Abstract
Renal fibrosis is the common pathological feature of chronic kidney diseases, which is in parallel with increasing energy demand in proximal tubular epithelial cells during the metabolic shift from fatty acid oxidation to glycolysis. Shen-Shuai-II-Recipe (SSR) is a traditional Chinese medicine formula with known renal benefits in patients with chronic kidney disease (CKD) and has been shown to intervene in renal energy metabolism in a rodent model of CKD. We aimed to explore the mechanism underlying the protective effect of SSR against CKD. A 5/6 ablation/infarction (A/I) renal failure model was established in rats, followed by 8 weeks of gavage feeding with SSR or Losartan, a positive control. For in vitro experiments, normal rat kidney-52E (NRK-52E) cells were cultured under hypoxic condition (1% O2) or normoxic condition. The expression of fibrotic markers and aerobic glycolysis-related enzymes were determined by Western blotting analysis. The concentration of metabolites was also measured. The concentration of lactic acid was increased, and the concentration of pyruvic acid was decreased in vitro and in vivo models, which were correlated with increased expression of glycolysis-related enzymes Hexokinase2 (HK2) and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3), SSR treatment reversed the changes of renal glycolysis in cells and animals, which was associated with reduced expression of fibrotic markers such as fibronectin and α-SMA. Mechanistically, SSR treatment enhanced the expression of Sirtuin 1 (SIRT1) and reduced the expression of Hypoxia-Inducible Factor 1-alpha (HIF-1α) in vitro and in vivo models. Downregulation of SIRT1 by small interfering ribonucleic acid (siRNA) reversed the anti-fibrotic effect and anti-glycolysis effect of SSR in renal cells. SSR exerts an anti-fibrotic effect and inhibits renal aerobic glycolysis in CKD via the regulation of SIRT1/HIF-1α.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is mainly defined as kidney damage or decreased glomerular filtration rate (GFR) over three months1. A variety of different conditions may lead to CKD, such as diabetes, hypertension, acute kidney injury, sepsis, renal ischemia-reperfusion, and ureteral obstruction2. Irreversible nephron loss, microvascular injury, inflammation, oxidative stress, and metabolic changes widespread throughout the pathological process of CKD regardless of its origin, while the ultimate outcome invariably points to renal fibrosis3. The incidence of CKD has been increasing each year with more than 3.5 million people worldwide receiving maintenance dialysis as the treatment of chronic renal failure4. It has become a major global public health issue and has imposed a considerable socioeconomic burden. Therapeutic intervention of development of renal fibrosis is crucial for the prevention and treatment of chronic kidney disease5.
Recurrent or persistent epithelial injury leads to cell cycle arrest and senescence of tubular epithelial cells, resulting in an increase in production of profibrotic factors6,7. Renal fibrosis is characterized by proliferation of myofibroblasts, upregulation of epithelial-mesenchymal transition (EMT) markers such as α-smooth muscle actin (α-SMA), and massive accumulation of extracellular matrix (ECM) proteins such as fibronectin (FN) and type I collagen (Col-I)8.
Mounting evidence suggests that abnormal energy metabolism changes in tubular epithelial cells (TECs) during chronic kidney disease is one of the major underlying mechanisms of renal fibrosis9. TECs are the major cell type undergoing metabolic reprogramming in the process of renal fibrosis10,11. Upon kidney injury, renal TECs undergo a metabolic switch to upregulated aerobic glycolysis, which enhances glucose uptake and lactate production leading to CKD progression12,13. Inhibition of aerobic glycolysis is regarded as a promising therapeutic strategy for renal fibrosis14,15,16.
Hypoxia-inducible factor-1α (HIF-1α) is an important transcription factor that can sense the oxygen status and induce adaptive changes in cell metabolism, playing a crucial role in renal fibrosis and glucose metabolism17. Studies have shown that glycolysis is driven by the activation of HIF-1α signaling, which transcriptional regulates a series of glycolysis genes18,19. Silent information regulator 1 (SIRT1), a highly conserved nicotinamide adenine dinucleotide (NAD+) dependent deacetylase, is a sensor of cellular energy and metabolism, which is upstream of HIF-1α20. SIRT1 deacetylates HIF-1α leading to its degradation21,22.
Shen-Shuai-II-Recipe (SSR) is a traditional Chinese medicine formula for treating CKD. Several randomized controlled trials demonstrated that SSR delayed the disease progression of CKD patients to the end stage of renal failure23,24. In a CKD rat model, SSR alleviated renal interstitial fibrosis under hypoxia condition25,26, Further research indicates the protective effect of SSR might be partially associated with reduced glycolysis27. In addition, co-culture experiments showed that SSR inhibited the activation of renal fibroblasts and the deposition of extracellular matrix by ameliorating glycolysis of renal tubular cells28.
In this study, we aimed to study the mechanism underlying the inhibitory effect of SSR on aerobic glycolysis in chronic kidney disease.
Materials and methods
Animal studies
Healthy specific pathogen-Free (SPF) male Sprague-Dawley (SD) rats aged 6–8 weeks (purchased from Shanghai Sippe-Bk Lab Animal Co., Ltd.) were housed in the Laboratory Animal Center of Shanghai University of Traditional Chinese Medicine under standard temperature and laboratory diet conditions.
After 7 days of adaptive feeding, the rats were anesthetized by intraperitoneal injection of 2% pentobarbital sodium (40 mg/ kg). Incisions approximately 1.5 cm was made under the left costal arch, and the left kidneys were exposed aseptically. Then, the renal artery and vein were separated and two-thirds of the branches of the left renal artery were ligated. One week later, an incision was made under each rat’s right costal arch to expose the right kidneys, The right kidneys were removed after ligating the renal pedicle.
30 days later, Serum creatinine (SCr) testing and urine creatinine measurement showed a 50% decrease in calculated CCr (creatinine clearance rate) compared to previous levels, indicating successful establishment of the rat model of renal failure. 5/6 ablation/infarction (A/I) rats were randomly divided into 3 groups (n = 8): 5/6 (A/I), 5/6 (A/I) + SSR, and 5/6 (A/I) + Losartan. Another 8 rats were included as the sham operation group. Rats were treated with SSR (4 g/mL, 3.825 mL/kg) or losartan potassium (3.33 mg/mL, 10 mL/kg) once a day by gavage25. SSR freeze-dried powder was prepared by the Formulation Department of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine. Rats in the sham operation group or the 5/6 (A/I) group were orally fed with 0.9% normal saline as control. At 8 weeks after treatment, the rats were anesthetized with pentobarbital sodium (50 mg/kg), blood samples were collection and left kidneys were harvested. After perfusion with normal saline, the kidneys were cut transversely into halves. The upper half of the kidney specimens were placed in 4% paraformaldehyde, and the lower half were snap frozen in liquid nitrogen and then transferred to a -80 °C freezer for storage. Meanwhile, an additional intraperitoneal injection of sodium pentobarbital (100 mg/kg) were given after suturing the surgical incision to ensure humane euthanasia.
This study complied with all relevant ethical regulations and was approved by Shanghai University of Traditional Chinese Medicine Experimental Animal Ethics Committee (PZSHUTCM2303220003). Animal research followed the ARRIVE guidelines.
Masson’s trichrome
After the left kidney specimens were fixed in 4% paraformaldehyde for 24 h, samples were dehydrated with alcohol and then embedded in paraffin. Paraffin sections with a thickness of 3 μm were cut and placed successively in xylene (I) and xylene (II) for 10 min each, and then in 100% ethanol (I), 100% ethanol (II), 95% ethanol, 80% ethanol, 70% ethanol, and distilled water for 5 min each for gradient dewaxing. Subsequently, the paraffin sections were immersed in hematoxylin staining solution for 5 min, followed by rinsing under running water for 5 min, differentiation in 1% hydrochloric acid alcohol for 10 s. Then, the paraffin sections were placed in ponceau-acid fuchsin staining solution for 5–10 min and quickly rinsed with distilled water. After that, the paraffin sections were treated in phosphomolybdic acid aqueous solution for about 3–5 min, followed by counterstaining with aniline blue solution for 5 min and differentiation with 1% glacial acetic acid for 1 min. Finally, the paraffin sections were subjected to gradient dehydration in 90% alcohol, 95% alcohol, absolute ethanol (I), absolute ethanol (II), xylene (I), and xylene (II) for 5 min each. After drying, the sections were mounted with an appropriate amount of neutral balsam.
Cell culture
NRK-52E cells (obtained from the Cell Bank of the Chinese Academy of Medical Sciences, RRID: CVCL_0468) were cultured in dulbecco’s modified eagle’s medium(DMEM) supplemented with 5% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The NRK-52E cells were starved for 12 h in 0.5% FBS DMEM medium, and then exposed to a hypoxic environment of 37 °C in a 5% CO₂ and 1% O₂ incubator for 24 h. Cells were exposed to SSR serum (1%, 5%) according to the experimental design.
Preparation of drug-containing serum
SD rats were orally feed with SSR (4 g/ml, 3.825 ml/kg of the SSR, then diluted with normal saline) or vehicle at a dose of 10 ml/kg, twice a day for 3 days. After the rats were anesthetized by intraperitoneal injection of 2% pentobarbital sodium, the serum was collected from the abdominal aorta. The samples were centrifuged, and the supernatants were collected and stored at -80 °C.
Metabolite detection
Renal function measurement including SCr, blood urine nitrogen (BUN), urinary albumin and creatinine were detected by automatic biochemical analyzer from laboratory of Shuguang Hospital. Ccr was calculated as follows: Ccr (ml/min) = urine creatinine × 24 h urine volume (ml)/serum creatinine × 1440. UACR was calculated as Urinary albumin: creatinine ratio.
After homogenizing and centrifuging the tissue samples, the supernatant was taken for detection. Tissue protein concentration was determined by the Bicinchoninic Acid Assay (BCA) (P0009, Beyotime, China). According to the instructions of the lactic acid kit and the pyruvic acid determination kit (both purchased from Nanjing Jiancheng Bioengineering Institute), the concentrations of lactic acid and pyruvic acid were measured by the colorimetric method. The absorbance of each well was measured at wavelengths of 530 nm and 505 nm respectively using a microplate reader, and the specific concentrations were calculated using the corresponding formulas.
SiRNA transfection
The sequence of rat SIRT1 siRNA is 5-GCACUAAUUCCAAGUUCUATT-3′ (purchased from Shanghai Gene Pharma Co., Ltd.). The siRNA was transfected using the liposome transfection method. When cells reached 60–70% confluence, the siRNA-lipid complex was prepared according to the manufacturer’s instructions (Opti-MEM (1×), 31985-070, ThermoFisher Scientific, USA; Lipofectamine 3000, L3000015, ThermoFisher Scientific, USA). After incubating with the complex for 12 h, the cells were refreshed with new medium and further treated according to different experimental designs.
Western blotting analysis
After lysing renal tissues or cells with RIPA solution (P0013B, Beyotime, China), the protein concentration was measured using the BCA method. After separation by SDS-PAGE electrophoresis, the proteins were transferred onto a PVDF membrane (ISEQ00010, Millipore, USA). The membrane was blocked and cut, and then incubated overnight with primary antibodies (FN, Abcam Cat# ab45688, RRID: AB_732380; Boster Biological Technology Cat# BM0002, RRID: AB_2811044; proliferating cell nuclear antigen (PCNA), Antibodies-Online Cat# ABIN483995, RRID: AB_10848432; HIF-1α, Proteintech Cat# 20960-1-AP, RRID: AB_10732601; SIRT1, Proteintech Cat# 13161-1-AP, RRID: AB_10646436; Hexokinase2 (HK2), Abcam Cat# ab209847, RRID: AB_2904621; 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3), Abcam Cat# ab181861, RRID: AB_3095816; β-Actin, Proteintech Cat# 66009-1-Ig, RRID: AB_2687938) ; Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH), Proteintech Cat# 60004-1-Ig, RRID: AB_2107436) according to the dilution ratios recommended in the manufacturer’s protocols. After incubating with secondary antibodies, the blot was covered by ECL solution (P0018M, Beyotime, China) and signals were developed using Tanon-5200 software (BIOTANON, China).
Real-time PCR
Total RNA was extracted from kidney tissue using the Trizol method and reversely transcripted to cDNA using the Primescript™ RT Master Mix (Takara Bio Inc., RRO36A, Japan). The primer sequences for the quantitative PCR (qPCR) were designed and synthesized as follow: SIRT1 forward: 5’-GTTGTGTGCCTTCGTTTTGGA-3’ and reverse: 5’-AGGCCGGTTTGGCTTATACA-3’; HIF-1α forward: 5’-CGATGACACGGAAACTGAAG-3’ and reverse: 5’-CAGAGGCAGGTAATGGAGACA-3’ (Sangon, China). qPCR was conducted on a Step One Plus Real-Time System (ABI Company, USA) using SYBR Green RT-qPCR Mix (YEASEN, 11200ES08, China). All procedures were carried out according to the manufacturer’s protocols.
Statistical analysis
SPSS 25.0 software and GraphPad Prism 6 software were used for data processing and statistics. Results were presented as mean ± SD. Multiple group comparisons were conducted under one-way analysis of variance (ANOVA). For pairwise comparisons between groups, if the variances were equal, Tukey’s post hoc test method was used. While others with heterogeneous variances, logarithmic transformation was performed to make the variances equal, followed by Tukey’s post hoc test multiple comparisons. P < 0.05 was considered statistically significant.
Results
SSR inhibits disease progression in 5/6 (A/I) rat model
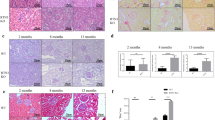
The effect of SSR on renal injury and interstitial fibrosis was studied in a rat 5/6 (A/I) renal failure model adhere strictly to the scheduled timeline (Fig. 1A). SCr and BUN levels, two renal functional parameters, were significantly elevated in 5/6 rats. Treatment with SSR or Losartan, a positive control agent, reduced SCr and BUN levels in 5/6 rats (Fig. 1B and C). Similarly, the level of UACR was increased after 5/6 operation, which was reduced by SSR or Losartan treatment (Fig. 1D). Intervention with SSR or Losartan reduced the interstitial collagen accumulation. HE staining revealed dilated renal tubules, atrophied epithelium and more inflammatory cells infiltration in 5/6 rat kidneys as compared with sham kidneys, which were attenuated by SSR or Losartan treatment. Masson staining showed that the collagen positive areas in 5/6 rat kidneys were significantly more than that in the sham kidneys (Fig. 1E). Furthermore, Western blotting analysis showed that the expressions of fibrotic markers such as FN, α-SMA, and PCNA in 5/6 rat kidneys were significantly enhanced as compared with that in sham kidneys, while SSR or Losartan inhibited the expressions of these fibrotic markers in 5/6 rat kidneys (Fig. 1F, G).
SSR inhibits disease progression in 5/6 (A/I) rat model. (A) 5/6 (A/I) rat model experimental program protocol. The levels of SCr (B), BUN (C), UACR (D) were measured after 8-week treatment (n = 8). (E) Representative HE and Masson’s trichrome staining. 200 × magnification. (F) The expression of fibronectin (FN), α-SMA and PCNA proteins were determined by Western blotting analysis. (G) Quantitative analysis of FN, α-SMA and PCNA expression (n = 4). Data were analyzed by One-way ANOVA. Values are mean ± SE. *P< 0.05; **P < 0.01. LOS, Losartan; SSR, Shen Shuai II Recipe; Scr, serum creatinine; BUN, blood urea nitrogen; UACR, urinary albumin: creatinine ratio.
SSR inhibits aerobic glycolysis in fibrotic kidneys
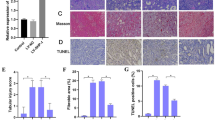
The expression of aerobic glycolysis enzymes was analyzed in 5/6 rat kidneys. Western blotting analysis showed that the 5/6 (A/I) rats induced the up-regulation of hexokinase-2 (HK-2) and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3), and the intervention of SSR or Losartan blocked this up-regulation (Fig. 2A-B). Next, renal lactate concentration, serum lactate concentration and renal lactic acid/pyruvic acid were examined in rat kidneys, and found to be increased in 5/6 kidneys as compared with sham kidneys (Fig. 2C-E). The intervention by SSR or Losartan significantly reversed all these metabolic changes in 5/6 rat kidneys (Fig. 2C-E). Together, these results indicate that SSR inhibits aerobic glycolysis in 5/6 rat kidneys.
SSR inhibits aerobic glycolysis in CKD rat kidneys. (A) The expression of HK2 and PFKFB3 proteins in rat kidneys were determined by Western blotting analysis. (B) Quantitative analysis of HK2 and PFKFB3 expression (n = 4). The levels of Serum LA (C), LA of kidney (D), LA/PYR (E) were measured after 8-week treatment (n = 6). Data were analyzed by One-way ANOVA. Values are mean ± SE. *P< 0.05; ** P < 0.01. LA, lactic acid; PYR, pyruvic acid.
SSR increases SIRT1 expression and inhibits HIF-1α expression in fibrotic kidneys
HIF-1α and its upstream regulatory factor SIRT1, two important factors involving in energy metabolism, were examined. SIRT1 was down-regulated and HIF-1α was up-regulated in 5/6 rat kidneys in mRNA and protein levels as compared with sham kidneys (Fig. 3A-C). Treatment with SSR or Losartan enhanced the mRNA (Fig. 3C) and protein expression (Fig. 3B) of SIRT1 and inhibited the expression of HIF-1α mRNA (Fig. 3C) and protein expression (Fig. 3B) in 5/6 kidneys.
SSR modulates SIRT1 and HIF-1α expression in fibrotic kidneys. (A) The expression of SIRT1 and HIF-1α proteins in rat kidneys were determined by Western blotting analysis. (B) Quantitative analysis of SIRT1 and HIF-1α expression (n = 4). (C) Quantitative analysis of SIRT1 and HIF-1α mRNA expression in rat kidneys (n = 6). Data were analyzed by One-way ANOVA. Values are mean ± SE. *P< 0.05; ** P < 0.01.
SSR inhibits fibrotic responses and glycolysis and increaseings SIRT1 expression in renal epithelial cells under hypoxia
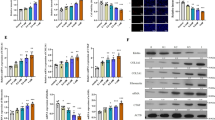
The effect of SSR on aerobic glycolysis was further studied through in vitro experiments. The expression of fibrotic markers FN and α-SMA were increased in rat renal epithelial cells (NRK-52E) upon hypoxic stimulation, which were associated with up-regulation of HK2, PFKFB3 and HIF-1α, and down-regulation of SIRT1 (Fig. 4A-C). SSR-containing serum dose-dependently reduced the expression of these fibrotic markers, HK2, PFKFB3 and HIF-1α, and dose-dependently increased SIRT1 expression (Fig. 4A-C). Upon hypoxia, lactate concentration was increased and glucose concentration was reduced in the supernatant of NRK-52E cells (Fig. 4D). SSR-containing serum dose-dependently reduced lactate concentration and increased glucose concentration in NRK-52E cells upon hypoxia (Fig. 4D).
SSR modulates SIRT1/HIF-1α and aerobic glycolysis in rat epithelial cells. (A) The expression of FN and α-SMA proteins were determined by western blot. Quantitative analysis of FN and α-SMA levels (n = 4). (B) The expression of HK2 and PFKFB3 proteins were determined by Western blotting analysis. Quantitative analysis of HK2 and PFKFB3 levels (n = 4). (C) The expression of SIRT1 and HIF-1α proteins were determined by Western blotting analysis. Quantitative analysis of SIRT1 and HIF-1α levels (n = 4). (D) The levels of Glucose, LA and PH in the cell culture medium (n = 4). Data were analyzed by One-way ANOVA. Values are mean ± SE. *P< 0.05; ** P < 0.01.
SSR inhibits fibrotic responses and glycolysis through SIRT1
Under hypoxic conditions, SSR reduced the expression of FN, α-SMA, HK2, PFKFB3 and HIF-1α and increased SIRT1 expression in NRK-52E cells (Fig. 5A-C). Downregulation of SIRT1 by siRNA reversed the expression of these fibrotic markers, HIF-1α and glycolysis enzymes in NRK-52E cells under hypoxia condition (Fig. 5A-C). Moreover, treatment with SSR reduced lactate concentration and increased glucose concentration in NRK-52E cells under hypoxic condition, which were reversed by SIRT1 siRNA (Fig. 5D).
All these data indicate that SSR inhibits fibrotic responses and glycolysis through SIRT1 in renal epithelial cells.
SSR inhibits renal fibrosis and glycolysis through SIRT1. (A) The expression of FN and α-SMA proteins were determined by western blot. Quantitative analysis of FN and α-SMA levels (n = 4). (B) The expression of HK2 and PFKFB3 proteins were determined by Western blotting analysis. Quantitative analysis of HK2 and PFKFB3 levels (n = 4). (C) The expression of SIRT1 and HIF-1α proteins were determined by Western blotting analysis. Quantitative analysis of SIRT1 and HIF-1α levels (n = 4). (D) The levels of Glucose, LA and PH in the cell culture medium (n = 4). Data were analyzed by One-way ANOVA. Values are mean ± SE. *P< 0.05; ** P < 0.01.
Discussion
In the current study, we revealed the renal protection of SSR in a rat CKD model as shown by improved renal function, reduced proteinuria and renal interstitial fibrosis. Mechanistically, we found the renal protective effect of SSR was correlated with increased expression of SIRT1 and reduced aerobic glycolysis in CKD kidneys. This correlation was further proved in vitro model of rat renal epithelial cells. The protective effect of SSR on HIF-1α and renal fibrosis was weakened when the SIRT1 gene was silenced, indicating that SIRT1 is a core molecular target for SSR’s renal protection during hypoxia. Additionally, it was found that SSR can down-regulate the expression of glycolysis-related proteins in the hypoxic epithelial cell model, reducing the consumption of glucose in the culture medium and the accumulation of lactic acid. When SIRT1 gene was silenced, this effect of SSR would also be unable to reverse the glycolytic phenotype.
SIRT1, the most well-studied member of the Sirtuin family, has its active center in the NAD+/NADH-binding region and is a NAD+-dependent deacetylase that alters the activity of specific enzymes or transcription factors by modifying their protein acetylation. It is a NAD+-dependent deacetylase that counteracts the acetyltransferases of specific enzymes or transcription factors through protein acetylation modification, thus altering their activities and playing a role in DNA damage repair, energy metabolism, apoptosis, autophagy, and inflammation29,30. Under physiological conditions, activation of SIRT1 at high NAD+ levels can inhibit the activation of downstream target genes by HIF-1α by deacetylating the K674 site of HIF-1α, promoting its binding to VHL proteins, accelerating ubiquitination degradation, and decreasing the stability of HIF-1α, as well as promoting the deacetylation of the Lys674 site in HIF-1α and inhibiting the activation of downstream target genes by HIF-1α21,31.Under hypoxia, mitochondrial oxidative phosphorylation is inhibited, NADH cannot be oxidized to NAD⁺, and the role of SIRT1 is inhibited. At the same time, HIF-1α as a hypoxia-dependent core transcription factor can contribute to the shift of metabolic reprogramming from oxidative phosphorylation to aerobic glycolysis by promoting the expression and activity of key enzymes for glucose uptake and utilization and glycolysis32 Down-regulation of SIRT1 expression was followed by increased acetylation of the HIF-lα promoter region and up-regulation of HIF-1α mRNA and protein expression, suggesting that SIRT1 can regulate HIF-1α expression at the transcriptional level30. In recent years, several studies have indicated that the SIRT1/HIF-lα axis plays an important role in the occurrence and development of Renal fibrosis22,32,33 (Fig. 6).
Energy metabolism changing in renal fibrosis under hypoxia. (A) Energy metabolism under hypoxia changes from oxidative phosphorylation to glycolysis in renal fibrosis. (B) Glycolysis under hypoxia is related to the inhibition of SIRT1/HIF-1α in renal fibrosis. Under physiological conditions, activation of SIRT1 at high NAD+ levels can inhibit the activation of downstream target genes by HIF-1α. Under hypoxia, NADH cannot be oxidized to NAD⁺, downregulated SIRT1 leading to upregulated of HIF-1α which promotes metabolic reprogramming from oxidative phosphorylation to glycolysis by enhancing the expression and activity of key enzymes involved in glucose uptake, utilization, and glycolysis.
Previous studies have shown that SSR contains active ingredients such as Salvianolic acid B, Berberine hydrochloride, Ferulic acid, Icariin, Tanshinone IIA, and Emodin34. It has been reported that Salvianolic acid B provided renal protection through activation of SIRT1-mediated autophagy in a unilateral nephrectomized rat model of renal fibrosis35. Berberine hydrochloride has been reported to improve the inflammatory responses in skeletal cells by activating the AMPKα-SIRT-1-PGC-1α pathway36. Ferulic acid improved glucose homeostasis, insulin resistance and chronic inflammation in a rodent model of diabetes mellitus by upregulation of SIRT1 mRNA expression37. Icariin played a role in regulation of energy metabolism through the activation of AMPK/ SIRT1/ NF-κB in rats with chronic kidney disease (CKD)38. Tanshinone IIA has been shown to inhibit endoplasmic reticulum stress in cardiomyocytes through SIRT1 in diabetic cardiomyopathy, thus exerting a cardioprotective effect39. Emodin has been found to improve fatty acid oxidation, oxidative stress, inflammation and complement activation system by upregulation of SIRT1 protein in influenza A virus pneumonia and sepsis40,41. Thus, SSR may activate SIRT1 signaling pathway in CKD kidneys through the synergistic effects of multiple active ingredients. Nevertheless, further studies are needed to determine the pharmacokinetic profile and therapeutic concentration of these compounds in renal tissue following oral administration. Notably, the multi-herb formulation may simultaneously target multiple regulatory sites of SIRT1 - a distinct advantage over single-component drugs.
In this study, we found that SSR inhibits aerobic glycolysis. It has been reported that Salvianolic acid B inhibited mTORC1-dependent glycolysis in the ischemia/reperfusion heart resulting in alleviation of inflammation and improved cardiac function42. Ferulic acid was shown to inhibit colorectal cancer through lncRNA 495,810/PKM2-mediated aerobic glycolysis43. Icariin activated the Wnt/β-catenin signaling pathway to increase the protein levels and activities of key glycolytic enzymes in Alzheimer’s disease models44. Tanshinone IIA inactivated Akt-c-Myc signal-mediated aerobic glycolysis resulting in inhibition of oral squamous cell carcinoma45. Emodin inhibited glycolysis through downregulation of GLUT1 by inactivating the ROS-mediated PI3K/AKT signaling pathway in renal cancer cells46. Thus, SSR may inhibits renal aerobic glycolysis in CKD kidneys through multiple signaling pathways, which warrants further investigations.
Previous studies have repeatedly observed that certain monomeric components within SSR can regulate SIRT1 and influence renal fibrosis, these investigations faced two primary limitations: the downstream pathways remained unexplored, and critically, the pharmacological actions of a compound herbal formula likely represent more than merely the additive effects of its individual constituents. Therefore, in the present study, we innovatively investigated the effects of SSR — a multi-component herbal formulation — on SIRT1 and its downstream targets HIF-1α and glycolysis through integrated in vivo and in vitro experiments. Our results demonstrate that SSR modulates glycolysis via the SIRT1/HIF-1α signaling axis, thereby attenuating renal fibrosis. Further research will characterize the biodistribution profile of SSR to assess its developability as a targeted metabolic reprogramming modulator.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 105, S117–S314. https://doi.org/10.1016/j.kint.2023.10.018 (2024).
Charles, C. & Ferris, A. H. Chronic kidney disease. Prim. Care. 47, 585–595. https://doi.org/10.1016/j.pop.2020.08.001 (2020).
Liu, B. C., Tang, T. T., Lv, L. L. & Lan, H. Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 93, 568–579. https://doi.org/10.1016/j.kint.2017.09.033 (2018).
Flythe, J. E. & Watnick, S. Dialysis for chronic kidney failure: a review. JAMA 332, 1559–1573. https://doi.org/10.1001/jama.2024.16338 (2024).
Ortiz, A. et al. Board of the EURECA-m working group of ERA-EDTA. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 383, 1831–1843. https://doi.org/10.1016/S0140-6736(14)60384-6 (2014).
Friedman, S. L., Sheppard, D., Duffield, J. S. & Violette, S. Therapy for fibrotic diseases: nearing the starting line. Sci. Transl. Med. 5, 167sr1. https://doi.org/10.1126/scitranslmed.3004700 (2013).
Huang, R., Fu, P. & Ma, L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal. Transduct. Target. Ther. 8, 129. https://doi.org/10.1038/s41392-023-01379-7 (2023).
Liu, B. C., Tang, T. T., Lv, L. L. & Lan, L. L. H. Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 93, 568–579. https://doi.org/10.1016/j.kint.2017.09.033 (2018).
Wang, W. et al. Metabolic reprogramming and renal fibrosis: what role might Chinese medicine play? Chin. Med. 19, 148. https://doi.org/10.1186/s13020-024-01004-x (2024).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37-46. https://doi.org/10.1038/nm.376220 (2015).
Dhillon, P. et al. The nuclear receptor ESRRA protects from kidney disease by coupling metabolism and differentiation. Cell Metabol. 33, 379–394e378. https://doi.org/10.1016/j.cmet.2020.11.011 (2021).
Wei, X., Hou, Y., Long, M., Jiang, L. & Du, Y. Advances in energy metabolism in renal fibrosis. Life Sci. 312, 121033. https://doi.org/10.1016/j.lfs.2022.121033 (2023).
Yang, S. et al. Inhibition of PFKP in renal tubular epithelial cell restrains TGF-β induced glycolysis and renal fibrosis. Cell Death Dis. 14, 816. https://doi.org/10.1038/s41419-023-06347-1 (2023).
Wei, Q. et al. Glycolysis inhibitors suppress renal interstitial fibrosis via divergent effects on fibroblasts and tubular cells. Am. J. Physiol. Ren. Physiol. 316, F1162–f1172. https://doi.org/10.1152/ajprenal.00422.2018 (2019).
Cai, T. et al. Sodium-glucose cotransporter 2 Inhibition suppresses HIF-1α-mediated metabolic switch from lipid oxidation to Glycolysis in kidney tubule cells of diabetic mice. Cell. Death Dis. 11, 390. https://doi.org/10.1038/s41419-020-2544-7 (2020).
Hewitson, T. D. & Smith, E. R. A metabolic reprogramming of Glycolysis and glutamine metabolism is a requisite for renal fibrogenesis-why and how? Front. Physiol. 12, 645857. https://doi.org/10.3389/fphys.2021.645857 (2021).
Van der Rijt, S., Leemans, J. C., Florquin, S., Houtkooper, R. H. & Tammaro, A. Immunometabolic rewiring of tubular epithelial cells in kidney disease. Nat. Rev. Nephrol. 18, 588–603. https://doi.org/10.1038/s41581-022-00592-x (2022).
Wei, X., Hou, Y., Long, M., Jiang, L. & Du, Y. Molecular mechanisms underlying the role of hypoxia-inducible factor-1 α in metabolic reprogramming in renal fibrosis. Front. Endocrinol. (Lausanne). 13, 927329. https://doi.org/10.3389/fendo.2022.927329 (2022).
Liu, D. et al. HIF-1α: a potential therapeutic opportunity in renal fibrosis. Chem. Biol. Interact. 387, 110808. https://doi.org/10.1016/j.cbi.2023.110808 (2024).
Joo, H. Y. et al. Lee. NADH elevation during chronic hypoxia leads to VHL-mediated HIF-1α degradation via SIRT1 Inhibition. Cell. Biosci. 13, 182. https://doi.org/10.1186/s13578-023-01130-3 (2023).
Lim, J. H. et al. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol. Cell. 38, 864–878. https://doi.org/10.1016/j.molcel.2010.05.023 (2010).
Ryu, D. R. et al. Sirt1-hypoxia-inducible factor-1α interaction is a key mediator of tubulointerstitial damage in the aged kidney. Aging Cell. 18, e12904. https://doi.org/10.1111/acel.12904 (2019).
Yang, J., Yan, R. J., Wang, C. & HE, L. Q. Effects of meliorated renal failure Decoction on renal function and inflammatory cytokines in patients with chronic kidney disease at 3–4 stage. Chin. J. Inform. Tradit. Chin. Med. 21 (12), 15–18 (2014).
Xu, Y. Y., He, Z., Zhou, Y., Yang, J. & Wang, C. Effects of the meliorated renal failure Decoction combined with Western medicine on renal function and renal perfusion in primary chronic kidney disease 3 or 4 stage patients. J. Tradit. Chin. Med. 59, 1480–1484. https://doi.org/10.13288/j.11-2166/r.2018.17.010 (2018). [Chinese].
Wang, M., Yang, J., Zhou, Y. & Wang, C. ShenShuai II recipe attenuates apoptosis and renal fibrosis in chronic kidney disease by increasing renal blood flow and improving oxygen consumption. Evid. Based Complement. Alternat Med. 13, 7602962. https://doi.org/10.1155/2018/7602962 (2018).
Wang, M. et al. Shen Shuai II recipe attenuates renal fibrosis in chronic kidney disease by improving hypoxia-induced the imbalance of mitochondrial dynamics via PGC-1α activation. Phytomedicine 98, 153947. https://doi.org/10.1016/j.phymed.2022.153947 (2022).
Wang, L., Feng, X., Ye, C., Wang, C. & Wang, M. Shen Shuai II recipe inhibits hypoxia-induced Glycolysis by preserving mitochondrial dynamics to attenuate kidney fibrosis. J. Ethnopharmacol. 308, 116271. https://doi.org/10.1016/j.jep.2023.116271 (2023).
Hong, Y. A., Kim, J. E., JO, M. & Ko, G. J. The role of sirtuins in kidney diseases. Int. J. Mol. Sci. 21, 6686. https://doi.org/10.3390/ijms21186686 (2020).
Morigi, M., Perico, L. & Benigni, A. Sirtuins in renal health and disease. J. Am. Soc. Nephrol. 29, 1799–1809. https://doi.org/10.1681/ASN.2017111218 (2018).
Han, X. et al. Targeting Sirtuin1 to treat aging-related tissue fibrosis: from prevention to therapy. Pharmacol. Ther. 229, 107983. https://doi.org/10.1016/j.pharmthera.2021.107983 (2022).
Wu, H. et al. Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1α-mediated glycolytic reprogramming. Nat. Commun. 14, 6858. https://doi.org/10.1038/s41467-023-42634-3 (2023).
Owczarek, A. et al. Melatonin lowers HIF-1α content in human proximal tubular cells (HK-2) due to preventing its deacetylation by sirtuin 1. Front. Physiol. 11, 572911. https://doi.org/10.3389/fphys.2020.572911 (2020).
Sun, X. H. et al. Connexin 43 prevents the progression of diabetic renal tubulointerstitial fibrosis by regulating the SIRT1-HIF-1α signaling pathway. Clin. Sci. (Lond.) 134, 1573–1592. https://doi.org/10.1042/CS20200171 (2020).
Yang, L. Y. et al. Shen Shuai II Recipe attenuates renal interstitial fibrosis by improving hypoxia via the IL-1β/c-Myc pathway. Evid. Based Complement Alternat. Med. 7, 5539584. https://doi.org/10.1155/2021/5539584 (2021).
He, Y. et al. Salvianolic acid B attenuates epithelial-mesenchymal transition in renal fibrosis rats through activating Sirt1-mediated autophagy. Biomed. Pharmacother. 128, 110241. https://doi.org/10.1016/j.biopha.2020.110241 (2020).
Poudel, A. et al. Berberine hydrochloride protects against cytokine-induced inflammation through multiple pathways in undifferentiated C2C12 myoblast cells. Can. J. Physiol. Pharmacol. 97, 699–707. https://doi.org/10.1139/cjpp-2018-0653 (2019).
Mehdi, S., Mehmood, M. H., Ahmed, M. G. & Ashfaq, U. A. Antidiabetic activity of berberis brandisiana is possibly mediated through modulation of insulin signaling pathway, inflammatory cytokines and adipocytokines in high fat diet and streptozotocin-administered rats. Front. Pharmacol. 14, 1085013. https://doi.org/10.3389/fphar.2023.1085013 (2023).
Zhao, Y., Yang, W., Zhang, X., Lv, C. & Lu, J. Icariin, the main prenylflavonoid of epimedii Folium, ameliorated chronic kidney disease by modulating energy metabolism via AMPK activation. J. Ethnopharmacol. 312, 116543. https://doi.org/10.1016/j.jep.2023.116543 (2023).
Wu, S. et al. Tanshinone IIA ameliorates experimental diabetic cardiomyopathy by inhibiting Endoplasmic reticulum stress in cardiomyocytes via SIRT1. Phytother. Res. 37, 3543–3558. https://doi.org/10.1002/ptr.7831 (2023).
Bei, Y. et al. Anti-influenza A virus effects and mechanisms of emodin and its analogs via regulating PPARα/γ-AMPK-SIRT1 pathway and fatty acid metabolism. Biomed. Res. Int. 2021, 9066938. https://doi.org/10.1155/2021/9066938 (2021).
Cui, J., Wang, S., Bi, S., Zhou, H. & Sun, L. Emodin-based regulation and control of serum complement C5a, oxidative stress, and inflammatory responses in rats with Urosepsis via AMPK/SIRT1. Iran. J. Allergy Asthma Immunol. 23, 550–562. https://doi.org/10.18502/ijaai.v23i5.16750 (2024).
Zhao, M. et al. Salvianolic acid B regulates macrophage polarization in ischemic/reperfused hearts by inhibiting mTORC1-induced Glycolysis. Eur. J. Pharmacol. 871, 172916. https://doi.org/10.1016/j.ejphar.2020.172916 (2020).
Cui, K. et al. The mixture of ferulic acid and P-Coumaric acid suppresses colorectal cancer through LncRNA 495810/PKM2 mediated aerobic Glycolysis. Int. J. Mol. Sci. 23, 12106. https://doi.org/10.3390/ijms232012106 (2022).
Liu, J. et al. Icariin ameliorates glycolytic dysfunction in alzheimer’s disease models by activating the Wnt/β-catenin signaling pathway. FEBS J. 291, 2221–2241. https://doi.org/10.1111/febs.17099 (2024).
Li, M. et al. Tanshinone IIA inhibits oral squamous cell carcinoma via reducing Akt-c-Myc signaling-mediated aerobic glycolysis. Cell. Death Dis. 11, 381. https://doi.org/10.1038/s41419-020-2579-9 (2020).
Wang, K. J. et al. Emodin induced necroptosis and inhibited glycolysis in the renal cancer cells by enhancing ROS. Oxid. Med. Cell. Longev. 19, 8840590. https://doi.org/10.1155/2021/8840590 (2021).
Funding
This work was financially supported by Youth Fund of the National Natural Science Foundation of China (No. 82205018) to Liuyi Yang, National Natural Science Foundation of China (No. 81973770) to Chen Wang.
Author information
Authors and Affiliations
Contributions
L performance for in vitro and in vivo experiments, Data analysis, Manuscript drafting. Z performance for in vivo experiments, Data analysis. L performance for in vivo experiments, Data analysis. W performance for manuscript drafting, Supervision. W performance for Conceptualization, experimental design. Y performance for Conceptualization, Data analysis, Performance for in vitro and in vivo experiments, Manuscript drafting, Project managing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lan, T., Zhang, X., Lyu, X. et al. Shen-Shuai-II-Recipe inhibits aerobic glycolysis through SIRT1 in 5/6 ablation/infarction renal failure model. Sci Rep 16, 5022 (2026). https://doi.org/10.1038/s41598-026-35061-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-026-35061-z