Abstract
Elevated iron in the SNpc may play a key role in Parkinson’s disease (PD) neurodegeneration, yet the underlying mechanism accounting for this iron accumulation is unclear. Although iron is an essential element, excessive amounts produce toxicity. Here, we focused on the role of iron and ATP13A2, the causative gene of PARK9 neurodegeneration with brain iron accumulation, using a cellular model. ATP13A2 deficiency resulted in impaired lysosomal function and iron accumulation in cell organelles. Further, we found dysfunction of mitophagy, which is involved in managing mitochondrial quality, as well as mitochondrial damage. Furthermore, we confirmed a decreased heme synthesis capacity, which is important to maintain intracellular iron homeostasis. Overall, our study indicates that lysosome-derived mitochondrial impairment can disrupt intracellular iron homeostasis in a cell model of PD pathology. This could help better understand the mechanisms underlying PD.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is characterized by loss of the midbrain dopaminergic neurons; its clinical features include motor and nonmotor symptoms such as akinesia, rigidity, tremor, cognitive impairment, sleep disorders, and dysautonomia1. Pathologically, Lewy bodies, a neuronal inclusion containing the presynaptic protein α-synuclein (α-Syn, encoded by SNCA) and other factors that accelerate α-Syn aggregation (e.g., ubiquitin, lipids, and iron) are mostly found in the affected regions, strongly implying that α-Syn aggregation is closely related to the neurodegeneration in PD2,3,4.
Although the precise mechanism underlying the onset of PD remains unclear, both genetic and environmental factors appear to contribute to the development of PD5. Two different forms of PD have been identified, i.e., sporadic and familial. Most PD cases are sporadic, of unknown etiology1. However, approximately 5–10% of PD cases seem to have monogenic forms of inheritance, including autosomal dominant (e.g., SNCA, LRRK2, VPS35, and CHCHD2) and recessive (e.g., PRKN, PINK1, DJ1, and ATP13A2) forms6,7,8. Analysis of causative genes and environmental factors has indicated an association between dysfunction of cellular organelles such as mitochondria and lysosomes and PD progression9,10. Since numerous PD-related genes are involved in common biological pathways, several critical cellular routes may be involved in the disease risk7. Mitochondrial dysfunction was one of the first biological processes associated with PD pathogenesis11. Familial PD can be caused by mutations in PINK/PARK6, PRKN/PARK2, and DJ-1/PARK7, each contributing to the selective removal of dysfunctional mitochondria by autophagy (mitophagy)7. During mitophagy, compromised mitochondria are flagged by autophagy receptors, recognized and engulfed by phagophores, which then mature into autophagosomes, being delivered to lysosomes for degradation12. Further, PD-related mutations in genes regulating lysosomal functions have indirect effects on mitochondria. In addition, recent studies have pointed out a high genetic risk burden for PD in lysosomal pathways7,12,13,14,15,16.
ATPase cation transporting 13A2 (ATP13A2) has been identified as the causative gene of PARK9 for familial PD17. In addition, several pathogenic mutations in ATP13A2 have been implicated in Kufor-Rakeb Syndrome18. Kufor-Rakeb syndrome is classified as neurodegeneration with brain iron accumulation (NBIA), a neurodegenerative disease characterized by iron deposition in the basal ganglia18. ATP13A2 is a lysosome-localized ATPase possibly involved in membrane transport of cationic molecules such as polyamines and protons, being involved in maintaining lysosomal function19,20,21. Further, iron accumulation is observed in the brain of PARK9 patients18 and ATP13A2 overexpression decreases iron-derived cytotoxicity in a cell model of PD22. Additionally, in patients with PD there is high lysosomal pH caused by loss-of-function mutations of ATP13A2, leading to proteolytic failure19. Therefore, given that mitophagy is a lysosome-mediated mechanism, decreased ATP13A2 function can result in mitophagy failure. In such a case, defective mitochondria may accumulate, causing oxidative stress, and impairing mitochondrial function. Moreover, mitochondria may be key in the pathogenesis of PARK9, given the reported relationship between PD and mitophagy23,24. Accordingly, ATP13A2 may contribute to intracellular iron homeostasis through lysosome dysfunction. However, the relationship between ATP13A2 deficiency and intracellular iron homeostasis remains unclear.
Iron is an essential element that contributes not only to oxygen transport but also to physiological functions such as electron transfer in mitochondria and DNA synthesis25. Iron has two stable oxidative states, Fe2+ and Fe3+, which are readily convertible via accepting or donating electron. The redox activity of iron enables many enzymatic redox reactions in collaboration with proteins but can produce free radicals through Fenton reaction26. Emerging evidence suggests that ferroptosis, an iron-dependent non-apoptotic cell death process, may be involved in the progression of PD. On the other hand, α-Syn aggregation has been shown to generate reactive oxygen species and lipid peroxides within cellular membranes, which may potentially trigger ferroptosis3,27. Thus, the disruption of intracellular iron homeostasis contributes to neurodegenerative diseases3,28. The heme, synthesized in mitochondria and with iron ions coordinated to its active center, plays an important role in the maintenance of intracellular iron homeostasis. Iron regulatory protein 2 (IRP2), which regulates the expression of iron-related factors such as DMT1, transferrin receptor, and ferroportin, is degraded in a heme-dependent manner; thus, heme synthesis in mitochondria is involved in the intracellular iron concentration sensing mechanism29. Therefore, mitochondrial dysfunction could be a potential cause of disruption of intracellular iron homeostasis, as increased iron levels in neurons have been reported not only in PARK9 but also in common neurodegenerative diseases such as sporadic PD, Alzheimer’s disease, and amyotrophic lateral sclerosis.
This study aimed to elucidate one aspect of the mechanism of iron accumulation in PARK9. We applied the cell organelle specific fluorescent probes for Fe2+ and labile heme30,31,32,33,34 to investigating how ATP13A2 deficiency affects intracellular iron homeostasis under pathological conditions. In this study, we found that lysosomal dysfunction caused by ATP13A2 deficiency induces mitochondrial dysfunction, which in turn disrupts intracellular iron homeostasis.
Materials/Subjects and methods
Cell culture experiment
The human neuroblastoma cell line SH-SY5Y (European Collection of Authenticated Cell Cultures, Catalogue number: 94030304) was cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich) supplemented with 10% FBS at 37 °C in a 5% CO2/95% air incubator. For the overexpression experiment, α-Syn-SH cells were prepared as previously described33.
SiRNA transfection
For ATP13A2 KD experiments, SH-SY5Y or α-Syn-SH cells were transfected using a siGENOME pool targeted against human ATP13A2 (ATP13A2-siRNA; EHU141031, Sigma-Aldrich), siGENOME pool targeted against human PINK1 (PINK1-siRNA; EHU057101, Sigma-Aldrich), or MISSION® siRNA Universal Negative Control (NC, SIC001-10NMOL, Sigma-Aldrich), using Lipofectamine RNAiMAX reagent (13778150, ThermoFisher Scientific), and incubated for 48 h following manufacturer’s instructions (Thermo Fisher Scientific).
RT-qPCR
Total cellular RNA was isolated using TRI reagent according to manufacturer’s instructions (Sigma-Aldrich). Reverse transcription was performed using a ReverTra Ace qPCR RT Master Mix in accordance with manufacturer’s instructions (TOYOBO). Similarly, RT-qPCR was performed using SYBR Green on a StepOne Real-Time PCR System according to manufacturer’s instructions (Life Technologies). Table S1 shows the sequences of gene-specific primer sets. mRNA expression levels were normalized to those of GAPDH mRNA.
Western blot
Treated cells were lysed with RIPA buffer [50 mM Tris-HCl (pH. 7.4), 150 mM NaCl, 0.1% Triton X, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), protease inhibitor cocktail, and phosphatase inhibitor] before centrifugation at 15,000 ×g and 4 °C for 30 min. The supernatant was collected as it contained the protein samples. Protein concentration was determined using the Pierce BCA protein assay kit (Thermo Fisher Scientific). Samples underwent SDS-PAGE to separate proteins based on molecular weight. SDS-PAGE was performed under constant voltage at 200 V at room temperature for 60 min. The separated proteins in polyacrylamide gel were transferred to a Poly Vinylidene Di-Fluoride membrane in transfer buffer (0.3% Tris, 1.44% glycine, 20% methanol) under constant voltage at 100 V at 4 °C for 60 min. The transferred membrane was incubated in 5% skim milk (Nakarai Tesque) or Blocking One (Nakarai Tesque) at room temperature for 60 min. After blocking, the membrane was incubated with the following primary antibodies: the mouse monoclonal antibody transferrin receptor (1:500, Invitrogen), and β-actin (1:2000, Santa Cruz Biotechnology); rabbit polyclonal antibodies: ATP13A2 C-terminal region (1:1000, Sigma-Aldrich), LC3 (1:1000, MBL), SQSTM1/p62 (1:1000, Cell Signaling), IRP2 (1:1000, Novus Biologicals), ferritin and DMT1 (1:1000, Abcam) α-Synuclein (1:2000, Abcam) dissolved in 5% skim milk or Reagent A of Immuno-enhancer (FUJIFILM) at 4 °C overnight. After the primary antibody reaction, the membrane was incubated with the secondary antibody, namely, goat anti-rabbit antibody conjugated with HRP (1:2500, Santa Cruz Biotechnology), and goat anti-mouse antibody conjugated with HRP (1:2500, Santa Cruz Biotechnology) dissolved in 3% skim milk or Reagent B of Immuno-enhancer for 60 min. Next, membranes were incubated in ECL prime (GE Healthcare, Buckinghamshire, UK) to generate chemiluminescence from HRP antibodies. Chemiluminescence was detected by Fusion System (Vilber-Lourmat), and the band density was measured using ImageJ.
Autophagy flux experiments
α-Syn-SH cells were seeded in 12-well plates and transfected with ATP13A2 siRNA for 48 h, and 50 µM Chloroquine Diphosphate (Tokyo Chemical Industry Co., Ltd.) was treated for 1 h. After that, LC3 was analyzed by WB.
Assessing the acidity of lysosomes
α-Syn-SH cells were seeded in 3.5 cm, 4 compartments, glass bottom dish (Greiner bio-one, 627870) and transfected with ATP13A2 siRNA for 48 h. After siRNA transfection, cells were washed with HBSS (140 mg/L CaCl2, 100 mg/L MgCl2 6H2O, 100 mg/L MgSO4 7H2O, 400 mg/L KCl, 60 mg/L KH2PO4, 350 mg/L NaHCO3, 8000 mg/L NaCl, 48 mg/L Na2HPO4, 1000 mg/L D-Glucose) and treated with Lysosonsor™ Green-DND-189 (100 nM in HBSS, Thermo Fisher Scientific) for 15 min at 37 °C. After incubation, cells treated with Hoechst 33342 (1.0 µg/mL in HBSS, Thermo Fisher Scientific) for 15 min at 37 °C. Next, cells were washed 3x with HBSS. Fluorescent microscopy images were acquired using a confocal fluorescence microscope (LSM700; Carl Zeiss) and the fluorescence intensity of each image was analyzed using Image J.
Live cell imaging of Fe2+ by fluorescent probes
α-Syn-SH cells were seeded in 3.5 cm, four compartments, glass bottom dishes (Greiner bio-one, 627870) and transfected with ATP13A2 or PINK1 siRNA for 48 h. Next, cells were washed with HBSS and treated with Rho-Nox 4 (1.0 µM in HBSS), MtFluNox (5.0 µM in HBSS), or HM-RhoNox-M (3.0 µM in HBSS) for 15 min at 37 °C. After incubation, cells were treated with Hoechst 33342 (1.0 µg/mL in HBSS, Thermo Fisher Scientific) for 15 min at 37 °C. Then, cells were washed 3x with HBSS. Fluorescent microscopy images were acquired using a confocal fluorescence microscope (LSM700 or LSM 900; Carl Zeiss) and the fluorescence intensity of each image was analyzed using Image J.
Atomic absorption spectrometry to measure intracellular iron content
α-Syn-SH cells seeded in 6-well plates (NIPPON genetics, TR5000) were transfected with ATP13A2 siRNA for 48 h. The medium was then washed 3x with cold phosphate-buffered saline (PBS, 6 mL times). Then, cells were removed from the dishes using a scraper, and the number of cells was counted with an automated cell counter (Countess II® FL, Thermo Fisher Scientific). Next, the suspension was centrifuged (1,000 rpm, 5 min) and the supernatant carefully removed, before resuspending the cells into conc. HNO3 (100 µL). Then, the suspension was heated at 90 °C for 4 h to dissolve the cell bodies. Next, the lysate was diluted to 5 mL with distilled water. Iron concentrations in the samples were measured by furnace atomic absorption spectroscopy with a Shimadzu AA-7000 atomic absorption spectrometer. The obtained values (ng/mL) were normalized to the cell number (per 105 cells).
Immunofluorescence staining
Treated cells were fixed with 4% paraformaldehyde for 10 min. Subsequently, these cells were permeabilized with 0.1% Triton X/PBS for 30 min at RT. A 2% goat serum was used for blocking for 60 min. Then, cells were incubated with a rabbit polyclonal antibody against Ferritin (1:100, Abcam). Subsequently, cells were incubated with secondary antibodies (goat anti-rabbit IgG (H + L) cross-adsorbed secondary antibody, Alexa Fluor 555 (1:200; Life Technologies Co.) at RT for 30 min. In addition, cells were incubated with Hoechst 33342 (Molecular Probes) for 15 min at room temperature. Finally, fluorescence was observed using a confocal fluorescence microscope (LSM700; Carl Zeiss). Fluorescence intensity of each image was analyzed by ImageJ, and the data were normalized by the number of nuclei.
Measurement of oxidative stress
To detect oxidative stress, we used a red mitochondrial superoxide indicator, MitoSOX® Red (Thermo Fisher Scientific). First, α-Syn-SH cells were seeded in 3.5 cm, four compartment, glass bottom dishes (Greiner bio-one, 627870) and transfected with ATP13A2 siRNA for 48 h. After transfection, MitoSOX® Red was added to the cell culture to a final concentration of 5 µM and incubated for 30 min at 37 °C following manufacturer’s instructions (Thermo Fisher Scientific). Fluorescent microscopy images were acquired using a confocal fluorescence microscope (LSM700, Carl Zeiss) and the fluorescence intensity of each image was analyzed using Image J.
Measurement of cell viability
Cell viability was measured using a Cell Counting Kit-8 (Dojindo). α-Syn-SH cells were seeded in 96-well plates and transfected with ATP13A2 siRNA for 48 h. Thereafter, cell viability was measured with or without DFO, apo Transferrin, Glibenclamide or Ferrostatin-1, as per the CCK-8 protocol.
Seahorse XF mitostress test
The XF Extracellular Flux Analyzer (Seahorse Bioscience) was used to detect oxygen consumption rates. α-Syn-SH cells were seeded in 6-well plates (NIPPON genetics, TR5000) and transfected with ATP13A2 siRNA for 48 h. After transfection, cells were re-seeded in a Seahorse plate coated with Cell-Tak (CORNING), and centrifuged at 200 g for 1 min. Then, cells were equilibrated with XF Base media (Seahorse, catalog number 102353-100) at 37 °C for 1 h in an CO2 free incubator. Mitochondrial stress was measured using an Agilent Seahorse XF Cell Mito Stress Test Kit (Seahorse) following the manufacturer’s protocol before sequential treatment with oligomycin (1.0 µM), FCCP (1.0 µM), and rotenone/antimycinA (0.5 µM) (Seahorse).
Live cell Heme imaging by fluorescent probes
α-Syn-SH cells were first seeded in 3.5 cm, four compartments, glass bottom dishes (Greiner bio-one, 627870) and transfected with ATP13A2 or PINK1 siRNA for 48 h. Next, cells were treated with 5-amino levulinic acid for 24 h to induce heme synthesis. Thereafter, cells were washed with HBSS and treated with AcH-FluNox (1.0 µM in HBSS) for 15 min at 37 °C. After incubation, cells we treated with Hoechst 33,342 (1.0 µg/mL in HBSS, Thermo Fisher Scientific) for 15 min at 37 °C. Finally, cells were washed 3x with HBSS. Fluorescent microscopy images were acquired using a confocal fluorescence microscope (LSM700 or LSM 900; Carl Zeiss) and the fluorescence intensity of each image was analyzed using Image J.
Mitophagy detection kit
Mitophagy was detected under starvation conditions using a mitophagy detection kit (DOJINDO, MD01). α-Syn-SH cells were first seeded in 3.5 cm, four compartments, glass bottom dishes (Greiner bio-one, 627870) and transfected with ATP13A2 siRNA for 48 h. Then, cells were washed 2x with HBSS and treated with 100 nM mitophagy dye working solution for 30 min at 37 °C. Thereafter, cells were washed 2x with HBSS and starvation was induced by an amino acid-free medium including pepstatin A (7.5 µM) and E-64d (1.0 µM) for 6 h at 37 °C. Then, cells were treated with 1 µM Lyso dye working solution for 30 min at 37 °C. After washing 2x with HBSS, fluorescent microscopy images were acquired using a confocal fluorescence microscope (LSM700 or LSM 900; Carl Zeiss) and analyzed the intensity of Mitophagy Dye using Image J.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). Significance was determined using the Student’s t-test or ANOVA. Post-hoc comparisons were conducted utilizing the Bonferroni/Dunn test (StatView, Abacus). p-values < 0.05 were considered statistically significant (n.s. not significant. *p < 0.05, **p < 0.01, ***p < 0.001).
Results
Lysosomal dysfunction in SH-SY5Y cells overexpressing α-Syn with ATP13A2 knockdown
Previous studies have established the crucial role of lysosomal function in the degradation of protein aggregates35,36. Therefore, we investigated whether ATP13A2 loss accelerates α-Syn accumulation in human neuroblastoma SH-SY5Y cells. These cells are a common model to investigate the molecular basis of PD-associated genes, including ATP13A237. Initially, we analyzed the intracellular levels of endogenous α-Syn in both control and ATP13A2 knockdown (KD) cells (Figure S1). In control cells, endogenous α-Syn was hardly detectable under our experimental conditions. (Figure S1). This result may be due to the low expression level of endogenous α-Syn in SH-SY5Y cells. Subsequently, we explored the effects of ATP13A2 KD after α-Syn overexpression through lentivirus infection for human wild-type α-Syn in SH-SY5Y cells (hereafter named α-Syn-SH cells)33. ATP13A2 KD was confirmed by Western blot and real-time PCR in α-Syn-SH cells (Fig. 1A–1C). As ATP13A2 is a lysosomal localized ATPase, we also analyzed lysosome function. Using LysoSensor™ Green, we detected an increase in lysosomal pH in ATP13A2 KD cells (Figs. 1D and E). In α-Syn-SH cells with ATP13A2 KD, the levels of autophagy markers, such as LC3 and p62, were significantly elevated, indicating a reduction in autophagy capacity (Fig. 1F–1H). In addition, we performed autophagy flux experiments with Chloroquine, which inhibits fusion of autophagosomes with lysosomes (Figure S2). LC3 increase in Chloroquine-treated groups was similar between NC and ATP13A2 KD. This result indicates that the increase in LC3 in the basal condition was due to impaired fusion between autophagosomes and lysosomes, rather than increased biogenesis. It has been widely reported that lysosomal function is impaired, and autophagy is inhibited when ATP13A2 is deficient38,39,40. Therefore, autophagy would also be inhibited in our α-Syn-SH cells with ATP13A2 KD in this study. Additionally, a significant α-Syn accumulation was confirmed in α-Syn-SH cells with ATP13A2 KD (Figure S1). These results suggest that the decreased ATP13A2 expression by siRNA caused lysosomal impairment in α-Syn-SH cells. For subsequent experiments we used a siRNA ATP13A2 KD in α-Syn-SH cells as a PARK9 cell model.
Lysosome function decreases in SH-SY5Y cells overexpressing α-synuclein with of ATP13A2 knockdown. SH-SY5Y cells overexpressing α-synuclein were transfected with siRNA targeting ATP13A2 for 48 h. We evaluated lysosomal functions. (A) Knocking down ATP13A2 with siRNA in α-Syn-SH cells. Knockdown (KD) was confirmed at mRNA level (qRT-PCR analysis). Data were normalized to GAPDH level (n = 4, biological replicates, NC = 1.0, siATP = 0.277). (B) ATP13A2 KD confirmed at the protein level (Western blot). (C) Quantification of (B). Data were normalized to β-actin content (n = 3, biological replicates, NC = 1.0, siATP = 0.237). (D) Lysosensor™ Green and Hoechst staining for analysis of lysosomal pH in α-Syn-SH cells with ATP13A2 KD (Green: LysoSensor™ Green, Blue: Hoechst) (Scale bar, 10 μm). (E) Quantification of (D) (n = 4, biological replicates, Cell number of NC for each biological replicate: 68, 49, 65, 58, Cell number of siATP for each biological replicate: 55, 57, 55, 54, NC = 1.0, siATP = 0.882). (F) Western blot of autophagy markers (LC3-Ⅰ, LC3-Ⅱ, p62) and β-actin in α-Syn-SH cells with ATP13A2 KD. (G) Quantification of (F). Data were normalized to β-actin levels (n = 3, biological replicates, LC3: NC = 1.0, siATP = 1.644, p62: NC = 1.0, siATP = 3.706). Each value represents the mean ± SEM. Student’s t-test was used to test the significance of differences (*p < 0.05, **p < 0.01, ***p < 0.001). siATP: siRNA targeting ATP13A2. NC: negative control, scrambled RNA was added to the cells instead of the targeted siRNA.
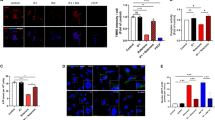
Cytotoxicity induced by increased intracellular iron levels in α-Syn-SH cells with ATP13A2 KD
As the pathophysiology of PARK9 appears closely related to intracellular iron homeostasis17,18, we analyzed the total levels of iron and labile Fe2+ in α-Syn-SH cells with ATP13A2 knockdown (KD) using atomic absorption spectrometry and RhoNox-4, a novel chemical probe designed for the detection of intracellular labile Fe2+, respectively30. In α-Syn-SH cells with ATP13A2 KD, we detected a significant increase in both total iron levels (Fig. 2A) and labile Fe2+ (Fig. 2B and C). We analyzed expression levels of ferritin, a protein that stores labile Fe2+ within its structure to avoid iron toxicity41, using fluorescent immunostaining and Western blot in α-Syn-SH cells with ATP13A2 KD. Ferritin expression was significantly increased (Fig. 2D and G), suggesting that the elevated ferritin levels could be due to inadequate ferritin degradation in α-Syn-SH cells with ATP13A2 KD. Additionally, labile Fe2+ is an important component in essential cellular processes such as mitochondrial respiration, DNA synthesis, and various enzymatic reactions26. The distribution of labile Fe2+ is crucial for intracellular iron homeostasis. Therefore, to monitor mitochondrial and lysosomal labile Fe2+ levels, we used two organelle-specific Fe2+ fluorescent probes: MtFluNox for mitochondria and HM-RhoNox-M for lysosomes31,32. MtFluNox exhibited significantly higher fluorescence in the mitochondria of ATP13A2 KD cells than in control cells (Fig. 2H and I), indicating an increased basal level of labile Fe2+ in the mitochondria due to ATP13A2 KD. Similarly, imaging with HM-RhoNox-M revealed a higher fluorescence signal in ATP13A2 KD cells than control cells (Fig. 2J and K), suggesting that ATP13A2 KD induced lysosomal accumulation of labile Fe2+.
Cytotoxicity due to increased intracellular iron level in α-Syn-SH cells with ATP13A2 KD. (A) Atomic absorption spectrometry for the analysis of intracellular total iron level in α-Syn-SH cells with ATP13A2 KD. Quantification of iron content per 1.0 × 105 cells (n = 3, biological replicates, NC = 1.241, siATP = 2.177). (B) Staining of RhoNox-4, which is a selective Fe2+ probe in α-Syn-SH cells with ATP13A2 KD (Red: Rho-Nox4, Blue: Hoechst) (Scale bar, 10 μm). (C) Quantification of (B) (n = 4, biological replicates, Cell number of NC for each biological replicate: 124, 104, 77, 74, Cell number of siATP for each biological replicate: 104, 92, 83, 81, NC = 1.0, siATP = 1.528). (D) Immunocytochemistry for Ferritin which is iron storage protein in α-Syn-SH cells with ATP13A2 KD. (Red: Ferritin, Blue: Hoechst) (Scale bar, 10 μm) (E) Quantification of (D) (n = 5, biological replicates, Cell number of NC for each biological replicate: 22, 29, 39, 25, 29, Cell number of siATP for each biological replicate: 21, 22, 20, 24, 31, NC = 1.0, siATP = 2.369). (F) Immunoblot for Ferritin in in α-Syn-SH cells with ATP13A2 KD. (G) Quantification of (F). Data were normalized to β-actin (n = 3, biological replicates, NC = 1.0, siATP = 1.917). (H) Staining of MtFluNox, which is a selective mitochondrial Fe2+ probe in α-Syn-SH cells with ATP13A2 KD (Green: MtFluNox, Blue: Hoechst) (Scale bar, 10 μm). (I) Quantification of (H) (n = 7, biological replicates, Cell number of NC for each biological replicate: 85, 101, 93, 92, 83, 73, 67, Cell number of siATP for each biological replicate: 73, 82, 76, 84, 64, 89, 80, NC = 1.0, siATP = 1.098). (J) Staining of HM-RhoNox M, which is a selective lysosomal Fe2+ probe in α-Syn-SH cells with ATP13A2 KD (Red: HM-RhoNox M, Blue: Hoechst) (Scale bar, 10 μm). (K) Quantification of (J) (n = 8, biological replicates, Cell number of NC for each biological replicate: 73, 53, 51, 69, 47, 54, 71, 48, Cell number of siATP for each biological replicate: 57, 68, 71, 63, 64, 56, 62, 64, NC = 1.0, siATP = 1.134). (L) MitoSOX dye in α-Syn-SH cells treated with DFO (50 µM, 24 h) after ATP13A2 KD for evaluation of mitochondrial ROS (Red: MitoSOX, Blue: Hoechst) (Scale bar, 10 μm). (M) Quantification of (L) (n = 4, biological replicates, Cell number of NC for each biological replicate: 92, 99, 85, 127, Cell number of NC DFO for each biological replicate: 66, 49, 55, 62, Cell number of siATP for each biological replicate: 80, 102, 88, 68, Cell number of siATP DFO for each biological replicate: 131, 96, 89, 125, NC = 1.0, NC DFO = 0.865, siATP = 1.649 siATP DFO = 1.314). (N) Cell viability was measured with the CCK-8 assay α-Syn-SH cells treated DFO (50 µM, 24 h) after ATP13A2 KD (n = 4, biological replicates, NC = 100, NC DFO = 101.2, siATP = 67.28, siATP DFO = 76.92). Each value represents the mean ± SEM. Student’s t-test or analysis of variance (ANOVA), followed by the Bonferroni/Dunn post-hoc test was used to test the significance of differences (*p < 0.05, **p < 0.01, ***p < 0.001). siATP: siRNA targeting ATP13A2, NC: siRNA of negative control, IC: Immunocytochemistry, DFO: deferoxamine.
Next, we investigated the presence of increased oxidative stress in mitochondria due to labile Fe2+ accumulation using MitoSOX Red. We observed increased oxidative stress in α-Syn-SH cells with ATP13A2 KD; this increase was ameliorated by treatment with deferoxamine mesylate (DFO), a clinically used iron chelator (Fig. 2L and M). Moreover, cell viability decreased in α-Syn-SH cells with ATP13A2 KD, improving with DFO treatment (Fig. 2N). These results suggest that excessive iron accumulation, particularly labile Fe2+, within cellular organelles, including mitochondria and lysosomes, in α-Syn-SH cells with ATP13A2 KD results in cellular toxicity. Using a ferroptosis inhibitor to quantify cell viability, we observed that the toxicity of α-Syn-SH cells with ATP13A2 KD was improved (Figure S3). This suggests that the cellular damage observed in α-Syn-SH cells with ATP13A2 KD is partially related to ferroptosis.
Disruption of intracellular iron homeostasis in α-Syn-SH cells with ATP13A2 KD
To investigate the mechanism whereby ATP13A2 KD increases intracellular iron levels, we focused on the transferrin receptor (TfR) and divalent metal transporter 1 (DMT1), both involved in iron influx into cells, and ferroportin (FPN), involved in iron efflux from cells, respectively. Initially, we performed real-time PCR to examine the changes in mRNA level due to ATP13A2 KD. We observed that ATP13A2 KD significantly increased mRNA expression of TfR and DMT1, but did not affect FPN expression at the mRNA level (Figs. 3A–3C). TfR protein levels also increased, but those of DMT1 were not affected (Fig. 3D–3G). These findings indicate that intracellular iron homeostasis would be disrupted in α-Syn-SH cells with ATP13A2 KD, as iron influx is not inhibited despite elevated intracellular iron concentrations. In healthy cells, to prevent cellular damage caused by iron accumulation, intracellular iron levels are maintained through an IRP2-based iron-responsive feedback system regulating the expression of iron-related genes42. With increasing iron levels in cells, heme-dependent IRP2 degradation destabilizes mRNA of the proteins involved in iron influx, such as TfR and DMT1, resulting in reduced intracellular iron levels43. Thus, the IRP2 expression level in α-Syn-SH cells with ATP13A2 KD was examined using Western blotting. Although IRP2 must be decreased due to the increased intracellular iron levels in α-Syn-SH cells with ATP13A2 KD, the IRP2 protein level was not significantly changed upon ATP13A2 KD (Fig. 3D and H). This suggests that intracellular iron levels increased in α-Syn-SH cells with ATP13A2 KD, but IRP2 does not respond to the upregulation of labile iron, which failed to reduce the expression of genes involved in iron influx, such as DMT1 and TfR.
ATP13A2 KD disrupts intracellular iron homeostasis in α-Syn-SH cells. We confirmed expression level of iron related genes for analysis of the effect of ATP13A2 on iron homeostasis. (A–C) mRNA expression of TfR (A), DMT1 (B), FPN (C) analyzed by qRT-PCR in α-Syn-SH cells with ATP13A2 KD. Data were normalized to the GAPDH level (n = 4, biological replicates, TfR: NC = 1.0, siATP = 1.423, DMT1: NC = 1.0, siATP = 1.362, FPN: NC = 1.0, siATP = 0.835). (D) Protein expression of TfR, DMT1, FPN, and IRP2 detected by Western blot in α-Syn-SH cells with ATP13A2 KD. (E–H) Quantification of (D). Data were normalized to β-actin levels (n = 3, biological replicates, TfR: NC = 1.0, siATP = 1.648, DMT1: NC = 1.0, siATP = 0.971, FPN: NC = 1.0, siATP = 0.921, IRP2: NC = 1.0, siATP = 0.927). Each value represents the mean ± SEM. Student’s t-test was used to test the significance of differences (n.s. means not significant. *p < 0.05, **p < 0.01, ***p < 0.001). siATP: siRNA targeting ATP13A2, NC: siRNA of negative control, TfR: Transferrin receptor, DMT1: Divalent Metal Transporter 1, FPN: Ferroportin, IRP2: Iron Regulatory protein 2.
Protective effect of iron influx Inhibition for cytotoxicity of α-Syn-SH cells with ATP13A2 KD
To investigate whether the iron influx into cells through the TfR and DMT1 pathways is implicated in cellular injury, we examined whether inhibiting it mitigates cellular injury. First, we utilized apo Transferrin (apo-Tf), the iron-free variant of transferrin, to competitively inhibit the transferrin-mediated iron uptake via TfR44. Additionally, to inhibit iron influx via the DMT1 pathway, we used glibenclamide (GBD), which can decrease DMT1 activity45,46. Next, we determined the total intracellular iron level using atomic absorption spectrometry. The increased intracellular iron level caused by ATP13A2 KD was significantly reduced by treatment with each of apo-Tf and GBD (Fig. 4A and G). In addition, we measured intracellular Fe2+ levels using RhoNox-4 in α-Syn-SH cells with ATP13A2 KD. Both apo-Tf and GBD significantly decreased the increased intracellular Fe2+ levels due to ATP13A2 KD (Fig. 4B, C, H and I). In NC group, intracellular iron homeostasis may contribute to the consistent levels of iron even by treatment of apo-Tf and GBD. Then, using MitoSOX Red, we found improvements in the upregulated mitochondrial oxidative stress by ATP13A2 KD due to apo-Tf and GBD (Fig. 4D, E, J and K). Further, apo-Tf and GBD ameliorated cell viability in α-Syn-SH cells with ATP13A2 KD in a concentration-dependent manner (Fig. 4F and L).
Protective effect of ATP13A2 KD in α-Syn-SH cells by inhibiting iron influx. Apo Transferrin or Glibenclamide (GBD) were administered for 24 h after ATP13A2 KD in α-Syn-SH cells. (A) Atomic absorption spectrometry for evaluation of total intracellular iron level in α-Syn-SH cells treated with apo Transferrin for 24 h (0.10 mg/mL) after ATP13A2 KD. Quantification of iron content per 1.0 × 105 cells (n = 3, biological replicates, NC = 1.579, NC apo-Tf = 1.645, siATP = 2.671, siATP apo-Tf = 1.984). (B) Staining of RhoNox-4 for evaluation of intracellular Fe2+ level in α-Syn-SH cells treated with apo Transferrin for 24 h (0.10 mg/mL) after ATP13A2 KD (Red: Rho-Nox4, Blue: Hoechst) (Scale bar, 10 μm). (C) Quantification of (B) (n = 5, biological replicates, Cell number of NC for each biological replicate: 156, 89, 93, 94, 116, Cell number of NC apo-Tf for each biological replicate: 146, 101, 117, 91, 76, Cell number of siATP for each biological replicate: 90, 134, 94, 108, 126, Cell number of siATP apo-Tf for each biological replicate: 135, 103, 68, 120, 112, NC = 1.0, NC apo-Tf = 1.069, siATP = 1.510, siATP apo-Tf = 1.121). (D) MitoSOX dye for detection of mitochondrial ROS in α-Syn-SH cells treated with apo Transferrin for 24 h (0.10 mg/mL) after ATP13A2 KD (Red: MitoSOX, Blue: Hoechst) (Scale bar, 20 μm). (E) Quantification of (D) (n = 4, biological replicates, Cell number of NC for each biological replicate: 203, 180, 99, 163, Cell number of NC apo-Tf for each biological replicate: 236, 223, 208, 199, Cell number of siATP for each biological replicate: 187, 188, 175, 145, Cell number of siATP for each biological replicate: 187, 188, 175, 145, Cell number of siATP apo-Tf for each biological replicate: 162, 181, 173, 166, NC = 1.0, NC apo-Tf = 1.013, siATP = 2.729, siATP apo-Tf = 1.552). (F) Cell viability was measured by CCK-8 assay in α-Syn-SH cells treated with apo Transferrin for 24 h (0.01, 0.10, 0.50, or 1.00 mg/mL) after ATP13A2 KD (n = 4, biological replicates, NC = 100, siATP = 78.34, siATP apo-Tf 0.01 mg/mL = 91.84, siATP apo-Tf 0.10 mg/mL = 102.3, siATP apo-Tf 0.50 mg/mL = 105.0, siATP apo-Tf 1.0 mg/mL = 101.8). (G) Atomic absorption spectrometry in α-Syn-SH cells treated with Glibenclamide for 24 h (30.0 µM) after ATP13A2 KD. Quantification of iron content per 1.0 × 105 cells (n = 3, biological replicates, NC = 1.276, NC GBD = 1.130, siATP = 1.882, siATP GBD = 1.268). (H) Staining of RhoNox-4 in α-Syn-SH cells treated with Glibenclamide for 24 h (30.0 µM) after ATP13A2 KD (Red: Rho-Nox4, Blue: Hoechst) (Scale bar, 10 μm). (I) Quantification of (H) (n = 5, biological replicates, Cell number of NC for each biological replicate: 73, 52, 60, 77, 54, Cell number of NC GBD for each biological replicate: 62, 86, 99, 96, 94, Cell number of siATP for each biological replicate: 123, 65, 80, 115, 97, Cell number of siATP GBD for each biological replicate: 82, 64, 81, 127, 95, NC = 1.0, NC GBD = 1.073, siATP = 1.849, siATP GBD = 1.126). (J) MitoSOX dye in α-Syn-SH cells treated with Glibenclamide for 24 h (30.0 µM) after ATP13A2 KD (Red: MitoSOX, Blue: Hoechst) (Scale bar, 20 μm). (K) Quantification of (J) (n = 4, biological replicates, Cell number of NC for each biological replicate: 121, 119, 153,128, Cell number of NC GBD for each biological replicate: 115, 158, 125, 139, Cell number of siATP for each biological replicate: 106, 109, 83, 99, Cell number of siATP GBD for each biological replicate: 119, 122, 128, 97, NC = 1.0, NC GBD = 0.904, siATP = 2.325, siATP GBD = 1.839). (L) Cell viability was measured by CCK-8 assay in α-Syn-SH cells treated with Glibenclamide for 24 h (3.0, 10.0, or 30.0 µM) after ATP13A2 KD (n = 6, biological replicates, NC = 100, NC GBD 10 µM = 118.1, siATP = 73.66, siATP GBD 3 µM = 87.66, siATP GBD 10 µM = 94.94, siATP GBD 30 µM = 95.94). Each value represents the mean ± SEM. An ANOVA, followed by the Bonferroni/Dunn post-hoc test was used to test the significance of differences (n.s. not significant. *p < 0.05, **p < 0.01, ***p < 0.001). siATP: siRNA targeting ATP13A2, NC: siRNA of negative control.
Decreased heme-synthetic capacity due to mitochondrial defects in α-Syn-SH cells with ATP13A2 KD
Next, we assessed the mitophagy activity induced by starvation in α-Syn-SH cells with ATP13A2 KD. Starvation was induced by amino acid-free DMEM, pepstatin A, and E-64d. We observed that mitophagy was not induced to the same extent in α-Syn-SH cells with ATP13A2 KD as in control cells (Fig. 5A and B). We also analyzed mitochondrial function using an extracellular flux analyzer, examining parameters such as basal respiration, maximal respiration, ATP production, and spare respiratory capacity. All decreased compared to controls, suggesting the occurrence of mitochondrial dysfunction in α-Syn-SH cells with ATP13A2 KD (Fig. 5C and G). We next employed a labile heme-specific fluorescent probe, AcH-FluNox34, to assess subcellular heme synthesis of mitochondria in α-Syn-SH cells with ATP13A2 KD. 5-aminolevulinic acid (5-ALA), a substrate for heme biosynthesis, induced heme synthesis in control cells, but it was smaller upregulate in α-Syn-SH cells with ATP13A2 KD (Fig. 5H and I). We similarly performed intracellular Fe2+ levels and the capacity of heme synthesis in SH-SY5Y with knockdown of PINK1 (Figure S4A). It is known that PINK1 deficiency induced mitophagy dysfunction47. In the results, we confirmed the accumulation of Fe2+ using Rhonox-4 (Figure S4B, C). Additionally, we also confirmed the reduced heme synthesis capacity using AcH-FluNox (Figure S4D, E). IRP2 degradation is heme-dependent43. Therefore, we analyzed IRP2 expression in α-Syn-SH cells with ATP13A2 KD and control cells with or without ferric ammonium citrate (FAC). We used FAC for assessment of IRP2 reactivity to iron. FAC treatment decreased IRP2 levels in control cells but not in α-Syn-SH cells with ATP13A2 KD (Fig. 5J and K). These findings indicate that the weak response IRP2 to subcellular iron would be due to reduced heme synthesis capacity in mitochondria.
Heme-synthetic capacity decreased with mitochondrial disorders in α-Syn-SH cells with ATP13A2 KD. (A) Detection of mitophagy using Mitophagy detection kit in α-Syn-SH cells with ATP13A2 KD. (Red: Mitophagy Dye, Green: Lyso Dye, Blue: Hoechst) (scale bar, 20 μm). (B) Quantification of (A, Mitophagy Dye) (n = 6, biological replicates, Cell number of NC for each biological replicate: 138, 113, 111, 146, 147, 139, Cell number of NC Starvation for each biological replicate: 102, 108, 82, 91, 141, 132, Cell number of siATP for each biological replicate: 125, 123, 94, 103, 124, 88, Cell number of siATP Starvation for each biological replicate: 118, 151, 125, 102, 129, 134, NC = 1.0, NC Starvation = 2.855, siATP = 0.833, siATP Starvation = 1.759). (C) Analysis of mitochondrial respiration using Extracellular Flux Analyzer in α-Syn-SH cells with ATP13A2 KD. (D–G) Quantification of basal respiration (D, n = 3, biological replicates, NC = 71.56, siATP = 62.94), maximal respiration (E, n = 3, biological replicates, NC = 91.04, siATP = 77.97), ATP production (F, n = 3, biological replicates, NC = 53.46, siATP = 46.18), and spare respiratory capacity (G, n = 3, biological replicates, NC = 19.47, siATP = 15.03) in (C). (H) Staining of AcH-FluNox, which is a selective heme probe in α-Syn-SH cells treated with 5-ALA for 24 h (1.0 mM) after ATP13A2 KD for analysis heme synthesis capacity (Green: Ach-FluNox, Blue: Hoechst) (Scale bar, 20 μm). (I) Quantification of (H) (n = 5, biological replicates, Cell number of NC for each biological replicate: 70, 118, 87, 54, 84, Cell number of NC 5-ALA for each biological replicate: 60, 72, 62, 66, 97, Cell number of siATP for each biological replicate: 76, 89, 71, 63, 67, Cell number of siATP 5-ALA for each biological replicate: 81, 76, 58, 67, 61, NC = 1.0, NC 5-ALA = 2.957, siATP = 0.787, siATP 5-ALA = 1.868). (J) IRP2 protein expression as detected by Western blot in α-Syn-SH cells treated with FAC for 24 h (100 µM) after ATP13A2 KD. (K) Quantification of (J). Data were normalized to β-actin levels (n = 3, biological replicates, NC = 1.0, NC FAC = 0.444, siATP = 0.970, siATP FAC = 0.761). Each value represents the mean ± SEM, based on NC. A Student’s t-test or ANOVA, followed by the Bonferroni/Dunn post-hoc test was used to test the significance of differences (n.s. means not significant. *p < 0.05, **p < 0.01, ***p < 0.001). siATP: siRNA targeting ATP13A2, NC: siRNA of negative control, 5-ALA: 5-aminolevulinic acid.
No phenotypic differences induced by ATP13A2 deficiency between Naïve SH-SY5Y cells and α-Syn-SH-SY5Y cells
We also examined whether ATP13A2 knockdown induces phenotypic differences between naïve SH-SY5Y cells (hereafter named naïve-SH cells) and α-Syn-SH cells. To this end, a subset of experiments was conducted using both cell types. As shown in Supplementary Fig. 5, ATP13A2 knockdown resulted in no significant phenotypic differences between naïve-SH and α-Syn-SH cells. First, efficient ATP13A2 knockdown was confirmed in both cell types (Figure S5A and S5B). Both exhibited comparable reductions in cell viability (Figure S5C), as well as similar increases in mitochondrial ROS (Figure S5D and S5E) and labile Fe²⁺ levels (Figure S5F and S5G). Moreover, ATP13A2 knockdown impaired heme synthesis to a similar extent in both cell types (Figure S5H and S5I). These findings suggest that although α-Syn-SH cells stably express wild-type α-synuclein, the expression level is not sufficient to significantly alter cellular phenotypes compared to naïve-SH cells under our experimental conditions.
Discussion
This study aimed to characterize the mechanism of iron accumulation in PARK9 using α-Syn overexpressing human neuroblastoma SH-SY5Y cells, α-Syn-SH, with ATP13A2 KD. The increased total intracellular iron content detected in ATP13A2 KD cells, aligns with previous reports, confirming the suitability of this model for PARK9 cell studies19,48. In addition, ATP13A2 KD induced lysosomal and mitochondrial accumulation of labile Fe2+. Despite intracellular iron accumulation, IRP2 protein levels remained unchanged in ATP13A2 KD cells, resulting in dysregulation of these iron transporters. In ATP13A2 KD cells, we observed that mitophagy deficiency leads to mitochondrial dysfunction. Additionally, we observed impaired heme synthesis in ATP13A2 KD cells. We think reduced heme synthesis capacity leads to reduced responsiveness of IRP2 to iron. Furthermore, the impaired heme synthesis in mitochondria led to abnormal IRP2-mediated iron transporter expression, contributing to neurodegeneration in NBIA, characterized by abnormal intracellular iron accumulation. The present results indicate that defects in heme synthesis within malfunctioning mitochondria may underlie the abnormal iron accumulation observed in ATP13A2 KD cells.
In our study, fluorescent probes selectively targeting Fe2+ not only increased total iron levels but also increased those of Fe2+ in ATP13A2 KD cells. Furthermore, organelle-specific Fe2+ probes revealed Fe2+ accumulation in both mitochondria and lysosomes in ATP13A2 KD cells. Indeed, mitochondrial oxidative stress was increased in ATP13A2 KD cells, which was ameliorated by treatment with the iron chelator DFO. In addition, cell viability was decreased in ATP13A2 KD cells but recovered with DFO treatment. These results indicate that increased oxidative stress due to the accumulation of iron, especially Fe2+, is involved in the cytotoxic pathway of ATP13A2 KD cells. The mechanism of increased iron level in lysosomes and mitochondria remains unclear. However, as iron is essential for the function of lysosomes and mitochondria, these organelles may become iron-demanding due to dysfunction. Further studies are needed to elucidate the mechanism in detail.
The detailed mechanisms of the relationship between iron accumulation and the pathogenesis of neurodegenerative diseases, including PD, remain unclear. Iron accumulation and genetic polymorphisms of transferrin, involved in iron transport, have been described in brains of patients with PD49,50. Further, increased DMT1 expression has been reported in the substantia nigra of PD patients51. This study found that ATP13A2 KD increased TfR expression level, which is consistent with the lesion changes observed in PD patients. In addition, inhibiting iron influx through the DMT1 and TfR pathways decreased mitochondrial oxidative stress and ameliorated neurotoxicity in ATP13A2KD cells. These findings suggest that DMT1 and TfR are involved in neurotoxicity in ATP13A2 KD cells and may serve as potential therapeutic targets for PARK9 and other diseases characterized by iron accumulation such as NBIA. Therefore, we inferred that ferroptosis is occurring in this model. Actually, treatment with iron chelators and Ferrostatin-1 partially improved cell viability, suggesting that ferroptosis may be involved. However, the improvement in viability by Ferrostatin-1 was limited. We speculate that the extent of ferroptosis induction in this model may be insufficient to produce a detectable phenotype. We need to investigate this in more detail using ATP13A2 knockout mice. Despite intracellular iron accumulation in ATP13A2 KD cells, IRP2 protein levels were not affected in this study. However, mitochondrial function and mitophagy were impaired in ATP13A2 KD cells. Furthermore, imaging experiments using heme-specific fluorescent probes revealed decreased heme synthesis in ATP13A2 KD cells. These findings indicate compromised heme synthesis in ATP13A2 KD cells due to mitochondrial dysfunction, leading to insufficient IRP2 degradation. In the PINK1 deficiency model, which is known as impaired mitophagy model, heme synthesis was also decreased. These data support our hypothesis.
Consequently, this produced a sustained intracellular iron influx without suppression of TfR or DMT1 expression, ultimately contributing to increased intracellular iron content. We think the suppression of IRP2 degradation stabilizes TfR mRNA and enhances TfR expression. Iron accumulation may further induce mitochondrial damage, establishing a feedback loop involved in the pathophysiology of PARK9. We hypothesize that lysosomal dysfunction occurs first, as ATP13A2 is localized to lysosomes, and is subsequently followed by mitochondrial impairment. Previous studies have demonstrated that lysosomal and mitophagy dysfunctions are associated with mitochondrial damage14. Consistent with these findings, our data indicates that ATP13A2 deficiency induces both lysosomal and mitophagy dysfunction. Given that mitochondrial homeostasis is regulated by a balance between biogenesis and degradation, with mitophagy serving as a critical mechanism for mitochondrial quality control, disruption of this process may lead to mitochondrial dysfunction. Nevertheless, as the precise function of ATP13A2 has not yet been fully elucidated, we cannot exclude the possibility that ATP13A2 may also directly influence mitochondrial function.
Iron chelators, such as deferiprone have been used to try to decrease the increased brain iron levels in patients with PD52, but without much success. Hence, therapeutic strategies targeting the preservation of intracellular iron homeostasis are imperative. The decreased heme synthesis capacity in the mitochondria of PARK9 and impaired IRP2 degradation observed in this study, may contribute to the disruption of intracellular iron balance. Thus, we advocate for the development of drug targets increasing heme synthesis capacity in mitochondria.
We employed α-Syn-SH cells in this study due to our focus on iron metabolism in the context of PD pathology, because endogenous α-Syn was hardly detectable in naïve SH-SY5Y. However, the expression of α-Synuclein may influence our hypothesis. So, we conducted parts of the experiments using naïve-SH and α-Syn-SH cells (Figure S5). We didn’t find the differences of phenotype between these by knockdown of ATP13A2. Therefore, under the experimental conditions described above, we assume that ATP13A2 deficiency affects cellular phenotype independently of α-synuclein expression. However, ATP13A2 deficiency induced α-synuclein increase, it may enhance cytotoxicity. In the future, oligomeric or fibrillar α-synuclein accumulation should also be assessed. Another limitation is using human neuroblastoma SH-SY5Y cells as model in an artificial ATP13A2 deficient state. However, SH-SY5Y cells have been previously used as a model to investigate the molecular basis of PD-related genes, including ATP13A237. It will be necessary to validate the results of the present study using disease-specific iPS cells and appropriate animal models.
In conclusion, decreased ATP13A2 expression caused lysosomal dysfunction and autophagy failure in our cell model. Additionally, reduced lysosome function induces mitochondrial dysfunction, attributed to the inadequate mitophagy function due to autophagy failure. Moreover, decreased mitochondrial respiration and ATP synthesis capacity, coupled with decreased heme synthesis capacity. The diminished heme synthesis capacity, in turn, contributes to defective IRP2 degradation, enabling a persistent iron influx into the cell. Subsequently, these increased iron levels fail to inhibit further iron influx, increasing intracellular iron content (Fig. 6). This excess iron induces oxidative stress, potentially amplifying mitochondrial damage and perpetuating a detrimental cycle. The insights derived from this study contribute to understanding the pathogenesis of PD and other brain disorders characterized by iron deposition, facilitating the identification of new drug targets.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PD:
-
Parkinson’s disease
- α-Syn:
-
α-synuclein
- ATP13A2:
-
ATPase cation transporting 13A2
- NBIA:
-
neurodegeneration with brain iron accumulation
- IRP2:
-
Iron regulatory protein 2
- SDS:
-
sodium dodecyl sulfate
- PBS:
-
phosphate-buffered saline
- KD:
-
knockdown
- α-Syn-SH cells:
-
human wild-type α-Syn in SH-SY5Y cells
- DFO:
-
deferoxamine mesylate
- TfR:
-
transferrin receptor
- DMT1:
-
divalent metal transporter 1
- FPN:
-
ferroportin
- apo-Tf apo:
-
Transferrin
- GBD:
-
glibenclamide
- 5-ALA:
-
5-aminolevulinic acid
- FAC:
-
ferric ammonium citrate
- naïve-SH:
-
cells naïve SH-SY5Y cells
- siATP:
-
siRNA targeting ATP13A2
- NC:
-
siRNA of negative control
References
Braak, H. et al. Staging of brain pathology related to sporadic parkinson’s disease. Neurobiol. Aging. 24, 197–211. https://doi.org/10.1016/s0197-4580(02)00065-9 (2003).
Charvin, D., Medori, R., Hauser, R. A. & Rascol, O. Therapeutic strategies for Parkinson disease: beyond dopaminergic drugs. Nat. Rev. Drug Discov. 17, 804–822. https://doi.org/10.1038/nrd.2018.136 (2018).
Ding, X. S. et al. Ferroptosis in parkinson’s disease: molecular mechanisms and therapeutic potential. Ageing Res. Rev. 91, 102077. https://doi.org/10.1016/j.arr.2023.102077 (2023).
Wise, R. M. et al. Interactions of dopamine, iron, and alpha-synuclein linked to dopaminergic neuron vulnerability in parkinson’s disease and neurodegeneration with brain iron accumulation disorders. Neurobiol. Dis. 175, 105920. https://doi.org/10.1016/j.nbd.2022.105920 (2022).
Zeng, X. S., Geng, W. S., Jia, J. J., Chen, L. & Zhang, P. P. Cellular and molecular basis of neurodegeneration in Parkinson disease. Front. Aging Neurosci. 10, 109. https://doi.org/10.3389/fnagi.2018.00109 (2018).
Perinan, M. T. et al. Effect modification between genes and environment and parkinson’s disease risk. Ann. Neurol. 92, 715–724. https://doi.org/10.1002/ana.26467 (2022).
Bandres-Ciga, S., Diez-Fairen, M., Kim, J. J. & Singleton, A. B. Genetics of parkinson’s disease: an introspection of its journey towards precision medicine. Neurobiol. Dis. 137, 104782. https://doi.org/10.1016/j.nbd.2020.104782 (2020).
Gialluisi, A. et al. Identification of sixteen novel candidate genes for late onset Parkinson’s disease. Mol. Neurodegener. 16 https://doi.org/10.1186/s13024-021-00455-2 (2021).
Matsui, H. et al. Cytosolic DsDNA of mitochondrial origin induces cytotoxicity and neurodegeneration in cellular and zebrafish models of parkinson’s disease. Nat. Commun. 12, 3101. https://doi.org/10.1038/s41467-021-23452-x (2021).
Meng, Y. et al. Putaminal Recombinant glucocerebrosidase delivery with magnetic Resonance-Guided focused ultrasound in parkinson’s disease: A phase I study. Mov. Disord. 37, 2134–2139. https://doi.org/10.1002/mds.29190 (2022).
Henrich, M. T., Oertel, W. H., Surmeier, D. J. & Geibl, F. F. Mitochondrial dysfunction in parkinson’s disease - a key disease hallmark with therapeutic potential. Mol. Neurodegener. 18, 83. https://doi.org/10.1186/s13024-023-00676-7 (2023).
Clark, E. H., de la Torre, V., Hoshikawa, A., Briston, T. & T. & Targeting mitophagy in parkinson’s disease. J. Biol. Chem. 296, 100209. https://doi.org/10.1074/jbc.REV120.014294 (2021).
Lin, M. K. & Farrer, M. J. Genetics and genomics of parkinson’s disease. Genome Med. 6, 48. https://doi.org/10.1186/gm566 (2014).
Li, H. et al. Mitochondrial dysfunction and mitophagy defect triggered by heterozygous GBA mutations. Autophagy 15, 113–130. https://doi.org/10.1080/15548627.2018.1509818 (2019).
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X (2021).
Tian, Y. et al. Iron metabolism in aging and Age-Related diseases. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23073612 (2022).
Ramirez, A. et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 38, 1184–1191. https://doi.org/10.1038/ng1884 (2006).
Schneider, S. A. et al. ATP13A2 mutations (PARK9) cause neurodegeneration with brain iron accumulation. Mov. Disord. 25, 979–984. https://doi.org/10.1002/mds.22947 (2010).
Dehay, B. et al. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc. Natl. Acad. Sci. U S A. 109, 9611–9616. https://doi.org/10.1073/pnas.1112368109 (2012).
van Veen, S. et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature 578, 419–424. https://doi.org/10.1038/s41586-020-1968-7 (2020).
Fujii, T. et al. Parkinson’s disease-associated ATP13A2/PARK9 functions as a lysosomal H(+),K(+)-ATPase. Nat. Commun. 14, 2174. https://doi.org/10.1038/s41467-023-37815-z (2023).
Rinaldi, D. E., Corradi, G. R., Cuesta, L. M. & Adamo, H. P. Tezanos Pinto, F. The Parkinson-associated human P5B-ATPase ATP13A2 protects against the iron-induced cytotoxicity. Biochim. Biophys. Acta. 1848, 1646–1655. https://doi.org/10.1016/j.bbamem.2015.04.008 (2015). de.
Ryan, B. J., Hoek, S., Fon, E. A. & Wade-Martins, R. Mitochondrial dysfunction and mitophagy in parkinson’s: from Familial to sporadic disease. Trends Biochem. Sci. 40, 200–210. https://doi.org/10.1016/j.tibs.2015.02.003 (2015).
Hsieh, C. H. et al. Functional impairment in Miro degradation and mitophagy is a shared feature in Familial and sporadic parkinson’s disease. Cell. Stem Cell. 19, 709–724. https://doi.org/10.1016/j.stem.2016.08.002 (2016).
Costas, M., Mehn, M. P., Jensen, M. P. & Que, L. Jr. Dioxygen activation at mononuclear nonheme iron active sites: enzymes, models, and intermediates. Chem. Rev. 104, 939–986. https://doi.org/10.1021/cr020628n (2004).
Enami, S., Sakamoto, Y. & Colussi, A. J. Fenton chemistry at aqueous interfaces. Proc. Natl. Acad. Sci. U S A. 111, 623–628. https://doi.org/10.1073/pnas.1314885111 (2014).
Lee, J. & Hyun, D. H. The interplay between intracellular iron homeostasis and neuroinflammation in neurodegenerative diseases. Antioxid. (Basel). 12 https://doi.org/10.3390/antiox12040918 (2023).
Zecca, L., Youdim, M. B., Riederer, P., Connor, J. R. & Crichton, R. R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 5, 863–873. https://doi.org/10.1038/nrn1537 (2004).
Ishikawa, H. et al. Involvement of Heme regulatory motif in Heme-mediated ubiquitination and degradation of IRP2. Mol. Cell. 19, 171–181. https://doi.org/10.1016/j.molcel.2005.05.027 (2005).
Hirayama, T., Niwa, M., Hirosawa, S. & Nagasawa, H. High-Throughput screening for the discovery of iron homeostasis modulators using an extremely sensitive fluorescent probe. ACS Sens. 5, 2950–2958. https://doi.org/10.1021/acssensors.0c01445 (2020).
Hirayama, T., Kadota, S., Niwa, M. & Nagasawa, H. A mitochondria-targeted fluorescent probe for selective detection of mitochondrial labile Fe(ii). Metallomics 10, 794–801. https://doi.org/10.1039/c8mt00049b (2018).
Niwa, M., Hirayama, T., Okuda, K. & Nagasawa, H. A new class of high-contrast Fe(II) selective fluorescent probes based on spirocyclized scaffolds for visualization of intracellular labile iron delivered by transferrin. Org. Biomol. Chem. 12, 6590–6597. https://doi.org/10.1039/c4ob00935e (2014).
Hirayama, T. et al. A Golgi-targeting fluorescent probe for labile Fe(ii) to reveal an abnormal cellular iron distribution induced by dysfunction of VPS35. Chem. Sci. 10, 1514–1521. https://doi.org/10.1039/c8sc04386h (2019).
Kawai, K. et al. Molecular imaging of labile Heme in living cells using a small molecule fluorescent probe. J. Am. Chem. Soc. 144, 3793–3803. https://doi.org/10.1021/jacs.1c08485 (2022).
Xie, Y. X. et al. Lysosomal exocytosis releases pathogenic alpha-synuclein species from neurons in synucleinopathy models. Nat. Commun. 13, 4918. https://doi.org/10.1038/s41467-022-32625-1 (2022).
Rivero-Rios, P., Madero-Perez, J., Fernandez, B. & Hilfiker, S. Targeting the Autophagy/Lysosomal degradation pathway in parkinson’s disease. Curr. Neuropharmacol. 14, 238–249. https://doi.org/10.2174/1570159x13666151030103027 (2016).
Krishna, A. et al. Systems genomics evaluation of the SH-SY5Y neuroblastoma cell line as a model for parkinson’s disease. BMC Genom. 15, 1154. https://doi.org/10.1186/1471-2164-15-1154 (2014).
Usenovic, M., Tresse, E., Mazzulli, J. R., Taylor, J. P. & Krainc, D. Deficiency of ATP13A2 leads to lysosomal dysfunction, alpha-synuclein accumulation, and neurotoxicity. J. Neurosci. 32, 4240–4246. https://doi.org/10.1523/JNEUROSCI.5575-11.2012 (2012).
Erb, M. L., Sipple, K., Levine, N., Chen, X. & Moore, D. J. Adult-onset deletion of ATP13A2 in mice induces progressive nigrostriatal pathway dopaminergic degeneration and lysosomal abnormalities. NPJ Parkinsons Dis. 10, 133. https://doi.org/10.1038/s41531-024-00748-5 (2024).
Wang, R. et al. ATP13A2 facilitates HDAC6 recruitment to lysosome to promote autophagosome-lysosome fusion. J. Cell. Biol. 218, 267–284. https://doi.org/10.1083/jcb.201804165 (2019).
Kolnagou, A., Kontoghiorghe, C. N. & Kontoghiorghes, G. J. New targeted therapies and diagnostic methods for iron overload diseases. Front. Biosci. (Schol Ed). 10, 1–20. https://doi.org/10.2741/s498 (2018).
Terzi, E. M., Sviderskiy, V. O., Alvarez, S. W., Whiten, G. C. & Possemato, R. Iron-sulfur cluster deficiency can be sensed by IRP2 and regulates iron homeostasis and sensitivity to ferroptosis independent of IRP1 and FBXL5. Sci. Adv. 7 https://doi.org/10.1126/sciadv.abg4302 (2021).
Muckenthaler, M. U., Galy, B. & Hentze, M. W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 28, 197–213. https://doi.org/10.1146/annurev.nutr.28.061807.155521 (2008).
Klausner, R. D., Ashwell, G., van Renswoude, J., Harford, J. B. & Bridges, K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc. Natl. Acad. Sci. U S A. 80, 2263–2266. https://doi.org/10.1073/pnas.80.8.2263 (1983).
Du, X. et al. Activation of ATP-sensitive potassium channels enhances DMT1-mediated iron uptake in SK-N-SH cells in vitro. Sci. Rep. 6, 33674. https://doi.org/10.1038/srep33674 (2016).
Zhang, Q. et al. Deletion of Kir6.2/SUR1 potassium channels rescues diminishing of DA neurons via decreasing iron accumulation in PD. Mol. Cell. Neurosci. 92, 164–176. https://doi.org/10.1016/j.mcn.2018.08.006 (2018).
Kim, J. et al. miR-27a and miR-27b regulate autophagic clearance of damaged mitochondria by targeting PTEN-induced putative kinase 1 (PINK1). Mol. Neurodegener. 11, 55. https://doi.org/10.1186/s13024-016-0121-4 (2016).
Rajagopalan, S., Rane, A., Chinta, S. J. & Andersen, J. K. Regulation of ATP13A2 via PHD2-HIF1alpha signaling is critical for cellular iron homeostasis: implications for parkinson’s disease. J. Neurosci. 36, 1086–1095. https://doi.org/10.1523/JNEUROSCI.3117-15.2016 (2016).
Mochizuki, H. & Yasuda, T. Iron accumulation in parkinson’s disease. J. Neural Transm (Vienna). 119, 1511–1514. https://doi.org/10.1007/s00702-012-0905-9 (2012).
Borie, C. et al. Association study between iron-related genes polymorphisms and parkinson’s disease. J. Neurol. 249, 801–804. https://doi.org/10.1007/s00415-002-0704-6 (2002).
Salazar, J. et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of parkinson’s disease. Proc. Natl. Acad. Sci. U S A. 105, 18578–18583. https://doi.org/10.1073/pnas.0804373105 (2008).
Devos, D. et al. Trial of deferiprone in parkinson’s disease. N Engl. J. Med. 387, 2045–2055. https://doi.org/10.1056/NEJMoa2209254 (2022).
Acknowledgements
We thank the members of Inden’s lab for helpful discussions. This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant No. 22K06744 to MI), a Grant-in-Aid for Scientific Research on Innovative Areas JSPS KAKENHI (JP19H05767A02 to IH) a grant from the Smoking Research Foundation (Grant No. 2021G021) to MI, JST SPRING (Grant No. JPMJSP2142 to TM), and a grant from the Takeda Science Foundation to MI.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant No. 22K06744 to MI), a Grant-in-Aid for Scientific Research on Innovative Areas JSPS KAKENHI (JP19H05767A02 to IH), a grant from the Smoking Research Foundation (Grant No. 2021G021) to MI, JST SPRING (Grant No. JPMJSP2142 to TM) and a grant from the Takeda Science Foundation to MI.
Author information
Authors and Affiliations
Contributions
TM, KO and MI. designed the experiments. TM, KO, MK, HK, KK, RK, and ZW performed the experiments. TM, KO, HK, TH, ZW and MI analyzed the data. TM, KO and MI wrote and edited the manuscript. HN, YM and IH supervised the project. IH and MI acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Murakami, T., Ohuchi, K., Kiuchi, M. et al. Disruption of intracellular iron homeostasis through mitochondrial dysfunction associated with suppression of ATP 13A2 expression. Sci Rep 16, 5007 (2026). https://doi.org/10.1038/s41598-026-35368-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-026-35368-x