Abstract
In this study, based on the escalating demand for thermally stable scale inhibitors in high-pressure/high-temperature (HPHT) water–gas reservoirs, an organic–inorganic composite scale inhibitor (CT-5) was successfully synthesized via solution polymerization-mediated in situ intercalation using acrylic acid (AA), 2-acrylamido-2-methylpropanesulfonic acid (AMPS) and diallyldimethylammonium chloride (DMDAAC) as monomers, with surface-modified montmorillonite (MMT) as reactive filler. Orthogonal optimization established ideal synthesis parameters, which including a reaction temperature of 75 °C, an initiator dosage of 0.6%, a solution pH of 7, a reaction time of 12 h, and a monomer ratio of m (AMPS): m (AA): m (DMDAAC): m (MMT) = 48:25:23:4. Moreover, the molecular structure and thermal stability of CT-5 were characterized by FTIR, XRD, and TG-DTG, as a result, the polymer intercalated MMT was successful, and CT-5 had a composite intercalation structure of organic polymer/inorganic montmorillonite, with a thermal decomposition temperature of 235.24 °C. Salt tolerance evaluation demonstrated robust performance under saline conditions. The scale inhibition mechanism of CT-5 was explored through scale inhibition rate testing, interlayer spacing testing at different temperatures, characterization of CaCO3 scale crystal structure and morphology, and chemical binding energy testing of CaCO3 scale crystals. The CT-5 can release effective chelating groups in the intercalation layer at high temperature, which inhibits the formation of CaCO3 scale by chelating Ca2+ to form chelates, and also forms an adsorption layer on the surface of CaCO3 scale crystals to interfere with the normal growth of CaCO3 scale crystals and change the lattice structure of CaCO3 scale crystals, thereby achieving the scale inhibition effect.
Similar content being viewed by others
Introduction
Longwangmiao Gas Reservoir in Sichuan Basin, China is a typical high temperature and high-pressure gas reservoir with ultra-large low porosity and high pressure with water. The buried depth is 4600 ~ 4900 m, the temperature in the middle of the gas reservoir is 145 °C, and the formation pressure is as high as 74 ~ 78 MPa1. Progressive exploitation of the Longwangmiao gas reservoir has exacerbated aquifer encroachment, wherein high-TDS formation water under high-pressure, high-temperature (HPHT) downhole conditions triggers calcium carbonate scaling, as a result, an increase in the friction coefficient of the wellbore and the throttle phenomenon in the wellbore were formed, which will seriously affect the normal production of the producing wells and seriously restrict the upper production of the gas field2. However, the current studies comprehensively address scale formation mechanisms in gas reservoirs, and the critical knowledge gaps persist regarding design and preparation, structure–activity relationship, and mechanism of action of scale inhibitors in specific environments of gas reservoirs.
Currently, the scale inhibitor solution with certain concentration was applied to solve the problem of scaling inside the wellbore depends on the combination of functional groups in the scale inhibitor, and the main mechanism is that the combination of functional groups in the scale inhibitor (such as phosphonic acid, carboxylic acid, sulfonic acid, and amide groups) and Ca2+ to delay the growth of CaCO3 scale crystals and inhibit the formation of CaCO3 scale. Presently, the scale inhibitors, such as natural polymers, phosphates, copolymers and environmental-friendly types, are widely used3,4. Undeniably, the formation of CaCO3 scale has been effectively reduced to some extent by these traditional types of scale inhibitors, but, there are also some drawbacks that limit its further promotion and application, such as the easy decomposition of natural polymer scale inhibitors at high temperatures5. Besides, the principal limitation of phosphate-based scale inhibitors resides in their propensity to undergo pronounced thermal hydrolysis under high temperature, which subsequently induces aquatic eutrophication through phosphate release6,7. Moreover, the copolymer scale inhibitors are further challenged by suboptimal thermal tolerance and non-negligible eco-toxicity, particularly under prolonged operational conditions. Furthermore, the environment-friendly scale inhibitors demonstrate favorable environmental compatibility, but they exhibit compromised scale inhibition efficacy under elevated-temperature conditions8. Recently, some organic scale inhibitors were developed by adding functional groups, such as sulfonic acid groups, which with excellent performance under high temperature. However, the organic scale inhibitors are main used in cooling water circulation system, and the application temperature is basically not more than 100 °C9,10. Scale inhibition performance under elevated thermal conditions is inherently compromised by structural heterogeneity within organic polymer matrices, resulting in suboptimal or negligible scaling mitigation efficacy11.
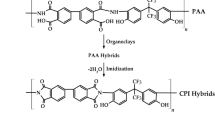
Montmorillonite (MMT) functions as an active polymerization filler primarily due to its expandable interlayer spaces, high-aspect-ratio nanolayers, and intrinsic cation-exchange capability, synergistically enhance polymer nucleation and matrix integration. Secondly, which distinct from conventional fillers, such as silica or carbon black, MMT’s structural anisotropy facilitates monomer intercalation within its aluminosilicate galleries, enabling in situ exfoliation during polymerization to achieve homogeneous dispersion. Moreover, its superior interfacial interactions with initiators or monomers, coupled with exceptional thermal stability ensure structural integrity under high-temperature polymerization conditions12. Regarding the problem of poor temperature resistance of the scale inhibitor mentioned above, based on the characteristics of MMT, during this study, a polymerizable quaternary ammonium salt was used to modify montmorillonite, which could use as an active polymerizable filler12. Then, the intercalated MMT served as reactive filler for synthesizing high-temperature scale inhibitor CT-5 via in-situ polymerization of AMPS, AA, and DMDAAC monomers. Structural characterization (XRD, FTIR, TGA) and mechanistic analysis revealed enhanced thermal stability. This organic–inorganic composite broadens the chemical diversity of scaling inhibitors, establishing a design framework for thermally adaptive materials.
Materials and methods
Materials
2-acrylamido-2-methylpropanesulfonic acid (AMPS), diallyldimethylammonium chloride (DMDAAC), acrylic acid (AA), sodium bisulfite (NaHSO3), potassium persulfate (K2S2O8) is all analytical grade, which purchased from Kelong Chemical plant (Chengdu, China). Montmorillonite (MMT) is an industrial product, which purchased from Xinjiang Zhongfei Xiazi Street Bentonite Co., Ltd.
Synthesis of CT-5
Selection of co-monomers
Due to the presence of long-chain side groups and sulfonic acid groups with good temperature and salt resistance in the molecular structure of AMPS, it has a significant steric hindrance effect and strong coordination effect, which can effectively improve the temperature resistance of polymers. Therefore, AMPS is chosen as the main monomer13. The carboxylic acid groups in the molecular structure of AA have good coordination and strong electronegativity, and can form complexes with Ca2+ in the liquid phase, which can not only improve the hydrophilicity and coordination of polymer, but also enhance the scale inhibition performance14. DMDAAC is a cationic monomer, which can enlarge the layers spacing of montmorillonite, facilitating the insertion and release of polymers15. As discussed in above, the MMT has the characteristics of layers structure, hydrothermal expansion and high thermal stability12, which can not only improve high thermal stability of polymers, but also enhance the scale inhibition effect at high temperature through the polymer intercalation bound release function.
The mechanism of polymer intercalated organic montmorillonite is as follows:
(R—Organic group; Rʹ—H; X—Cl−, Br−, I−; M—Na+, Ca2+, Mg2+).
The polymerization reaction of AA/AMPS/DMDAAC is shown in Fig. 1, and the preparation process of polymer/montmorillonite is shown in Fig. 2.
The synthesis process of CT-5
a: Pretreatment of MMT
Add a certain amount of DMDAAC to a suspension of montmorillonite with a solid content of 5%, and after ultrasonic dispersion, centrifuging, washing, and drying, the pre-treated modified MMT is obtained.
b: The preparation process of CT-5
Mix a certain amount of pre-treated modified MMT with deionized water and place it into a flask. Then weigh 12.5 g of AMPS, 8.3 g of AA, and 4.2 g of DMDAAC respectively, dissolve the weighed mixed monomers in deionized water (monomer mass fraction is 25%), adjust its pH value to 7, and then pour the mixed solution into the flask. Then, nitrogen was continuously injected for 10 min, stirred at 40 °C for 20 min, and then heated to 70 °C, and 0.6% initiator solution was added. Then, the CT-5 can be obtained after the reaction at this temperature for 5 h and cooling to room temperature. For the subsequent application of scale inhibition and analysis of scale inhibition related mechanisms, the solubility of CT-5 was measured, as a result, the solubility of CT-5 is very good within the temperature range of 70 ~ 130 °C, reaching 100%.
Structure characterization and scale inhibition performance evaluation method of CT-5
The product obtained after reaction is firstly added to ethanol in batches, and with leaching, drying, grinding, and then dissolved in distilled water. Moreover, the product is leached by acetone to obtain orange powder, which could be used to structure characterization.
Infrared analysis
The obtained orange powder was characterized by infrared spectroscopy using a WQF-520 Fourier transform infrared spectrometer (Nicolette, USA) with a scanning range of 400 ~ 4000 cm-1.
Thermogravimetric analysis
The thermal stability of the powder was analyzed by using the STA449F3 synchronous comprehensive thermal analyzer (NETZSCH, Germany) at a temperature of 50 ~ 450 °C with the heating rate of 5 °C /min, and nitrogen atmosphere used.
X-ray diffraction analysis
The phase composition of the powder was characterized by an X’Pert MPD PRO X-ray diffractometer (Panat, Netherlands) with a tube voltage of 40 kV, a tube current of 40 mA, a scanning rate of 2°/min, and a scanning range of 1° ~ 10°.
Scale inhibition performance test
During this study, the CaCO3 scale type standard saline water was prepared depending on the method described in SY/T 5673–2020 "General Technical Conditions for Oilfield Scale Inhibitors", then, the CaCO3 scale samples were simulated according to the method described in this standard. Furthermore, the scale inhibition rate of CaCO3 scale was investigated.
Chelation amount and layer spacing test
After the experiment on the scale inhibition rate of CT-5 at different temperatures, the reacted solution was centrifuged at high speed, and the free Ca2+ content in the supernatant was measured to calculate the chelation amount of Ca2+.
Scanning electron microscope
The microscopic morphology of CaCO3 scale crystals with or without CT-5 was analyzed by using a FlexSEM1000 compact intelligent scanning electron microscope. Before running testing, the gold spraying was required to enhance the conductivity of the sample.
X-ray photoelectron spectroscopy
The X-ray photoelectron spectroscopy (XPS) is a typical surface analysis method that can qualitatively analyze the elemental composition and chemical state of material surfaces. Moreover, it can quantitatively analyze the elemental content on the surface of materials. Static scale inhibition experiments were conducted, and the adding amount of CT-5 is 0 and 4%. After filtering and separating a small amount of solid in the bottle, it was dried in a vacuum chamber and analyzed for changes in the binding energy of Ca 2p and O 1 s orbitals using X-ray photoelectron spectroscopy.
Results and discussion
Optimization of preparation conditions of CT-5
Taking the scale inhibition rate as the main evaluation index, the optimal conditions for the synthesis of CT-5 were optimized, and the orthogonal experiment of copolymerization reaction was designed, as shown in Table 1. According to the principle of free radical polymerization, the main factors affecting the properties of co-polymer products are monomer mass ratio A [m (AMPS): m (AA): m (DMDAAC): m (MMT)], reaction temperature B (°C), initiator dosage C (%), reaction time D (h), and solution E (pH). Based on this, a five factor four level orthogonal experiment was constructed to analyze the main factors affecting copolymerization and the optimal synthesis conditions. Here, the K1*, K2*, K3* and K4* is the average scale inhibition at levels 1, 2, 3 and 4, respectively. Besides, the R is the range between different levels under the same factor conditions.
Which as shown in Table 1, the orthogonal analysis revealed reaction temperature as the predominant factor affecting inhibitor performance (highest range value in Table 1), followed sequentially by monomer mass ratio, initiator dosage (%), pH and reaction time (h). The optimal synthesis protocol (B4A2C3E3D4) derived from orthogonal mean analysis comprised, namely, the reaction temperature is 75 °C, the monomer mass ratio is 48:25:23:4, the initiator dosage is 0.6%, the pH is 7, and the reaction time is 12 h. CT-5 synthesized under these conditions underwent subsequent analytical characterization.
Structural characterization of CT-5
Infrared spectrum characterization of CT-5
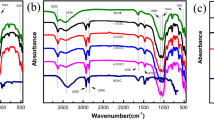
The infrared spectrum characterization results of CT-5 are shown in Fig. 3.
In Fig. 3, 3477 cm-1 is the N–H characteristic peak contained by the amide group in the molecular structure of AMPS, and the stretching vibration absorption peak of –CH3 is located at about 2936 cm-1. The stretching vibration absorption peaks of C = O in AA and AMPS are located at about 1724 and 1655 cm-1, respectively. The deformation vibration stretching peak of N–H is located at about 1552 cm-1, and the stretching vibration absorption peaks of C-N in AMPS and DMDAAC are located at about 1438 and 1368 cm-1. The stretching vibration absorption peaks of SO3− are located at about 1230, 1043, and 1175 cm-1, respectively. At 630 cm-1 is the deformation vibration peak of C-N five-membered ring in DMDAAC. However, compared to P (AA/AMPS/DMDAAC), the infrared spectrum of CT-5 shows distinct peaks at 1106 cm-1 and 463 cm-1, with the peak at 1106 cm-1 being the stretching vibration peak of Si–O and the peak at 463 cm-1 being the bending vibration peak of Si–O16. It can be seen from the above analysis that the functional groups of three monomers and MMT appear in the spectrum, which indicates that the polymer and MMT are successfully combined, and the CT-5 is the target product.
XRD characterization of CT-5
The XRD characterization results of MMT and scale inhibitor CT-5 are shown in Fig. 4.
From Fig. 4, it can be seen that the layer spacing of MMT is 1.208 nm (2θ = 7.31). The diffraction peak of CT-5 shifted to the left and the intensity significantly decreased, and the peak shape showed a wide and diffuse raised package shape, with an increased interlayer spacing of 1.522 nm (2θ = 5.80). According to the literature17,18, when the interlayer spacing increases beyond the small angle X-ray diffraction range, only the intercalated portion can be diffracted. Figure 4 shows a decrease in the intensity of the CT-5 diffraction peak, indicating that the polymer has been successfully intercalated between the layers of MMT, forming a composite material with an intercalation structure.
Thermal stability analysis of CT-5
The thermal stability analysis of CT-5 is listed in Fig. 5. Figure 5a,b shows the initial thermal decomposition temperature of P (AMPS/AA/DMDAAC) and CT-5 is about 218.42 and 235.24 °C. The above phenomenon indicates that the addition of MMT significantly improves the thermal stability of CT-5, making the composite intercalated polymer CT-5 have good temperature resistance and resistance characteristics. On the one hand, it is due to the excellent thermal stability of MMT. On the other hand, due to the intercalation of the polymer between the layers of MMT, the layers not only can effectively shield thermal decomposition effects, but also hinder the movement of small molecules generated by polymer molecules during thermal decomposition. Therefore, CT-5 exhibits good thermal stability.
Performance evaluation of CT-5 application
Scale inhibition effect of CT-5 at high temperature
Excellent scale inhibition performance at high temperature is the main performance indicator of high temperature scale inhibitors, however, polymer scale inhibitors are prone to functional group decomposition, molecular chain breakage, and high-temperature desorption under high temperature conditions, resulting in a significant reduction or even complete loss of scale inhibition effect. In this section, the scale inhibition rates are tested at different temperature points and CT-5 dosage to investigate the scale inhibition performance of CT-5 at high temperatures, and the results are shown in Table 2.
Which as shown in Table 2, on the one hand, the scale inhibition rates of P(AMPS/AA/DMDAAC) and CT-5 show a trend of first increasing and then decreasing with the increase of dosage, the scale inhibition rate of P(AMPS/AA/DMDAAC) and CT-5 with 4% adding amount is 93.6 and 89.7% at the temperature of 70 °C, of course, which shows similar patterns at other temperatures ( the adding amount will be used for subsequent evaluation and mechanism research experiments). Moreover, it was also demonstrated that the scale inhibition rate of CT-5 is slightly lower than that of P(AMPS/AA/DMDAAC) at low temperature, such as 70 °C, which may due to the layered structure of MMT in CT-5 affecting the chelating ability of anionic groups to Ca2+. However, it was also demonstrated that the scale inhibition rate of CT-5 is higher than that of P(AMPS/AA/DMDAAC) at high temperature, the scale inhibition rate of CT-5 with 4% adding amount is higher than that of P(AMPS/AA/DMDAAC) by 1.2, 4.7 and 14.1% at the temperature of 90, 110 and 130 °C, respectively. Besides, the scale inhibition rate of P(AMPS/AA/DMDAAC) decreases from 93.6% to 59.3%, a decrease of 34.3% was observed, while the scale inhibition rate of CT-5 decreases from 89.7% to 73.4%, a decrease of only 16.3%. Based on the data from the above two aspects, CT-5 has the good high-temperature resistance performance.
Additionally, which as discussed above, the scale inhibition rates of CT-5 with 4% adding amount is larger than that 2 and 6% at the temperature of 110 and 130 °C, the main reason is that scale inhibitors form soluble complexes by combining functional groups (such as sulfonic acid and carboxylic acid groups) with scaling ions (such as Ca 2 ⁺) to inhibit crystal growth, and when the adding amount of CT-5 is 4%, the molar ratio of CT-5 molecules to Ca2+ approaches stoichiometric equilibrium, achieving maximum complexation efficiency. However, exceeding the optimal concentration (6%), excessive CT-5 molecules may compete for binding sites or form molecular aggregates, thereby reducing effective activity. Simultaneously, the reason for scale inhibition rates of CT-5 decreased when the temperature beyond 130 °C is that high temperature causes thermal cracking of active functional groups (such as sulfonic acid groups and carboxylic acid groups) and molecular main chains in CT-5 molecules, on the other hand, the high temperatures may also intensify the thermal motion of polymer chains, leading to disordered molecular conformation and masking of active sites of CT-5, resulting in a decrease in scale inhibition effectiveness.
Evaluation of salt resistance of CT-5
The high mineralization environment in deep and ultra deep wells can affect the extension of polymer molecular chains, causing a sharp change in the scale inhibition effect of scale inhibitors. Therefore, scale inhibitors need to have excellent salt resistance performance. In this section, the scale inhibition rate of CT-5 was measured at 130 °C with different NaCl contents to investigate the salt resistance performance of CT-5, and the results are shown in Fig. 6.
The Fig. 6 shows that the scale inhibition rate of CT-5 presents a trend of first increasing and then stabilizing with the increase of NaCl content, and the scale inhibition rate of CT-5 with 4% adding amount is 73.4 and 76.9% by the salt content of 0 and 8%, and the change rate is 4.76%. Besides, when the NaCl content is less than 4%, the scale inhibition rate of CT-5 increases significantly with the increase of NaCl content, but when the NaCl content exceeds 4%, the scale inhibition rate of CT-5 increases slowly and tends to be stable with the increase of NaCl content, which is consistent with relevant literature19. The reason for the above phenomenon may be related to the molecular structure of the scale inhibitor. Due to the presence of both cationic and anionic groups in polymer molecules, there are two types of binding interactions within and between molecules. The shielding effect of NaCl hinders the binding interactions within and between the molecules, making the molecular chains of the polymer to unfold, enhancing the adsorption and chelation effects of molecular chains, disrupting the normal growth of scale crystals and increasing the scale inhibition rate20,21.
Study on scale inhibition mechanism of CT-5 at high temperature
Presently, it is believed that the scale inhibition mechanism of scale inhibitors as follows21,22. At first, the anionic groups within scale inhibitor molecules form soluble chelate complexes with scale-forming cations, such as Ca2⁺ in aqueous solutions, thereby inhibiting crystalline growth and exerting their scale inhibiting function. Moreover, through adsorption onto scale microcrystal surfaces, these anionic groups establish electrical double layers that generate surface electrostatic repulsion, which effectively preventing microcrystal aggregation into macroscopic deposits. Furthermore, scale inhibitors disrupt the regular lattice arrangement during crystal growth, inducing lattice strain that weakens structural integrity and promotes crystalline fragmentation, ultimately achieving scale inhibition through mechanical destabilization.
In this part, the release mechanism of CT-5 at high temperature and its effect on the crystal structure of CaCO3 scale were studied by measurement of chelation amount of Ca2+ at different temperatures, XRD, SEM and XPS tests, and then the scale inhibition mechanism of CT-5 was further studied.
Release mechanism of CT-5 at high temperature
To investigate the scale inhibition mechanism of CT-5, the changes in layer spacing and Ca2+ chelation amount of CT-5 at different temperatures were measured, and then compared with the chelation amount of Ca2+ by P(AA/AMPS/DMDAAC), as shown in Fig. 7.
Which as shown in Fig. 7, the results show that the interlayer spaces of CT-5 gradually increase with the increase of temperature, which is caused by thermal expansion of the layers of MMT in the CT-5 at high temperatures, the interlayer spaces increased by 63.49% when the temperature rises from 80 to 130 °C. Then, it was also found that the chelation amount of Ca2+ by P(AMPS/AA/DMDAAC) and CT-5 decreases with the increase of temperature, the reason is that the chelating groups of polymer molecules have a dynamic equilibrium process of adsorption and desorption. Specifically, at high temperature, it is manifested as a decrease in the chelation amount of Ca2+ when the desorption effect is greater than the adsorption effect. But the decreasing trend of the chelating amount of Ca2+ by CT-5 with increasing temperature is significantly slower than that of P(AMPS/AA/DMDAAC), which indicating that CT-5 is less sensitive to temperature. The reason is that the layer spacing of MMT expands by thermal expansion, the thermal motion of polymer molecules intensifies, and the polymer molecules originally curled up between the MMT layers is gradually released with the increase of the layer spacing, causing an increase of effective adsorption groups. Therefore, the CT-5 has a higher chelation amount to Ca2+, as a result, the trend of decreasing the chelation amount of Ca2+ with increasing temperature is slower.
Effect of CT-5 on the crystal structure of CaCO3
The effect of CT-5 on the crystal structure of CaCO3 scale was analyzed by XRD, and compared with the original CaCO3 scale. Figure 8 shows the XRD analysis results of CaCO3 scale crystals with and without CT-5.
According to the XRD analysis of CaCO3 scale crystals in the solution without CT-5 shown in Fig. 8, only the characteristic diffraction peaks of CaCO3 (calcite) are observed at 23.2°, 29.4°, 31.5°, 36.3°, 38.5°, 43.0°, 47.2° and 47.9°23,24. At the same time, the diffraction peak at 29.4° is sharper and higher than others, indicating that the CaCO3 scale crystals mainly grew along this crystal plane. The XRD analysis of CaCO3 scale crystals in solution with CT-5 in Fig. 8 shows that the characteristic diffraction peak of CaCO3 (calcite) only appears at 23.2°, the characteristic diffraction peak of CaCO3 (aracharite) appears at 36.0 and 47.6°25. Unlike the XRD results of CaCO3 scale crystals in solution without CT-5, the XRD analysis spectrum of CaCO3 scale crystals in solution with CT-5 shows characteristic diffraction peaks of CaCO3 (aragonite) with the character of loose structure and easy physical damage at 21.0°, 25.0°, 27.1°, 32.8°, 39.5° and 43.2°26,27. The characteristic peaks belonging to both CaCO3 (calcite) and CaCO3 (aragonite) decrease significantly, indicating that the main component in the crystal is CaCO3 (vaterite). On the one hand, the anion group in CT-5 can not only bind with Ca2+, hindering the binding of Ca2+ with other anions such as CO32-. On the other hand, the CT-5 can also change the crystal structure of CaCO3 scale crystals and affect the growth of crystals, thereby improving the scale inhibition effect28,29.
Analysis of the effect of CT-5 on the crystal morphology of CaCO3
The CaCO3 crystals primarily as calcite (rhombohedral), aragonite (acicular), and vaterite (spheroidal aggregates with polydispersity)30,31,32. SEM analysis revealed CT-5-induced morphological modification of CaCO3 deposits, elucidating its scale inhibition mechanism. Figure 9 contrasts the crystallographic features of CaCO3 scale crystals with and without CT-5. The magnification used in the SEM of CaCO3 scale crystals without CT-5 is 2000, 4000 and 10,000, the magnification used in the SEM of CaCO3 scale crystals with 2.0% CT-5 is 1000, 4000 and 10,000, the magnification used in the SEM of CaCO3 scale crystals with 4.0% CT-5 is 1000, 2000 and 4000, which has been also added in the revised manuscript.
Figure 9a shows the CaCO3 scale crystal without adding CT-5 is regular cubic shape with dense structure, indicating that the type of CaCO3 crystals is calcite. However, Fig. 9b and Fig. 9c show that the morphology of CaCO3 scale crystals changes significantly after the addition of CT-5. Figure 9b shows that when the content of CT-5 is 2%, most CaCO3 crystals appear as irregular angular shapes, only a small number of CaCO3 crystals appear as rod-shaped, and there are almost no regular cubic CaCO3 crystals. As the content of CT-5 increases, the morphology of CaCO3 scale crystals continues to be changed. Figure 9c shows that when the content of CT-5 increases to 4%, the size of CaCO3 scale crystals varies and the crystals are irregular spherical, indicating that the type of CaCO3 crystals are vaterite. In addition, it can be clearly seen from Fig. 9c2 that there are obvious cracks on the surface of CaCO3 crystals, indicating that the structure of CaCO3 crystal is relatively loose, which is consistent with the description of the unstable thermodynamic state and structure of vaterite in the literature27. The above characterization results indicate that the CT-5 achieves scale inhibition by changing the crystal structure of CaCO3, which may due to the anionic groups of CT-5 adsorbing on the surface of CaCO3 scale crystals by chelation of Ca2+ and electrostatic interactions, occupying the growth active sites of CaCO3 scale crystals33,34, causing lattice distortion of CaCO3 scale crystal and achieving the scale inhibition effect.
Chemical binding energy analysis of CT-5 on CaCO3 crystals
XPS revealed CT-5-induced chemical binding energy modulation in CaCO3 scale crystals, elucidating its inhibition mechanism35. Figure 10 compares the XPS spectra of CaCO3 deposits in scale-inhibited and control systems, namely, the XPS results of CaCO3 scale crystals in solution with and without scale inhibitor CT-5.
As shown in Fig. 10a, only energy peaks of elements Ca, C and O appear in the XPS spectrum of CaCO3 scale crystals in solution without CT-5, however, in the XPS spectrum of CaCO3 scale crystals in the solution with CT-5, in addition to the energy peaks of elements Ca, C, and O, there are also energy peaks of elements N (391.5 eV) and S (169.3 eV), which may be resulting from the raw materials AMPS and DMDAAC. The high-resolution spectrum of Ca 2P in Fig. 10b shows that energy peaks belonging to Ca 2p1/2 and Ca 2p3/2 of CaCO3 scale crystals in solution without CT-5 appear at 350.6 and 347.1 eV, respectively36. The energy peak positions ascribed to Ca 2p1/2 and Ca 2p3/2 in CaCO3 scale crystals in solution with CT-5 are 350.1 and 346.7 eV, respectively. Compared with high-resolution spectrum of Ca 2P in CaCO3 scale crystals in solution without CT-5, the binding energy of Ca 2P in CaCO3 scale crystals in solution with CT-5 has shifted to a lower binding energy direction by 0.5 eV. This indicates that the CT-5 can change the chemical environment of element Ca, because the anionic groups in CT-5 will bind with Ca2+, transfer and contribute some electrons to Ca2+, thereby increasing the electron density of the outer layer of Ca, and causing a decrease in the binding energy of Ca16,37,38. The above experimental results show that the anionic groups in CT-5 are bound to Ca2+, thus hindering the scale formation of Ca2+ and other anions, and inhibiting the normal growth of CaCO3 scale crystals.
Scale inhibition mechanism of CT-5 at high temperature
According to the molecular chain of polymer P(AA/AMPS/DMDAAC) and the structure of MMT in Figs. 1 and 2, it can be seen that CT-5 has good high temperature resistance due to the high temperature resistance of MMT layer, as well as the existence of five membered ring structures of polymer molecular chain and the group of sulfonic acid root in molecular side chain. By studying the effect of temperature on the layer spacing of CT-5 and the chelation amount of Ca2+, it can be seen that the higher the temperature and the larger the layer spacing, the more scale inhibiting groups in free fluid, indicating that CT-5 can release different amounts of scale inhibiting groups at different temperatures. By utilizing the characteristics of the MMT layer and thermal expansion, CT-5 with good scale inhibition performance at high temperatures. By analyzing the effect of CT-5 on the structure of CaCO3 crystal scale through XRD, SEM and XPS, combined with the molecular structure of CT-5, it can be inferred that the sulfonic acid and carboxylic acid groups with strong coordination in the polymer will form chelates with free Ca2+ in solution. On the one hand, the concentration of Ca2+ in free liquid is reduced, and the probability of Ca2+ binding with CO32− and other scale ions is reduced. On the other hand, the anionic groups of CT-5 adsorbing on the surface of CaCO3 scale crystals, hindering the normal growth of CaCO3 scale crystals, changing the lattice structure of CaCO3 scale crystals, and achieving scale inhibition effect26,39,40. Besides, the scale inhibition mechanism of CT-5 is shown in Fig. 11.
Conclusion
During this study, the organic–inorganic composite scale inhibitor CT-5 was successfully synthesized via solution polymerization-mediated in situ intercalation using AA, AMPS and DMDAAC as monomers, with MMT as reactive filler. The optimized preparation process parameters include a reaction temperature of 75 °C, an initiator dosage of 0.6%, a solution pH of 7, a reaction time of 12 h, and a monomer ratio of m (AMPS): m (AA): m (DMDAAC): m (MMT) = 48:25:23:4. The results of FTIR, XRD, and TG-DTG shows that the CT-5 intercalated MMT was successful, which shows a composite intercalation structure of organic polymer/inorganic montmorillonite, and the initial thermal decomposition temperature CT-5 was increased to 235.24 °C. Performance evaluation of CT-5 application shows that CT-5 has the good high-temperature resistance performance, a decrease of only 16.3% was observed with the temperature increased from 70 to 130 °C, meanwhile, the scale inhibition rate of CT-5 presents a trend of first increasing and then stabilizing with the increase of NaCl content (0 ~ 8%). Furthermore, the results of release mechanism of CT-5 at high temperature, effect of CT-5 on the crystal structure and morphology of CaCO3 show that the interlayer space of MMT in CT-5 molecules expands at higher temperatures (90 ~ 130 °C), and the polymer molecules in the intercalation are released, thus increasing the free adsorption groups, reducing the content of Ca2+ in the free liquid by forming a complex with Ca2+, and inhibiting the normal growth of CaCO3 crystals to achieve scale inhibition effect. On the other hand, the anionic groups of CT-5 adsorbing on the surface of CaCO3 scale crystals, hindering the normal growth of CaCO3 scale crystals, changing the lattice structure of CaCO3 scale crystals, and achieving scale inhibition effect.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Fang, F. et al. Experimental study on water invasion mechanism of fractured carbonate gas reservoirs in Longwangmiao Formation, Moxi block, Sichuan Basin. Environ. Earth Sci. 78, 316 (2019).
Zhao, L. et al. Study on the mechanism of wellbore blockage and scaling trend prediction of keshen block. Processes. 12, 782 (2024).
Liu, G. et al. Acrylic acid-allylpolyethoxy carboxylate copolymer as an effective inhibitor for calcium phosphate and iron(III) scales in cooling water systems. Clean: Soil, Air, Water 43, 989–994 (2015).
Zhang, M. et al. Controllable synthesis of polyaspartic acid: Studying into the chain length effect for calcium scale inhibition. Desalination 570, 117080 (2024).
Yu, W., Song, D., Li, A. & Yang, H. Control of gypsum-dominated scaling in reverse osmosis system using carboxymethyl cellulose. J. Membr. Sci. 577, 20–30 (2019).
Yang, L. et al. Synthesis and scale inhibition performance of a novel environmental friendly and hydrophilic terpolymer inhibitor. Desalination 416, 166–174 (2017).
Guo, X. et al. Preparation and application of copolymer modified with the palygorskite as inhibitor for calcium carbonate scale. Appl. Clay Sci. 99, 187–193 (2014).
Huang, H., Yao, Q., Jiao, Q., Liu, B. & Chen, H. Polyepoxysuccinic acid with hyper-branched structure as an environmentally friendly scale inhibitor and its scale inhibition mechanism. J. Saudi Chem. Soc. 23, 61–74 (2019).
Zhang, K. et al. Evaluation of arginine-modified polyepoxysuccinic acid as anti-scaling and anti-corrosion agent. Chem. Eng. Technol. 44, 1131–1140 (2021).
Chen, Y., Chen, X., Liang, Y. & Gao, Y. Synthesis of polyaspartic acid-oxidized starch copolymer and evaluation of its inhibition performance and dispersion capacity. J. Disper. Sci. Technol. 42, 1926–1935 (2021).
Ganguly, S. et al. Sustainable calcite scale inhibitors via oxidation of lignosulfonates. ACS Omega 9, 25162–25171 (2024).
Masoud, E. M., Abolibda, T. Z. & Khairy, M. Nano Na-montmorillonite @ polyaniline hybrid composite: effect of nano Na-montmorillonite content on structure, thermal stability and electrical properties. J. Inorg. Organomet. P. (2024).
Conrod, I. D., Topcuoglu, B., Penlidis, A. & Scott, A. J. Impact of ionic strength (sodium chloride concentration) on homopolymerization and copolymerization kinetics of acrylamide and 2-acrylamido-2-methylpropane sulfonic acid. Macromol. React. Eng. 18, 2300058 (2024).
Janicki, R., Mondry, A. & Starynowicz, P. Carboxylates of rare earth elements. Coordin. Chem. Rev. 340, 98–133 (2017).
Wang, L. et al. Flocculation performance and mechanism of P (DMDAAC-AM) on clay mineral layer: Insights from DFT calculation and experiment. Appl. Surf. Sci. 607, 155089 (2023).
Zhang, B., Peng, Z., Zou, C., Feng, Q. & Zhang, J. Study on surface modification of CaSO4 whisker and mechanism of enhancing mechanical properties of oil-well cement. Colloids Surf A Physicochem. Eng. Asp. 618, 126408 (2021).
Xu, W., Ge, M. & He, P. Nonisothermal crystallization kinetics of polyoxymethylene /montmorillonite nanocomposite. J. Appl. Polym. Sci. 82, 2281–2289 (2001).
Ray, S. & Bhowmick, A. K. Synthesis, characterization and properties of montmorillonite clay-polyacrylate hybrid material and its effect on the properties of engage-clay hybrid composite. Rubber Chem. Technol. 74, 835–845 (2001).
Kabanda, M. M. & Bahadur, I. Preferred intermolecular cation–anion interactions within the [EMIM][DCA] ionic liquid and its interaction with a water co-solvent molecule. J. Mol. Liq. 381, 121804 (2023).
Li, Y., Peng, W., Guan, Z. & Ding, Q. Micro-mechanical properties of individual phases in cement pastes under brine solution using nanoindentation and scanning electron microscopy. J. Nano Res.-Sw. 46, 31–44 (2017).
Dobberschütz, S. et al. The mechanisms of crystal growth inhibition by organic and inorganic inhibitors. Nat. Commun. 9, 1578 (2018).
Hsieh, I. & Malmali, M. Scaling behavior in membrane distillation: Effect of biopolymers and antiscalants. Water Res. 255, 121456 (2024).
Zuo, Y. et al. Performance and mechanism of 1-hydroxy ethylidene-1, 1-diphosphonic acid and 2-phosphonobutane-1, 2, 4-tricarboxylic acid in the inhibition of calcium carbonate scale. J. Mol. Liq. 334, 116093 (2021).
Zhang, W. et al. Performance and mechanism of a composite scaling–corrosion inhibitor used in seawater: 10-methylacridinium iodide and sodium citrate. Desalination 486, 114482 (2020).
Zuo, Z. et al. Effect of scale inhibitors on the structure and morphology of CaCO3 crystal electrochemically deposited on TA1 alloy. J. Colloid Interface Sci. 562, 558–566 (2020).
El Housse, M., Hadfi, A., Karmal, I., Ibrahimi, E. L. & B., Ben-aazza, S., Errami, M., Belattar, M., Mohareb, S., Driouiche, A.,. Experimental investigation and molecular dynamic simulation of Tannic acid as an eco-friendly inhibitor for calcium carbonate scale. J. Mol. Liq. 340, 117225 (2021).
Cui, K., Li, C., Yao, B., Yang, F. & Sun, G. Synthesis and evaluation of an environment-friendly terpolymer CaCO3 scale inhibitor for oilfield produced water with better salt and temperature resistance. J. Appl. Polym. Sci. 137, 48460 (2020).
Zhang, Y., Yin, H., Zhang, Q., Li, Y. & Yao, P. Synthesis and characterization of novel polyaspartic acid/urea graft copolymer with acylamino group and its scale inhibition performance. Desalination 395, 92–98 (2016).
Khormali, A., Petrakov, D. G. & Afshari Moein, M. J. Experimental analysis of calcium carbonate scale formation and inhibition in waterflooding of carbonate reservoirs. J. Petrol. Sci. Eng. 147, 843–850 (2016).
Macedo, R. G. M. D. et al. Water-soluble carboxymethylchitosan as green scale inhibitor in oil wells. Carbohydr. Polym. 215, 137–142 (2019).
Hamdi, R., Tlili, M. M. Influence of Foreign Salts and Antiscalants on Calcium Carbonate Crystallization. In Crystals. 13 (2023).
Yu, X. et al. Synthesis, scale and corrosion inhibition evaluation and mechanism of 2-aminobenzimidazole modified polyaspartic acid. J. Environ. Chem. Eng. 12, 112950 (2024).
Horner, O. et al. Antiscalant properties of Herniaria glabra aqueous solution. Desalination 409, 157–162 (2017).
Hu, Y., Chen, C. & Liu, S. Evaluation of microbial agents as corrosion and scale inhibitor for industrial cooling water applications. Water Sci. Technol. 85, 1904–1919 (2022).
Sayed, A., Ashmawy, A., Elgammal, W., Hassan, S. & Deyab, M. Synthesis, description, and application of novel corrosion inhibitors for CS AISI1095 in 1.0 M HCl based on benzoquinoline derivatives. Sci. Rep-UK. 13, 13761 (2023).
Gao, R. et al. ZIF-8@s-EPS as a novel hydrophilic multifunctional biomaterial for efficient scale inhibition, antibacterial and antifouling in water treatment. Sci. Total Environ. 773, 145706 (2021).
Bai, H. et al. Effect of interlayer cations on exfoliating 2D montmorillonite nanosheets with high aspect ratio: From experiment to molecular calculation. Ceram. Int. 45, 17054–17063 (2019).
Guo, X. et al. The synthesis of polyaspartic acid derivative PASP-Im and investigation of its scale inhibition performance and mechanism in industrial circulating water. RSC Adv. 10, 33595–33601 (2020).
Li, S. et al. Bacillus cereus s-EPS as a dual bio-functional corrosion and scale inhibitor in artificial seawater. Water Res. 166, 115094 (2019).
Du, Q., Wang, Y., Li, A. & Yang, H. Scale-inhibition and flocculation dual-functionality of poly(acrylic acid) grafted starch. J. Environ. Manag. 210, 273–279 (2018).
Funding
The research is financially supported by Sichuan Province Science and Technology Plan Project (No. 2023YFG0092).
Author information
Authors and Affiliations
Contributions
Bojian Zhang: Data curation, Formal analysis, Writing—original draft. Youquan Liu: Supervision, Writing—review & editing. Ying Xiong: Supervision, Writing—review & editing. Cheng Fu: Supervision, Validation, Investigation. Xianbing Wang: Data curation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, B., Liu, Y., Xiong, Y. et al. Preparation and performance evaluation of polymer intercalated montmorillonite composite high temperature scale inhibitor. Sci Rep 15, 15923 (2025). https://doi.org/10.1038/s41598-025-99968-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99968-9