regulated on activation, normal T cell expressed and secreted (RANTES), soluble CD40 ligand (sCD40L), interferon alpha 2 (IFN-a2), interferon gamma (IFN-g), interleukin 5 (IL-5), interleukin 9 (IL-9), interleukin 13 (IL-13), interleukin 15 (IL-15), Interferon gamma-induced Protein 10 (IP-10), reflecting a diverse range of immune responses in the oral environment.
Statistical analysis
Quantities of immune markers and colony-forming-units of S. mutans and C. albicans were converted to natural log data to normalize their distribution. The R software version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) was utilized for all computations. Univariate analysis highlighted individual marker characteristics. Latent Profile Analysis then clustered subjects based on these markers, revealing distinct immune response patterns. Further univariate analysis compared these clusters, uncovering significant differences in marker levels, Chi squared test was utilized for binary variables while an unpaired t-test was used for continuous variables. L1-penalized Logistic Regression was applied to explore the relationship between clusters and various covariates, enhancing model accuracy and interpretability. Post-selection inference provided reliable p-values, ensuring robust association tests between clusters and covariates, thus offering a comprehensive understanding of the characteristics’ impact on immune markers.
Results
181 pregnant women completed in this study. Among the participants, 70% were ≤ 30 years old, 58% had either middle school or high school education level, and 78% were unmarried. Regarding the participants’ race, 53% were Black, 29.3% were White, and 17.7% were from other racial backgrounds.
Univariate analysis of individual immune markers
In this study, we analyzed the detection rates and concentrations of various immune markers in 181 pregnant women. The immune markers evaluated showed varying levels of detectability and concentrations (Table 1). Markers such as EGF, IL-1a, IL-1ra, IL-8, IL-18, MCP-1, IL-1B, and IL-6 had high detection rates of over 90%, with IL-1a, IL-1ra, and EGF being detected in all participants. The mean concentrations of these markers varied widely, with IL-1ra and IL-1a showing notably high mean values of 2009.6 ± 2027.7 pg/mL and 1870.4 ± 2298 pg/mL, respectively. Other markers, such as TGF-a, Eotaxin-1, and CD40L, had moderate detection rates ranging from 70 to 87%, with corresponding mean concentrations of 7.7 ± 7 pg/mL, 14.6 ± 13.1 pg/mL, and 77.4 ± 88.8 pg/mL. Notably, several markers including IL-4, IL-5, and IL-2 had low detection rates of 20–33%, reflecting lower overall presence in the cohort. The median concentrations and ranges also varied significantly, with markers like IL-1ra and EGF exhibiting wide concentration ranges (55.8–13147.7 pg/mL and 28.5–4789.4 pg/mL, respectively). These findings illustrate the diverse immune profiles among pregnant women, highlighting both prevalent and less common immune markers in this population.
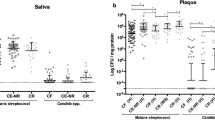
Pregnant women with ≥ 4 decayed teeth had lower salivary level of IFN-g and TNF-b, compared to those with < 4 decayed teeth (p < 0.05) (Fig. 1A). Among women with more than 105 CFU/ml S. mutans in saliva, FLT-3 L, IL-17a, TNF-B, and VEGF-a levels were significantly decreased (p < 0.05), while G-CSF levels were significantly increased (p < 0.05) (Fig. 1B). A lower level of Eotaxin, VEGF-a and IL-9 was found among the individuals with salivary C. albicans (p < 0.05) (Fig. 1C). The findings underscore the distinct immune responses associated with dental decay, microbial load, and fungal presence, highlighting their potential role as biomarkers for oral health and infection.
The results indicate significant differences in immune marker levels under various conditions. (A) reveals that levels of TNF-b and IFN-g are significantly higher in individuals with fewer than 4 decayed teeth compared to those with 4 or more decayed teeth. (B) Shows that levels of FLT-3 L, IL-17a, TNF-b, and VEGF-a are significantly higher in samples with CFU/ml < 10^5 compared to those with CFU/ml ≥ 10^5, while G-CSF levels are significantly lower in samples with CFU/ml < 10^5 compared to those with CFU/ml ≥ 10^5. Figure (c) demonstrates that levels of Eotaxin, VEGF-a, and IL-9 are significantly higher in C. albicans positive samples compared to negative ones.
Immune markers levels and clusters of saliva during pregnancy
In order to study 36 immune markers simultaneously, we conducted the Latent Profile Analysis (LPA) using the natural log-transformed value of all immune markers to generate clusters and analyze variations of covariates between clusters. As most of the popular clustering methods, LPA requires pre-specified number of clusters. Among the study population, two clusters were identified, cluster with higher immune markers levels (Cluster high, n = 54) and cluster with lower immune markers levels (Cluster low, n = 127) (Fig. 2). Moreover, immune markers were organized based on function as anti-inflammatory, dual function, and pro-inflammatory markers.
Based on salivary immune markers, two clusters of pregnant women were generated using a latent model. Two distinct clusters emerged from 181 samples: one with low immune marker levels (n = 127) and one with high immune marker levels (n = 54). Despite the minimal overlap between these two clusters, the classification effectively differentiates between clusters based on immune marker levels.
The comparison between the Low (n = 127) and High (n = 54) groups across various binary variables revealed several significant findings (Table 2). In terms of demographics, there were no significant differences observed in age > 30 (p = 0.148), Black race (p = 0.710), or Hispanic ethnicity (p = 1). Medical record variables, including yeast infection, antibiotic use, antifungal use, prenatal inhaler use, prenatal diabetes, prenatal asthma, prenatal emotional condition, depression score > 12, prenatal hypertension, and prenatal smoking, did not show significant differences between the groups (p > 0.05). In socioeconomic status, while employment status was similar between the groups (p = 1), marital status was significantly higher in the High group (33.33%) compared to the Low group (17.32%) (p < 0.05). Regarding birth outcomes, the incidence of low birth weight was higher in the High group (7.41%) compared to the Low group (2.36%), though this difference was not statistically significant (p = 0.234). Oral health variables displayed notable differences: the detection of Saliva Candida (p < 0.05), Saliva S. mutans (p < 0.05), Plaque Candida (p < 0.001), and Plaque S. mutans (p < 0.001) was significantly lower in the High group compared to the Low group, however, brushing twice daily did not show any significant difference. These results highlight that marital status and specific oral health indicators significantly differ between the Low and High groups, while other demographic, medical, socioeconomic, and birth outcome variables do not.
The comparison of various continuous variables between the Low (n = 127) and High (n = 54) groups revealed significant differences in several hormonal levels and microbial counts (Table 3). The mean age, gestational week and infant birth weight were similar between the two groups, with no significant differences observed. Dental health indicators such as DMFT, DMFS, and ICDAS scores also did not differ significantly. However, hormonal analysis showed significantly higher mean levels of cortisol (p < 0.0001), estradiol (p < 0.001), testosterone (p < 0.001), T3 (p < 0.001), and T4 (p < 0.05) in the Low group compared to the High group. Progesterone levels, though higher in the Low group, did not reach statistical significance (p = 0.133). Microbial analysis revealed significantly higher counts of C. albicans in both saliva (p < 0.05) and plaque (p < 0.0001) in the Low group. Additionally, the plaque counts of S. mutans were significantly higher in the Low group (p < 0.05), while saliva counts of S. mutans did not differ significantly. These findings suggest notable associations between the measured variables and the Low and High groups, particularly in the context of hormonal and microbial differences, warranting further investigation.
Variables associated with immune markers cluster
The logistic regression model with LASSO penalty identified several variables significantly associated with high immune markers, using low immune markers as the reference (Table 4). Positive coefficients were observed for being married (0.196), low birth weight (0.109), and prenatal diabetes (0.160), suggesting that these factors are associated with a higher likelihood of having high immune markers. Conversely, negative coefficients were observed for cortisol (-0.180), testosterone (-0.065), plaque C. albicans (-0.574), and plaque S. mutans (-0.763), indicating that higher levels of these variables are associated with a decreased likelihood of having high immune markers. These results highlight the complex interplay between various demographic, hormonal, and microbial factors in determining immune marker levels, emphasizing the need for a multifactorial approach in understanding and addressing the underlying mechanisms influencing immune status. Since LASSO penalty shrank the estimator toward zero, the coefficients were biased. To make inference, we applied the debiased procedure (Table 5)19. The debiased result is as follows: Two plaque-related variables (Plaque C. albicans and Plaque S. mutans) had p-values less than 0.05, indicating a statistically significant effect on the likelihood of having high immune markers. The negative debiased coefficients for these plaque-related variables suggest that higher levels of these variables are associated with a lower proportion of subjects having high immune markers.
Cytoscape software was utilized to construct and analyze the complex interactions between pathogens, immune markers and hormones20. The network analysis uncovered distinct associations between salivary and plaque levels of Candida and S. mutans with a diverse array of immune markers and hormones (Fig. 3). Candida exhibited strong correlations with both pro-inflammatory markers, including IL-1β, TNF-α, and IL-17 A, as well as anti-inflammatory markers such as IL-10 and TGF-α. This suggests that Candida plays a role in modulating a wide-ranging inflammatory response. In contrast, S. mutans, particularly in saliva, was primarily linked to pro-inflammatory cytokines like MCP-1, IL-12p40, and RANTES, indicating a more focused inflammatory activation. Additionally, hormones markers such as cortisol and progesterone were associated with both microbial species, implying systemic regulatory influences that may mediate the immune response to these microbes. Collectively, these findings emphasize the intricate interplay between oral microbiota, immune pathways, and systemic hormonal regulation, highlighting potential biomarkers for microbial-driven inflammation and overall systemic health.
This network illustrates the relationships between Candida and S. mutans levels (pink nodes) in saliva and plaque and various immune and hormonal markers. Immune markers are categorized as pro-inflammatory (green), anti-inflammatory (orange), and dual-function (purple), revealing their potential roles in microbial-host interactions.
Discussion
The feasibility of using salivary immune markers to monitor physiological changes during pregnancy and their impact on oral health, particularly dental caries, is supported by recent advancements in non-invasive diagnostic methods. Saliva collection is advantageous due to its non-invasive nature, lower biosafety requirements, and ease of repeated sampling, making it ideal for longitudinal studies in pregnant women21.Our study provides valuable insights into the immune changes that occur during the third trimester of pregnancy, focusing on salivary immune markers and their associations with hormonal levels and microbial counts. High detection rates (> 90%) were observed for markers such as EGF, IL-1a, IL-1ra, IL-8, IL-18, MCP-1, IL-1B, and IL-6, with IL-1ra and IL-1a showing notably high mean concentrations of 2009.6 ± 2027.7 pg/mL and 1870.4 ± 2298 pg/mL, respectively. Markers like TGF-a, Eotaxin-1, and CD40L had moderate detection rates (70-87%), while IL-4, IL-5, and IL-2 had low detection rates (20-33%). Significant associations were found between oral health indicators and immune responses, with lower salivary levels of IFN-g and TNF-b in women with ≥ 4 decayed teeth and variations in immune marker levels in relation to microbial loads and fungal presence. Latent Profile Analysis identified two clusters of immune marker levels, highlighting significant differences in demographic, socioeconomic, hormonal, and microbial variables, with higher cortisol, estradiol, testosterone, T3, and T4 levels and higher counts of C. albicans and S. mutans in the low-level cluster. Logistic regression with LASSO penalty revealed significant associations between marital status, low birth weight, diabetes, hormonal levels, and microbial counts with immune marker levels, underscoring the complex interplay of factors influencing immune status during pregnancy.
The rationale behind immune markers selection
The rationale for selecting this panel of 36 immune markers is based on current literature indicating multiple immune markers associated with caries. However, in our study, we were unable to include all immune markers mentioned in literature due to constraints in sample volume and the cross-reactivity of several markers when using the same assay kit. A study by Baker et al. examined 38 immune markers among children (EGF, FGF2, CCL11(eotaxin), TGFA, CSF3, CSF2, FLT3LG, VEGFA, CX3CL1 (fractalkine), CXCL1P1, CCL7, CCL22, CXCL8, CXCL10(IP-10), CCL2, CCL3, CCL4, IFNA2, IFNG, IL-1a, IL-1b, IL-1RN, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, CD40L, TNF, and LTA) finding that ten were elevated due to dental caries17. These markers include epidermal growth factor (EGF), interleukin 10 (IL-10), colony stimulating factor 3 (CSF3), interleukin 1 receptor antagonist (IL-1RN), colony stimulating factor 2 (CSF2), CCL22, interleukin 13 (IL-13), interleukin 15 (IL-15), and interleukin 6 (IL-6), all of which have significant roles in the development and progression of dental caries17. Another cross-sectional study examined 12 immune markers (IFN-γ, IL-1α, IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IP-10, TNF-α, VEGF-A), the concentrations of IL-1β, IL-6, IL-8, and IL-10 were markedly elevated among the caries group22. FGF expression plays a crucial role in various stages of tooth development, including the initiation of teeth and the formation of mineralized tissues. In rodents, FGFs are uniquely vital for maintaining the stem cell niche that supports the continuous growth of their incisors throughout their lives23. Moreover, previous studies have identified elevated levels of various cytokines in caries-affected dental pulp and/or odontoblasts. These include transforming growth factor-β1 (TGFβ1), vascular endothelial growth factor (VEGF), C-C chemokine ligand 2 (CCL2/MCP1), CCL20/MIP3α, interleukin 8 (IL8/CXCL8), CXC chemokine ligand 10 (CXCL10), epithelial cell-derived neutrophil attractant 78 (ENA78), IL-1β, IL2, IL4, IL6, IL10, IL11, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α)24.
In adults, the selected markers include TNFα, IFN-g, GM-CSF, IL-2, IL-4, IL-6, IL-8, and IL-10, reflecting the complex interactions between the immune system and cariogenic factors in adult populations25. This comprehensive panel aims to cover a broad spectrum of immune responses to provide a thorough understanding of caries-related immunological mechanisms. Furthermore, multiple studies indicated the role of IL-17 A in protection against C. albicans (an important cariogenic organism) and its regulation of antifungal immunity26, IL-17 A is crucial for combating candidiasis both at mucosal and systemic levels27. A single arm clinical trial on adult participants with candidiasis also revealed significant reduction in the levels of salivary cytokines Eotaxin and Fractalkine in the group receiving an antifungal intervention28.
The effect of salivary hormones on the immune markers
According to Mor and Cardenas (2010), the third trimester is characterized by a renewed pro-inflammatory state, essential for initiating labor and ensuring successful delivery. This phase involves elevated levels of pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6, which are crucial for processes like cervical ripening, membrane rupture, and uterine contractions29. Our analysis revealed that certain salivary hormones and microbial counts are significantly associated with immune marker levels during this critical period. Specifically, higher levels of cortisol and testosterone were negatively associated with high immune markers. Cortisol functions as an immunosuppressant by downregulating key inflammatory transcription factors such as NF-kB and AP-1, while simultaneously upregulating suppressor of cytokines (SOCS). This upregulation of SOCS inhibits STAT phosphorylation, leading to a reduction in downstream pro-inflammatory gene transcription, thereby weakening the pro-inflammatory response30,31. The observed negative association between cortisol and high immune markers is a significant finding that aligns with the well-documented immunosuppressive effects of cortisol. Cortisol, a glucocorticoid hormone, plays a crucial role in the body’s stress response and is known to suppress various components of the immune system, including cytokine production, antibody formation, and the activity of immune cells such as T-lymphocytes and macrophages32. This immunosuppressive effect can reduce the body’s ability to mount an effective immune response, which may explain why higher cortisol levels are associated with lower immune markers. Testosterone similarly exhibits immunosuppressive effects by inhibiting the production of pro-inflammatory cytokines and reducing the activity of various antimicrobial immune cells, contributing to the reduced immune marker levels33. The results of this study are consistent with previous research, for example a study by Yang et al. (2024), which identified significant associations between salivary hormones, dental caries, and cariogenic microorganisms during pregnancy13. Yang et al. reported that higher levels of salivary progesterone, estradiol, testosterone, and cortisol were positively associated with increased carriage of S. mutans and higher caries risk. Our study corroborates these findings, emphasizing the role of salivary hormones in modulating oral health and immune markers.
The influence of marital status and diabetes on the immune response
Conversely, demographic factors such as being married and having diabetes were positively associated with high immune markers. The social support associated with being married may help mitigate stress and its associated immunosuppressive effects, thereby contributing to higher immune markers. Additionally, the elevated immune markers observed in married pregnant women may reflect an enhanced ability to cope with these physiological demands due to reduced stress levels34. The third trimester’s pro-inflammatory state, while essential for labor, can also increase susceptibility to infections and inflammatory conditions34. Thus, the supportive environment provided by a stable marital relationship might play a crucial role in balancing the immune response, potentially reducing the risk of adverse outcomes related to excessive inflammation. Furthermore, the relationship between diabetes and altered immune marker levels during pregnancy highlights the complex interplay between metabolic and immune systems35. Pregnant women with diabetes, particularly gestational diabetes mellitus (GDM) and type 1 diabetes mellitus (T1DM), demonstrate a distinctive immune profile characterized by heightened inflammation. This pro-inflammatory state is evidenced by elevated levels of biomarkers such as CRP, IL-6, and PAI-135. Hence, diabetes was positively correlated with high immune marker levels in our study cohort. Previous studies have shown that individuals with diabetes mellitus (DM) tend to have a higher incidence of both coronal and root caries, particularly when their glycemic control is inadequate36. Additionally, DM is linked to changes in the immune response, which may affect salivary cytokine levels regardless of the presence of C. albicans or S. mutans.
Immune markers and low birth weight: potential predictive biomarkers
A paper by Padilla-Cáceres et al. examined the relationship between periodontal disease and adverse pregnancy outcomes, such as preterm birth and low birth weight. The study conducted a comprehensive review of systematic reviews and meta-analyses to explore this association. The findings indicated that periodontal disease in pregnant women is associated with an increased risk of preterm birth and low birth weight in newborns37. This association may be due to inflammatory processes or the translocation of periodontal pathogens. This explains the relationship between poor oral health and low birth weight. An unexpected finding was evaluated in our study indicating a positive relationship between high immune markers and low birth weight, this suggests that salivary immune status in pregnant women may serve as a predictive biomarker for adverse birth outcomes, such as low birth weight. However, this potential application requires further validation through future studies to confirm its accuracy and reliability.
Immune markers in saliva as indicators of caries and microbial imbalance
Certain cytokines and chemokines have shown promise as biomarkers for caries. These immune markers are part of the body’s response to inflammation and infection, and their presence in saliva can reflect the underlying pathological processes leading to caries formation 38. The number of decayed surfaces (DS) showed a trend towards significance (p = 0.055), suggesting women with high immune markers levels have less teeth surfaces affected by caries (Table 3). Also, the number of filled surfaces (FS) shows borderline significance (p = 0.053), indicating women with high immune markers levels have less restored teeth surfaces suggesting elevated immune markers showing a protective mechanism by reducing the need for restorative treatment. Microbial analysis highlighted that higher counts of plaque C. albicans and plaque S. mutans were negatively associated with high immune markers. The significantly lower S. mutans count in dental plaque among individuals with high immune markers, despite no difference in salivary levels, suggests that host immune responses may locally modulate bacterial colonization at the site of caries initiation. This supports the ecological plaque hypothesis and highlights the potential role of immunity in maintaining oral microbial balance39.
This suggests that an altered oral microbiome, potentially facilitated by a suppressed immune system due to higher cortisol and testosterone levels, can influence immune marker levels. The presence of these cariogenic microorganisms may reflect an imbalance in the oral environment due to hormonal changes and immune modulation during pregnancy.
Study limitations
The limitations of this study include the inherent constraints of a cross-sectional design, which preclude the determination of causality between salivary immune markers and the dental caries status or presence of cariogenic pathogens. Additionally, this study faced constraints in sample volume and did not account for all potential confounding variables such as dietary habits, detailed salivary characteristics, and the impact of diurnal fluctuations on immune markers. The cross-reactivity of several markers when using the same assay affected the immune markers selection process, limiting other important immune markers from being included in the analysis. The study’s participant pool was drawn exclusively from underserved communities in Upstate New York, limiting the generalizability of the findings to other populations or regions. Future research should consider longitudinal studies with more diverse populations and geographical settings to further elucidate the role of immune markers in oral health during pregnancy. Additionally, understanding the maternal influence on immunity in early life and its association with cariogenic microorganisms, as well as assessing the underlying mechanisms that link immune markers with caries risk and adverse birth outcomes, will be crucial.
Conclusions
In conclusion, our study highlights the complex interplay between hormonal levels, microbial counts, and immune markers in pregnant women. These findings suggest that monitoring salivary hormones and microbial presence could provide valuable insights into immune status and potential risks for adverse oral health outcomes during pregnancy. Overall, 36 immune markers were observed in saliva with varying detection rates among pregnant women from low-income and minoritized background, indicating the feasibility of using saliva as a diagnostic sample. Further research is warranted to comprehensively assess the role of salivary immune markers in understanding the immunological mechanisms between oral microorganisms and the host, and to develop targeted interventions to improve maternal and fetal health.
Data availability
A complete record of all the data collected or analyzed during this study is provided in this paper. The corresponding author can be contacted for further information.
References
Jahan, S. S. et al. Oral healthcare during pregnancy: its importance and challenges in Lower-Middle-Income countries (LMICs). Int. J. Environ. Res. Public. Health, 19(17). (2022).
Wu, T. T. et al. Metagenomic analysis examines oral Microbiome changes and interplay with immune response following prenatal total oral rehabilitation. J. Transl. Med. 21 (1), 172 (2023).
Schwendicke, F. et al. When to intervene in the caries process? An expert Delphi consensus statement. Clin. Oral Investig. 23 (10), 3691–3703 (2019).
Taso, E. et al. Effect of dental caries on periodontal inflammatory status: A split-mouth study. Arch. Oral Biol. 110, 104620 (2020).
Lo Giudice, G. et al. Correlation of s-IgA and IL-6 salivary with caries disease and oral hygiene parameters in children. Dent. J. (Basel), 8(1). (2019).
Alarcon-Sanchez, M. A. et al. Activity and levels of TNF-alpha, IL-6 and IL-8 in saliva of children and young adults with dental caries: A systematic review and meta-analysis. BMC Oral Health. 24 (1), 816 (2024).
Falsetta, M. L. et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 82 (5), 1968–1981 (2014).
Lu, Y. et al. Roles of Streptococcus mutans-Candida albicans interaction in early childhood caries: A literature review. Front. Cell. Infect. Microbiol. 13, 1151532 (2023).
Mitchell, S. C. et al. Maternal transmission of mutans Streptococci in severe-early childhood caries. Pediatr. Dent. 31 (3), 193–201 (2009).
Alkhars, N. et al. Multilocus sequence typing of Candida albicans oral isolates reveals high genetic relatedness of mother-child dyads in early life. PLoS ONE. 19 (1), e0290938 (2024).
Alkhars, N. et al. Oral Candida predicts Streptococcus mutans emergence in underserved US infants. J. Dent. Res. 101 (1), 54–62 (2022).
Al Jallad, N. et al. Racial and oral health disparity associated with perinatal oral health care utilization among underserved US pregnant women. Quintessence Int. 53 (10), 892–902 (2022).
Yang, R. et al. Association between salivary hormones, dental caries, and cariogenic microorganisms during pregnancy. J. Clin. Med., 13(11). (2024).
Xiao, J. et al. Oral health and Candida carriage in socioeconomically disadvantaged US pregnant women. BMC Pregnancy Childbirth. 19 (1), 480 (2019).
Xiao, J. et al. Candida albicans carriage in children with severe early childhood caries (S-ECC) and maternal relatedness. PLoS ONE. 11 (10), e0164242 (2016).
Chen, Z. et al. Development of species-specific primers for detection of Streptococcus mutans in mixed bacterial samples. FEMS Microbiol. Lett. 272 (2), 154–162 (2007).
Baker, J. L. et al. Deep metagenomics examines the oral Microbiome during dental caries, revealing novel taxa and co-occurrences with host molecules. Genome Res. 31 (1), 64–74 (2021).
Lee, K. T. et al. Salivary pro-Inflammatory markers and smoking status influences the treatment effectiveness of periodontal disease patients with hypertension. Int. J. Environ. Res. Public. Health, 18(14). (2021).
Taylor, J. & Tibshirani, R. Post-Selection inference for l(1)-Penalized likelihood models. Can. J. Stat. 46 (1), 41–61 (2018).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13 (11), 2498–2504 (2003).
Szabo, Y. Z. & Slavish, D. C. Measuring salivary markers of inflammation in health research: A review of methodological considerations and best practices. Psychoneuroendocrinology 124, 105069 (2021).
Rinderknecht, C. et al. Associations between salivary cytokines and oral health, age, and sex in healthy children. Sci. Rep. 12 (1), 15991 (2022).
Du, W., Du, W. & Yu, H. The Role of Fibroblast Growth Factors in Tooth Development and Incisor Renewal. Stem Cells Int, p. 7549160. (2018).
Horst, O. V. et al. Caries induced cytokine network in the odontoblast layer of human teeth. BMC Immunol. 12, 9 (2011).
Pakdemirli, A. Salivary Pro-Inflammatory cytokines and salivary bacterial challenge effect on dental caries: A clinico-molecular cross-sectional study. J. Basic. Clin. Health Sci. 1, 77–81 (2020).
Conti, H. R. & Gaffen, S. L. IL-17-Mediated immunity to the opportunistic fungal pathogen Candida albicans. J. Immunol. 195 (3), 780–788 (2015).
Mengesha, B. G. & Conti, H. R. The role of IL-17 in protection against mucosal Candida infections. J. Fungi (Basel), 3(4). (2017).
Aljaffary, M. et al. Effects of Nystatin oral rinse on oral Candida species and Streptococcus mutans among healthy adults. Clin. Oral Investig. 27 (7), 3557–3568 (2023).
Mor, G. & Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 63 (6), 425–433 (2010).
Heck, S. et al. I kappab alpha-independent downregulation of NF-kappaB activity by glucocorticoid receptor. EMBO J. 16 (15), 4698–4707 (1997).
Jonat, C. et al. Antitumor promotion and antiinflammation: Down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 62 (6), 1189–1204 (1990).
Strehl, C. et al. Glucocorticoids-All-Rounders tackling the versatile players of the immune system. Front. Immunol. 10, 1744 (2019).
Gubbels Bupp, M. R. & Jorgensen, T. N. Androgen-Induced Immunosuppression Front. Immunol., 9, 794. (2018).
Henriksen, R. E. & Thuen, F. Marital quality and stress in pregnancy predict the risk of infectious disease in the offspring: The Norwegian mother and child cohort study. PLoS One. 10 (9), e0137304 (2015).
Wierzchowska-Opoka, M. et al. Impact of obesity and diabetes in pregnant women on their immunity and vaccination. Vaccines (Basel) 11 (7). (2023).
de Lima, A. K. A. et al. Diabetes mellitus and poor glycemic control increase the occurrence of coronal and root caries: A systematic review and meta-analysis. Clin. Oral Investig. 24 (11), 3801–3812 (2020).
Padilla-Caceres, T. et al. Association between the risk of preterm birth and low birth weight with periodontal disease in pregnant women: An umbrella review. Dent. J. (Basel), 11(3). (2023).
Paque, P. N. et al. Salivary biomarkers for dental caries detection and personalized monitoring. J. Pers. Med., 11(3). (2021).
Marsh, P. D. Are dental diseases examples of ecological catastrophes? Microbiol. (Reading). 149 (Pt 2), 279–294 (2003).
Acknowledgments
We thank The University of Rochester Medical Center’s [Flow Cytometry Core], for their assistance with [Bio-Plex200 (Luminex)] technology. I acknowledge the assistance of OpenAI’s ChatGPT 2024 in providing editorial suggestions and support for refining the writing of this work.
Funding
This research was funded by the National Institutes of Health (R01DE031025).
Author information
Authors and Affiliations
Contributions
Conceptualization, N.A. and J.X.; methodology, N.A., J.X., X.M., R.Y., N.A, T.W., S.Q., A.S. and X.L. ; software, X.M., T.W.; validation, N.A., J.X. and T.W.; formal analysis, N.A. and X.M; investigation, N.A., J.X. and X.M; resources, N.A. and R.Y; data curation, N.A.; writing—original draft preparation, N.A.; writing—review and editing, N.A., J.X., X.M. and N.A.; visualization, N.A. and X.M.; supervision, J.X. and T.W.; project administration, N.A.; funding acquisition, J.X. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alomeir, N., Mao, X., Yang, R. et al. Association between salivary immune markers and oral health conditions in pregnant women. Sci Rep 15, 15776 (2025). https://doi.org/10.1038/s41598-025-99692-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99692-4





