Abstract
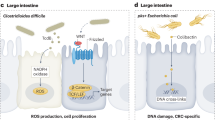
c-di-AMP is a bacterial second messenger recognized by host immune sensors such as the STING pathway, linking gut microbiota activity to tumor immunity. This interaction holds significant therapeutic potential particularly for oncologic patients, given the increasingly recognized relationship between gut microbiota and tumor immunity. Recent evidence shows that microbial c-di-AMP can enhance anti-tumor responses and improve the efficacy of PD-1/PD-L1 blockade and radiotherapy. This study identified gut microbial species capable of synthesizing c-di-AMP by mining the Unified Human Gastrointestinal Protein catalogue for diadenylate cyclases (DACs), generating a database of 4,228 DACs across 3,901 species out of 4,744 presents in the Unified Human Gastrointestinal Genome catalogue. Analysis of metagenomic data from 190 healthy subjects and 569 cancer patients (melanoma, NSCLC, renal carcinoma) revealed a significantly higher abundance of DAC-encoding species in healthy microbiota, with no differences between responders and non-responders to immunotherapy. These findings indicate that c-di-AMP-producing bacteria are depleted in cancer-associated microbiota, supporting further studies on their role in modulating anti-tumor immunity.
Similar content being viewed by others
Data availability
UHGP fasta file, UHGG Kraken2 database and UHGG reference genomes are available at UHGG repository (https://ftp.ebi.ac.uk/pub/databases/metagenomics/mgnify_genomes/human-gut/v2.0.1/). Accession numbers of metagenomes utilized in this work are reported in Suppl. Table 1.
References
Witte, G., Hartung, S., Buttner, K. & Hopfner, K. P. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol. Cell. 30, 167–178. https://doi.org/10.1016/j.molcel.2008.02.020 (2008).
Corrigan, R. M. & Gründling, A. Cyclic di-AMP: another second messenger enters the fray. Nat. Rev. Microbiol. 11, 513–524. https://doi.org/10.1038/nrmicro3069 (2013).
Römling, U. Great times for small molecules: c-di-AMP, a second messenger candidate in bacteria and archaea. Sci. Signal. 1, pe39. https://doi.org/10.1126/scisignal.133pe39 (2008).
Braun, F. et al. Cyclic nucleotides in archaea: Cyclic di-AMP in the archaeon haloferax volcanii and its putative role. Microbiologyopen 8(9), e00829. https://doi.org/10.1002/mbo3.829 (2019).
He, J., Yin, W., Galperin, M. Y. & Chou, S. H. Cyclic di-AMP, a second messenger of primary importance: tertiary structures and binding mechanisms. Nucleic Acids Res. 48, 2807–2829. https://doi.org/10.1093/nar/gkaa112 (2020).
Yin, W. et al. A decade of research on the second messenger c-di-AMP. FEMS Microbiol. Rev. 44, 701–724. https://doi.org/10.1093/femsre/fuaa019 (2020).
Stülke, J. & Krüger, L. Cyclic di-AMP signaling in bacteria. Annu. Rev. Microbiol. 74, 159–179. https://doi.org/10.1146/annurev-micro-020518-115943 (2020).
Oppenheimer-Shaanan, Y., Wexselblatt, E., Katzhendler, J., Yavin, E. & Ben-Yehuda, S. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Rep. 12, 594–601. https://doi.org/10.1038/embor.2011.77 (2011).
Zhang, L. & He, Z. G. Radiation-sensitive gene A (RadA) targets DisA, DNA integrity scanning protein A, to negatively affect cyclic di-AMP synthesis activity in Mycobacterium smegmatis. J. Biol. Chem. 288, 22426–22436. https://doi.org/10.1074/jbc.M113.464883 (2013).
Sureka, K. et al. The cyclic dinucleotide c-di-AMP is an allosteric regulator of metabolic enzyme function. Cell 158, 1389–1401. https://doi.org/10.1016/j.cell.2014.07.046 (2014).
Whiteley, A. T., Garelis, N. E., Peterson, B. N., Choi, P. H. & Tong, L. c-di-AMP modulates Listeria monocytogenes central metabolism to regulate growth, antibiotic resistance and osmoregulation. Mol. Microbiol. 104, 212–233. https://doi.org/10.1111/mmi.13622 (2017).
Tang, Q. et al. Functional analysis of a c-di-AMP-specific phosphodiesterase MsPDE from Mycobacterium smegmatis. Int. J. Biol. Sci. 11, 813–824. https://doi.org/10.7150/ijbs.11797 (2015).
Krüger, L. et al. Sustained control of pyruvate carboxylase by the essential second messenger cyclic di-AMP in Bacillus subtilis. mBio. 13, e0360221. https://doi.org/10.1128/mbio.03602-21 (2022).
Mehne, F. M. et al. Control of the diadenylate cyclase CdaS in Bacillus subtilis: an autoinhibitory domain limits cyclic di-AMP production. J. Biol. Chem. 289, 21098–21107. https://doi.org/10.1074/jbc.M114.562066 (2014).
Zheng, C. et al. Functional analysis of the sporulation-specific diadenylate cyclase CdaS in Bacillus thuringiensis. Front. Microbiol. 6, 908. https://doi.org/10.3389/fmicb.2015.00908 (2015).
Zarrella, T. M., Yang, J., Metzger, D. W. & Bai, G. The bacterial second messenger cyclic di-AMP modulates the competence state in Streptococcus pneumoniae. J. Bacteriol. 202, e00691–e00619. https://doi.org/10.1128/JB.00691-19 (2020).
Bowman, L., Zeden, M. S., Schuster, C. F., Kaever, V. & Gründling, A. New insights into the cyclic di-adenosine monophosphate (c-di-AMP) degradation pathway and the requirement of the cyclic dinucleotide for acid stress resistance in Staphylococcus aureus. J. Biol. Chem. 291, 26970–26986 (2016).
Rubin, B. E. et al. High-throughput interaction screens illuminate the role of c-di-AMP in cyanobacterial nighttime survival. PLoS Genet. 14, e1007301. https://doi.org/10.1371/journal.pgen.1007301 (2018).
Gundlach, J., Rath, H., Herzberg, C., Mäder, U. & Stülke, J. Second messenger signaling in Bacillus subtilis: accumulation of cyclic di-AMP inhibits biofilm formation. Front. Microbiol. 7, 804. https://doi.org/10.3389/fmicb.2016.00804 (2016).
Peng, X., Zhang, Y., Bai, G., Zhou, X. & Wu, H. Cyclic di-AMP mediates biofilm formation. Mol. Microbiol. 99, 945–959. https://doi.org/10.1111/mmi.13277 (2016).
Latoscha, A. et al. c-di-AMP hydrolysis by the phosphodiesterase AtaC promotes differentiation of multicellular bacteria. Proc. Natl. Acad. Sci. U S A. 117, 7392–7400. https://doi.org/10.1073/pnas.1917080117 (2020).
Bai, Y. et al. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence. J. Bacteriol. 195, 5123–5132. https://doi.org/10.1128/JB.00769-13 (2013).
Witte, C. E. et al. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. mBio 4, e00282–e00213. https://doi.org/10.1128/mBio.00282-13 (2013).
Yang, J. et al. Deletion of the cyclic di-AMP phosphodiesterase gene (cnpB) in Mycobacterium tuberculosis leads to reduced virulence in a mouse model of infection. Mol. Microbiol. 93, 65–79. https://doi.org/10.1111/mmi.12641 (2014).
Ye, M. et al. DhhP, a cyclic di-AMP phosphodiesterase of Borrelia burgdorferi, is essential for cell growth and virulence. Infect. Immun. 82, 1840–1849. https://doi.org/10.1128/IAI.00030-14 (2014).
Moradali, M. F. et al. Atypical cyclic di-AMP signaling is essential for Porphyromonas gingivalis growth and regulation of cell envelope homeostasis and virulence. NPJ Biofilms Microbiomes. 8, 53. https://doi.org/10.1038/s41522-022-00316-w (2022).
Lu, Y. et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J. Hematol. Oncol. 15(1), 47. https://doi.org/10.1186/s13045-022-01273-9 (2022).
Wang, M., Zhang, L., Chang, W. & Zhang, Y. The crosstalk between the gut microbiota and tumor immunity: implications for cancer progression and treatment outcomes. Front. Immunol. 13, 1096551. https://doi.org/10.3389/fimmu.2022.1096551 (2023).
Gazzaniga, F. S. & Kasper, D. L. The gut microbiome and cancer response to immune checkpoint inhibitors. J. Clin. Invest. 135(3), e184321. https://doi.org/10.1172/JCI184321 (2025).
Lam, K. C. et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 184(21), 5338–5356e21. https://doi.org/10.1016/j.cell.2021.09.019 (2021).
Li, Z. et al. Gut microbiota modulate radiotherapy-associated antitumor immune responses against hepatocellular carcinoma via STING signaling. Gut Microbes. 14(1), 2119055. https://doi.org/10.1080/19490976.2022.2119055 (2022).
Commichau, F. M., Heidemann, J. L., Ficner, R. & Stülke, J. Making and breaking of an essential poison: the cyclases and phosphodiesterases that produce and degrade the essential second messenger cyclic di-AMP in bacteria. J. Bacteriol. 201(1), e00462–e00418. https://doi.org/10.1128/JB.00462-18 (2018).
Galperin, M. Y. All DACs in a row: domain architectures of bacterial and archaeal diadenylate cyclases. J. Bacteriol. 205(4), e0002323. https://doi.org/10.1128/jb.00023-23 (2023).
Almeida, A. et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat. Biotechnol. 39(1), 105–114. https://doi.org/10.1038/s41587-020-0603-3 (2021).
Blum, M. et al. The interpro protein families and domains database: 20 years on. Nucleic Acids Res. 49(D1), D344–D354. https://doi.org/10.1093/nar/gkaa977 (2021).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30(9), 1236–1240. https://doi.org/10.1093/bioinformatics/btu031 (2014).
Steinegger, M. & Söding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35(11), 1026–1028. https://doi.org/10.1038/nbt.3988 (2017).
Andrews, S. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30(15), 2114–2120. https://doi.org/10.1093/bioinformatics/btu170 (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with bowtie 2. Nat. Methods. 9(4), 357–359. https://doi.org/10.1038/nmeth.1923 (2012).
Wood, D. E., Lu, J. & Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 20(1), 257. https://doi.org/10.1186/s13059-019-1891-0 (2019).
Lu, J., Breitwieser, F. P., Thielen, P. & Salzberg, S. L. Bracken: estimating species abundance in metagenomics data. PeerJ Comput. Sci. 3, e104. https://doi.org/10.7717/peerj-cs.104 (2017).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37(8), 852–857. https://doi.org/10.1038/s41587-019-0209-9 (2019).
Dabdoub, S. M. kraken-biom: Enabling interoperative format conversion for Kraken results (Version 1.2) [Software]. https://github.com/smdabdoub/kraken-biom (2016).
Ligges, U. & Mächler, M. Scatterplot3d - an R package for visualizing multivariate data. J. Stat. Softw. 8(11), 1–20. https://doi.org/10.18637/jss.v008.i11 (2003).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12(6), R60. https://doi.org/10.1186/gb-2011-12-6-r60 (2011).
Buchfink, B., Reuter, K. & Drost, H. G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods. 18, 366–368. https://doi.org/10.1038/s41592-021-01101-x (2021).
Jiang, Y., Li, X., Qian, F., Sun, B., Wang, X. & et Fine-tuning bacterial cyclic di-AMP production for durable antitumor effects through the activation of the STING pathway. Research 6, Article 0102. https://doi.org/10.34133/research.0102 (2023).
Huang, Y., Piao, L. & Liu, X. Enhancing tumor-specific immunity with SLdacA: A attenuated Salmonella-mediated c-di-AMP delivery system targeting the STING pathway. Int. J. Pharm. 666, 124759. https://doi.org/10.1016/j.ijpharm.2024.124759 (2024).
Wang, B. et al. Clinical applications of STING agonists in cancer immunotherapy: current progress and future prospects. Front. Immunol. 15, 1485546. https://doi.org/10.3389/fimmu.2024.1485546 (2024).
Cheng, X., Ning, J., Xu, X. & Zhou, X. The role of bacterial cyclic di-adenosine monophosphate in the host immune response. Front. Microbiol. 13, 958133. https://doi.org/10.3389/fmicb.2022.958133 (2022).
Zarrella, T. M. & Bai, G. The bacterial second messenger cyclic di-AMP and inflammation. In: (ed Wang, Y. X.) Inflammation. Springer, Cham. https://doi.org/10.1007/978-3-031-55254-0_1-1 (2025).
Woodward, J. J., Iavarone, A. T. & Portnoy, D. A. C-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328, 1703–1705. https://doi.org/10.1126/science.1189801 (2010).
Huynh, T. N. & Woodward, J. J. Too much of a good thing: regulated depletion of c-di-AMP in the bacterial cytoplasm. Curr. Opin. Microbiol. 30, 22–29. https://doi.org/10.1016/j.mib.2015.12.007 (2016).
Quillin, S. J., Schwartz, K. T. & Leber, J. H. The novel Listeria monocytogenes bile sensor BrtA controls expression of the cholic acid efflux pump MdrT. Mol Microbiol. 81(1), 129–142. https://doi.org/10.1111/j.1365-2958.2011.07683.x (2011).
Gries, C. M. et al. Cyclic di-AMP released from Staphylococcus aureus biofilm induces a macrophage type I interferon response. Infect. Immun. 84(12), 3564–3574. https://doi.org/10.1128/IAI.00447-16 (2016).
Funding
The research activities have been funded by PIANO NAZIONALE DI RIPRESA E RESILIENZA(PNRR) - MISSIONE 4 COMPONENTE 2, “Dalla ricerca all’impresa” INVESTIMENTO 1.3, Creazione di “Partenariati estesi alle università, ai centri di ricerca, alle aziende per il finanziamento di progetti di ricerca di base”, finanziato dall’Unione europea - NextGenerationEU” - Progetto identificato con codice PE00000019, Titolo “HEAL ITALIA” - Spoke 5 - CUP E93C22001860006 Avviso MUR DD. 341 del 15.03.2022.
Author information
Authors and Affiliations
Contributions
Conceptualization: Rossi M, Amaretti A, Raimondi SInvestigation: Candeliere F, Sola LFormal analysis: Candeliere F, Busi ESoftware: Candeliere FVisualization: Candeliere F, Amaretti A, Pedroni SWriting - original draft: Candeliere F, Rossi M, Pedroni S, Sola L, Amaretti A, Raimondi S, Greco S, Dominici MSupervision: Rossi MAll authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Candeliere, F., Sola, L., Busi, E. et al. Altered abundance in cancer patients gut of diadenylate cyclase-encoding bacteria. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35425-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35425-5