Abstract
Steroid-induced avascular necrosis of the femoral head (SANFH) significantly impairs patients’ quality of life, with long-term steroid use being a major contributing factor. The pathogenesis of SANFH remains complex and poorly understood. Bone marrow mesenchymal stem cells (BMSCs) possess multidirectional differentiation potential and are crucial for bone repair and regeneration, yet their precise mechanisms in SANFH treatment are not fully elucidated. In this study, 48 rabbits with early-stage SANFH were randomly assigned to four groups: control (Group A), core decompression (Group B), BMSCs embedded in fibrin sealant (FS) after decompression (Group C), and pravastatin administration combined with BMSCs in FS post-decompression (Group D). Blood lipid levels were monitored throughout the experiment, and femoral heads were assessed via MRI, HE staining, and VEGF immunohistochemical staining. Group C demonstrated reduced femoral head necrosis and increased new bone formation compared to the control. Group D showed significantly lower serum lipid content and femoral head necrosis than other groups, with HE staining revealing fewer empty lacunae and increased trabecular bone density. Additionally, VEGF expression was markedly elevated in Group D. These findings indicate that BMSCs embedded in biomaterials enhance the therapeutic efficacy for early SANFH, and the combination with pravastatin significantly improves outcomes, suggesting a promising new approach for clinical management of femoral head necrosis.
Similar content being viewed by others
Introduction
Osteonecrosis of the femoral head remains a challenging and unresolved disease in orthopedics due to its multifactorial etiology and complex pathogenesis1. Among the various causes, steroid-induced avascular necrosis of the femoral head (SANFH) has emerged as the leading cause of non-traumatic femoral head necrosis, primarily driven by the widespread clinical use of steroid medications2. The prolonged use of steroids disrupts lipid metabolism, resulting in fat embolism, increased intraosseous pressure, and subsequent degeneration and necrosis of bone cells within the femoral head3,4. In the absence of effective early interventions, patients with SANFH often face progressive joint deterioration, ultimately requiring total hip arthroplasty in their middle to late years5. This growing clinical burden underscores the urgent need for innovative therapeutic approaches to prevent and manage SANFH.
The development of SANFH involves multiple factors, with the dysregulation of lipid metabolism being a significant contributor.Lipid-lowering drugs are effective in correcting lipid metabolism disorders induced by hormones in animals, thereby mitigating damage to bone cells within the femoral head6. The primary focus of these drugs is on statins, which demonstrate enhanced efficacy and fewer side effects. These medications reduce the synthesis and secretion of triglyceride-rich lipoproteins, leading to a significant decrease in plasma LDL and triglyceride levels in patients with hyperlipidemia. Pravastatin, notable for its hydrophilicity, high protein binding rate, and short plasma half-life, offers several clinical advantages7. Consequently, this experiment selected pravastatin as the pharmacological intervention for early-stage steroid-induced osteonecrosis of the femoral head.
Research has demonstrated that one of the pathogenic mechanisms underlying femoral head necrosis may be associated with a decline in the function and quantity of bone marrow stem cells[8]. Bone marrow stromal stem cells (BMSCs) are a type of multipotent stem cell characterized by their robust proliferative capacity, residing in the non-hematopoietic tissues of the bone marrow. These cells exhibit multidirectional differentiation potential, enabling them to differentiate into osteoblasts, chondrocytes, and vascular endothelial cells, among others9. BMSCs have the ability to restore blood flow within the affected femoral head, enhance circulation in the surrounding areas of necrosis, and promote tissue repair, thus positioning them as ideal seed cells for therapeutic applications. the capability for osteogenesis in bone marrow stromal stem cells after in vivo implantation tends to be limited, mainly due to the lack of a supportive cell carrier10. This deficiency results in the diminished retention of bone marrow stromal stem cells and obstructs the development of an optimal cell concentration at the site of implantation. Fibrin Sealant(FS), a macromolecular biological material, is known for its durability against environmental factors and finds extensive application across various surgical disciplines11. Furthermore, it promotes the proliferation of capillary endothelial cells and fibroblasts, aiding in the formation of granulation tissue, with the biological protein network functioning as a scaffold that enhances wound healing12. This material exhibits remarkable biocompatibility, adaptability, and a swift degradation process. It creates an insoluble fibrin polymer by entwining polymers into a three-dimensional network structure in a brief period13. Significantly, it can change from a liquid to a solid form under specific conditions, which allows it to be combined with seed cells while in its liquid state. This mechanism improves in vivo tissue engineering, reduces the loss of seed cells, and is particularly advantageous for minimally invasive techniques, reflecting the crucial features of an injectable tissue cell transplantation scaffold.
Based on the above evidence and our previous research14, we speculate that pravastatin combined with fibrin sealant encapsulation of BMSCs has a good effect on the recovery of steroid-induced femoral head necrosis. Our research provides a theoretical basis for the development of new treatment strategies for SANFH.
Materials and methods
Animals Preparing
Total 48 New Zealand white rabbits (weight 2.8 oz, 0.5 kg, male or female) were successfully modeled, provided by the Experimental Animal Center of Kunming Medical University, Experimental Animal Production license No. 20090458, Experimental animal use license No. : 2009365895) were randomly divided into four groups: A (control group), B (core decompression group), C (core decompression + stem cell group) and D (core decompression + stem cell + pravastatin group), with 12 rabbits in each group. Pravastatin sodium tablets from Chung-Mei Shanghai SmithKline Beecham Pharmaceuticals company (No H20103215). Group A was fed conventionally without any treatment. In group B, only a bone puncture needle was used to decompress the core of the necrotic area of the femoral head under C-arm fluoroscopy. After decompression, BMSCs loaded FS (Shanghai Lys Co., Ltd.) was injected into the decompression hole in group C. Group D was given pravastatin daily based on group C, and the dose of pravastatin (Chung-Mei Shanghai SmithKline Beecham Pharmaceuticals Co., Ltd., batch number: H20103215) given to each white rabbit was 1.2 mg/kg body weight according to the dosage relationship of human surface area. All laboratory animals are treated and cared for in strict accordance with the Laboratory Animal Management Regulations and approved by the Laboratory Animal Ethics Committee.All methods comply with the requirements of relevant guidelines and regulations, and also meet the requirements of ARRIVE.
Core decompression and stem cell transplantation
The third-generation stem cells (Bone marrow samples were collected from the metaphyseal region of the femur of healthy adult New Zealand white rabbits, and centrifugation was performed. The cells were then placed in DMEM medium containing 10% fetal bovine serum for cultivation. The medium was completely replaced every three days to remove the non-adherent cells. When the adherent BMSCs reached a cell density of 80%−90%, trypsin was used to digest and pass the culture to the third generation.)were injected into fibrinogen solution at a cell density of 1 × 105/ml, mixed with the accompanying thrombin solution during transplantation, and quickly transplanted into the decompression core. Rabbits in the other three groups, except group A, had hair clipped on the lateral sides of both hips, were routinely disinfected and covered with tissue after successful anesthesia. Rabbits were underwent incision on the lateral side of the greater trochanter, and were fully exposed. Used X-ray positioning under an C-arm, drilled above the greater trochanter to the femoral head and the contents of the pulp core for decompression. Groups C and D were simultaneously injected with the BMSCs complex. The procedure emphasized an aseptic procedure and penicillin injection for 3 consecutive days after surgery.
Lipid detection
Four New Zealand white rabbits were randomly selected from each group before treatment and at the 4th, 8th, and 12th week after treatment. After disinfection, fasting venous blood of the rabbit ear margin was extracted by scalp needle. Total cholesterol and triacylglycerol were detected by Olympus automatic biochemical analyzer, and lipid changes of each group before and after treatment were observed.
Magnetic resonance
Imaging (MRI) (All scans were performed using a Siemens Magnetom Vision 1.5T superconducting MRI system. This ensures a stable and high-strength magnetic field as the foundation for image acquisition.Its radiofrequency coil is suitable for rabbit hip imaging.High-resolution sequences were utilized to observe the image more clearly)detection at 4, 8, and 12 weeks after treatment, 3 rabbits were randomly selected from each group to be anesthetized with 3% pentobarbital sodium (1 ml/kg) in the auricular vein and then placed in a supine position. Their lower limbs were flexed and fixed with tape for MRI scanning, and imaging changes of the bilateral femoral head were observed.
General observation
After each specimen of the femoral head is removed, the gross observation of the femoral head is carried out first, mainly to observe the appearance of the femoral head, the color and thickness of the articular surface of the cartilage, the change of the contents of the bone marrow and the degree of osteoporosis.
HE staining
The specimen of the femoral head was fixed in a 10% formaldehyde solution for 24 h, dehydrated by ethanol gradient, and embedded in paraffin, and a 5 μm section was prepared. Dewaxing was performed using xylene and ethanol gradients, followed by hematoxylin and eosin (HE) staining.HE staining was performed to observe the thickness of the articular surface cartilage of the femoral head, cartilage lacunae, subchondral region, and intramedullary bone trabecula, and other structures.
VEGF immunohistochemistry
Femoral head specimens were routinely fixed, degreased and decalcified, embedded, and sliced, then dewaxed to water and antigenic repair, stained according to the VEGF immunohistochemistry kit (R&D Systems, USA) operating instructions, and examined by sealing plate microscopy. The tan reaction product represented the location of the antigen, and the positive coloring depth and quantity determined the expression intensity of VEGF.
Statistics and analysis
SPSS17.0 statistical software was used to analyze and process the data. The data of each group is homogeneous number the standard deviation. A one-way analysis of variance was used to compare multiple groups, and an LSD test was used to do two comparisons. The test level α = 0.05 was used. A P value < 0.05 was considered statistically significant.
Results
General situation of model
No postoperative infection or death was observed in all 48 white rabbits. After core decompression surgery, C-arm fluoroscopy could accurately reach the necrotic area of the femoral head, and BMSCs combined with bioprotein glue successfully reached the femoral head area (Fig. 1).
Lipid changes across groups
Results of fasting serum cholesterol and triglyceride before treatment and at 4, 8 and 12 weeks after treatment in the four groups of white rabbits are shown in Tables 1 and 2. Before treatment, the serum total cholesterol and triglyceride contents in the four groups were not significantly different. With the extension of treatment time, the serum total cholesterol and triglyceride contents in the three groups A, B and C gradually increased, while the two indexes in the serum of group D gradually decreased. This result indicates that pravastatin can effectively lower blood lipids and improve lipid disorders.
Nuclear magnetic resonance findings across groups
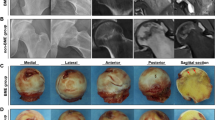
At 4, 8 and 12 weeks after treatment, an MRI examination of the femoral head of the white rabbit was performed on both sides. It was found that the signals of the femoral head in group A gradually decreased and became chaotic with the extension of treatment time, and the femoral head collapsed at 12 weeks. The signal in the femoral head of group B was more potent than before but weaker than that of group C. In group D, irregular signal bands in the femoral head decreased with the extension of treatment time, and no signs of femoral head collapse were found at 12 weeks(Fig. 2).This result indicates that Group D is effective in improving blood circulation and promoting healing of osteonecrosis of the femoral head.
The irregular low signal shadows in group A were observed in the femoral head at 4 weeks. At 8 weeks, the necrosis signals in the femoral head were aggravated, and the articular surface of cartilage signals were uneven. The image of femoral head collapse was evident at 12 weeks. In groups B and C, at 4 weeks, the low signal in the femoral head was still visible in the shape of map. At 8 and 12 weeks, the low signal imaging decreased, but there were still signs of femoral head necrosis. In group D, the low signal shadow was still visible in the femoral head at 4 weeks. At 8 weeks, the low signal shadow decreased significantly. At 12 weeks, the images of femoral head necrosis were not obvious.(red arrow: head of femur).
General observation of femoral head
At 4 weeks after treatment, the surface of the femoral head cartilage in group A was dark and gray. Group B and C were slightly grayish white. In group D, the surface of femoral head cartilage was darkened slightly. 8 weeks after treatment, the grayish-yellow steatosis of the subchondral area and necrotic bone tissue was enlarged in section of group A, and part of the necrotic bone tissue was muddy. The necrotic area in the bone marrow cavity increased in groups B and C. New bone tissue was found in the femoral head of group D. At 12 weeks after treatment, the surface cartilage of the femoral head in group A collapsed. The necrotic area in the bone marrow cavity of groups B and C was reduced, and a small amount of new bone was formed between normal bone tissue and necrotic bone tissue, which was increased in group C compared with group B. The area of necrosis in the bone marrow cavity of the femoral head in group D was reduced compared with 8 weeks, and no obvious abnormality was found in the morphology of the femoral head.This result indicates that group D is significantly more effective than the other groups in the repair of femoral head necrosis.
Histological changes in femoral head across groups
In group A, with the extension of time, the fat cells in the medullary cavity of the femoral head increased, the trabecular thinning and empty bone lacunae increased, and the damage degree of trabecular thinning was aggravated, and the cartilage surface necrosis collapsed at 12 weeks. In group B and C, 4 weeks after treatment, fat cells in the medullary cavity of the femoral head were proliferated with some fibrocytes, and trabeculae were still sparse and thin. With the extension of treatment time, the fat cells in the medullary cavity were slightly reduced, and a small amount of new bone formation was observed, and the empty bone lacunae were slightly reduced, and the amount of new bone formation in group C was more than that in group B. At 4 weeks after treatment, the number of adipocytes and empty bone lacunae in the medullary cavity in group D was significantly reduced compared with that in groups B and C, and the degree of bone trabecular sparsity was less than that in groups B and C. The formation of new bone tissue on the trabecular surface of bone was observed at 8 weeks after treatment, and it basically returned to normal at 12 weeks (Fig. 3).This result indicates that group D can significantly reduce the formation of fat cells compared to other groups, and the number of bone trabeculae becomes greater and denser.
HE staining smear results of the femoral head in each group at different time points, group A had the largest number of fat cells and the thinnest, bone trabeculae under the femoral head. Bone trabeculae in group B and group C gradually thickened. Group D is the thickest. At 8 weeks, the bone trabeculae in group A were the thinnest and the fat cells were the most filled. The trabeculae in group D were the thickest. At 12 weeks, group A had necrosis of articular surface, sparse subchondral bone, and many empty bone lacunae. Group B and C were relatively improved, but the articular surface of group C was thinner and broken than that of group D. The articular cartilage surface of group D was normal and thick, and the empty bone lacunae were the least.(: Bone trabeculae; ༚The articular cartilage).
Immunohistochemical results of VEGF in different groups
The positive evaluation criteria of vascular endothelial growth factor (VEGF) were the depth and quantity of Browning granules in the cytoplasm. In group A, there was no expression of VEGF in the femoral head 4 weeks after treatment. From 8 weeks to 12 weeks, VEGF expression was positive and gradually weakened. In group B, there was a weak positive expression in the femoral head at 4 weeks after treatment. It was positive at 8 weeks and then decreased. In group C, the expression of VEGF in the femoral head was weakly positive at 4 weeks after staining, and there were positive expression cells in the femoral head at 8 weeks, which gradually decreased. The expression of VEGF in the femoral head of group D was positive at 4 weeks after treatment, and the expression of VEGF-positive cells was the highest at 8 weeks and decreased at 12 weeks (Fig. 4).This result indicates that Group D is better than the other, significant increase in the expression of Vascular Endothelial Growth Factor (VEGF). This increase is associated with enhanced blood supply and promotes the healing process.
Immunohistochemical results of VEGF in the femoral head of each group at different time points, after 4 weeks of treatment in the four groups, VEGF positive staining in group D was the deepest and the largest, and there was basically no positive expression in group A. At 8 weeks, positive staining was observed in all four groups, group A was the least and shallowest, group B was the second, and group D was the most obvious and most obvious. At 12 weeks, there was a decrease in staining in the four groups compared to 8 weeks, but group D was still deeper than the other groups, with the most obvious.
Discussion
The present study demonstrates that a combination therapeutic approach —core decompression, delivered of BMSCs via a fibrin sealant (FS) scaffold, and systemic pravastatin administration (Group D) —achieves superior outcomes in a rabbit model of early-stage SANFH. This multimodal strategy significantly mitigated femoral head necrosis, stimulated new bone formation, and upregulated local VEGF expression compared to alternative interventions. Our findings highlight the synergistic potential of addressing both systemic metabolic dysregulation and local cellular repair mechanisms in SANFH, presenting a promising therapeutic paradigm consistent with the clinical trend of integrating cellular and pharmacological interventions13.
The pathogenesis of SANFH is multifactorial, encompassing intraosseous hypertension, compromised osteogenic differentiation of BMSCs, and disrupted vascular supply14,15.Currently, it is widely acknowledged that the functional impairment and quantitative decline of osteoblasts and BMSCs are pivotal in the progression of this disease16, thereby providing a compelling rationale for stem cell transplantation as a therapeutic approach. Core decompression stands as a foundational surgical intervention, designed to alleviate intraosseous pressure and disrupt the ischemic cycle that contributes to the disease17. Nevertheless, its effectiveness is frequently constrained by inadequate bone regeneration when employed alone. Our findings corroborate that combining core decompression with BMSCs embedded in FS (Group C) yields superior outcomes compared to decompression performed in isolation (Group B). This observation aligns with the results of a 10-year follow-up clinical study by Li et al., which furnished high-level evidence indicating that autologous bone marrow stem cell grafting, when combined with core decompression, results in significantly better long-term outcomes than decompression alone18. The utilization of BMSCs is justified by their well-documented pluripotency. Under appropriate condition, BMSCs have the capacity to differentiate into both osteoblasts and vascular endothelial cells, a process that is intricately regulated by complex molecular mechanisms19,20. For instance, Wang et al. demonstrated that antler polypeptide can promote the proliferation and osteogenic differentiation of BMSCs21, underscoring the critical importance of augmenting BMSC function for effective bone repair. Moreover, the FS scaffold likely serves as a three-dimensional matrix that enhances the retention and survival of BMSCs, thereby creating a conducive microenvironment for new bone formation.This notion is supported by studies on functionalized biomaterials for bone regeneration22.
The most notable improvement was evident in Group D. Where the superior efficacy of the treatment regimen can be ascribed to its dual-action mechanism. Firstly, pravastatin effectively rectified steroid-induced hyperlipidemia, thereby alleviating tow primary contributors to SANFH: fat embolism and intraosseous hypertension14,23. This finding corroborates the widely recognized theory that disorders in lipid metabolism play a pivotal role in the pathogenesis of steroid-induced osteonecrosis23. Notably, the search of pharmacological agents capable of correcting these metabolic disturbances remains an area of active research. For instance, recombinant human erythropoietin has been explored for its potential protective effects against steroid-induced osteonecrosis in rat models, with studies indicating that it may help mitigate the pathological process24,25. Our results with pravastatin add to this expanding body of evidence, showcasing a significant lipid-lowering effect that targets a fundamental aspect of SANFH pathogenesis.
Secondly, our study presents compelling evidence for a direct local synergistic effect between pravastatin and BMSCs. The notably expression of VEGF observed in the femoral heads of Group D indicates a robust promotion of angiogenesis. This is of critical important, gived that a decline in VEGF levels is a well-recognized risk factor for SANFH26. The interplay between hypoxia, its pivotal mediator HIF-1α, and osteocyte apoptosis has been thoroughlyinvestigated in the context of SANFH27. Our findings, which reveal that the combined therapy significantly upregulates VEGF expression, suggest that it may effectively modulate this hypoxic-apoptotic axis, thereby fostering a more conducive environment for tissue repair. The formation of new blood vessels is vital for supporting bone healing processes28,29. The role of growth factors in bone regeneration is well-established in the literature. As prior research has demonstrated, growth factors such EGF can significantly enhance the bone healing process in ONFH15. The combination of pravastatin and BMSCs elicted a more pronounced angiogenic response response compared to either agent alone. This synergy can be attributed to the known properties of both agents: BMSCs are known to secrete angiogenic factors17, while statins, in addition to lipid-lowering effects, possess functions such as protecting the vascular endothelium and promoting angiogenesis30. For instance, simvastatin has been shown to stimulate VEGF release31. Our findings with pravastatin, particularly its synergistic effect with BMSCs, underscore a promising therapeutic approach that combines systemic metabolic correction with enhanced local cellular repair and angiogenesis.
Our study is subject to several limitations. Firstly the sample size was comparatively small, and the follow-up period was restrited to 12 weeks.This short duration makes it impossible to evalute long-term outcomes, such as femoral head collapse. It is imperative to reconize that in advanced stages of SANFH, when femoral head collapse has already taken place, interventions like core decompression or biological therapies frequently prove to be inadequate. In such cases, arthroplasty emerges as the main treatment option. Research has demonstrated that artificial femoral head replacement can serve as an effective intervention for avascular necrosis at this terminal stage32. Moreover, the exact molecular mechanisms underlying the interaction between pravastatin and BMSCs remain unclear.For instance, it is still unknown whether this interaction involves pathways like Hippo signaling pathway, which has been linked to glucocorticoid-induced osteonecrosis16, These mechanisms warrant further exploration through in vitro studies.
In conclusion, our findings suggest that the combination of pravastatin and BMSCs-FS offers a highly promising synergistic approach for the early treatment of SANFH. This strategy effectively addresses systemic lipid disorder while simultaneously fostering local bone regeneration and angiogenesis. By intervening at an early stage of the disease, this combined therapy seeks to preserve the natural joint and may delay or even prevent progression to end-stage disease, which would otherwise necessitate joint replacement32. The positive results observed in this animal model lay a preclinical groundword for considering of such combined strategies in clinical practice, potentially enhancing the effectiveness of current surgical techniques13. Future research should be directed towards elucidating the detailed mechanistic interactions, including specific signaling pathways, and validating these findings in larger animal models over extended observation periods.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- BMSCs:
-
Bone marrow mesenchymal stem cells
- SANFH:
-
Steroid-induced avascular necrosis of the femoral head
- VEGF:
-
vascular endothelial growth factor
- FS:
-
Fibrin sealant
References
Jiao, M. et al. Circular RNA and messenger RNA expression profile and competing endogenous RNA network in subchondral bone in osteonecrosis of the femoral head. DNA Cell Biol. 40 (1), 61–69 (2021).
Wang, X., Li, J., Man, D., Liu, R. & Zhao, J. Early detection of steroid-induced femoral head necrosis using 99mTc-Cys-Annexin V-based apoptosis imaging in a rabbit model. Mol. Med. 26 (1), 120 (2020).
Jiang, Y. et al. Tetramethylpyrazine enhances vascularization and prevents osteonecrosis in steroid-treated rats. Biomed Res Int 2015:315850. (2015).
Aimaiti, A. et al. Can bisphenol A diglycidyl ether (BADGE) administration prevent steroid-induced femoral head osteonecrosis in the early stage? Med. Hypotheses. 77 (2), 282–285 (2011).
He, P. et al. Effectiveness and safety of traditional Chinese medicine in the treatment of steroid-osteonecrosis of femoral head: A protocol for systematic review and meta-analysis. Med. (Baltim). 100 (30), e26811 (2021).
Bian, Y. et al. Pathogenesis of glucocorticoid-induced avascular necrosis: A microarray analysis of gene expression in vitro. Int. J. Mol. Med. 36 (3), 678–684 (2015).
Ebisawa, S. et al. Impact of combination therapy with Statin and Ezetimibe on secondary prevention for post-acute myocardial infarction patients in the Statin era. Int. J. Cardiol. Heart Vasc. 8, 154–160 (2015).
H W, S. Z. & Z, Q. M. W. Y. L. Recent advances in osteonecrosis of the femoral head: a focus on mesenchymal stem cells and adipocytes. J. Translational Med. 23 (1), 592 (2025).
Wang, T. et al. Role of mesenchymal stem cells on differentiation in steroid-induced avascular necrosis of the femoral head. Exp. Ther. Med. 13 (2), 669–675 (2017).
Wang, W. et al. Chitosan derivatives and their application in biomedicine. Int J. Mol. Sci. 21 (2), 487 (2020).
Huo, S-C. & Yue, B. Approaches to promoting bone marrow mesenchymal stem cell osteogenesis on orthopedic implant surface. World J. Stem Cells. 12 (7), 545–561 (2020).
Albala, D. M. & Lawson, J. H. Recent clinical and investigational applications of fibrin sealant in selected surgical specialties. J. Am. Coll. Surg. 202 (4), 685–697 (2006).
Li S, Wang J, Ma R, Zhao C, Gao Z, Quan X, Zhang Q: Analysis of the efficacy of drilling decompression autologous bone marrow and allogeneic bone grafting in the treatment of HIV-positive patients with early osteonecrosis of the femoral head. BMC Musculoskelet. Disord. 24(1), 902 (2023).
Xi H, Tao W, Jian Z, Sun X, Gong X, Huang L, Dong T: Levodopa attenuates cellular apoptosis in steroid-associated necrosis of the femoral head. Exp. Ther. Med. 13(1), 69–74 (2017).
Wang T, Teng S, Zhang Y, Wang F, Ding H, Guo L: Role of mesenchymal stem cells on differentiation in steroid-induced avascular necrosis of the femoral head. Exp. Ther. Med. 13(2), 669–675.(2017).
Li Y, Xu Z, Chang S: Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway. Open Life Sci 16(1), 1130–1140 (2021).
Wang Z, Sun Q-M, Zhang F-Q, Zhang Q-L, Wang L-G, Wang W-J: Core decompression combined with autologous bone marrow stem cells versus core decompression alone for patients with osteonecrosis of the femoral head: A meta-analysis. Int. J. Surg. 69, 23–31 (2019).
Li M, Ma Y, Fu G, Zhang R, Li Q, Deng Z, Zheng M, Zheng Q: 10-year follow-up results of the prospective, double-blinded, randomized, controlled study on autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of the femoral head. Stem. Cell. Res. Ther. 11(1), 287 (2020).
Wang J, Wang T, Zhang F, Zhang Y, Guo Y, Jiang X, Yang B: Roles of circular RNAs in osteogenic differentiation of bone marrow mesenchymal stem cells (Review). Mol. Med. Rep. 26(1), (2022).
Zeng J, Deng J, He C, Xiong Q-A, Li X, Wang Z: IGF-1 Induces Osteogenic Differentiation of Rat Bone Marrow Mesenchymal Stem Cells by Promoting SOX4 via the MAPK/ERK Pathway. Int. J. Stem. Cells. 17(4), 418–426 (2024).
Wang P, Sun T-F, Li G, Zhang H-M, Liu F-J, Gao Z-H, Cao S-N, Sun G-D, Du H-T, Wang C-A et al: The Separation of Antler Polypeptide and Its Effects on the Proliferation and Osteogenetic Differentiation of Bone Marrow Mesenchymal Stem Cells. Evid. Based. Complement. Alternat. Med. 2020, 1294151 (2020).
Zhang H, Fu X, Zhang S, Li Q, Chen Y, Liu H, Zhou W, Wei S: Strategy of Stem Cell Transplantation for Bone Regeneration with Functionalized Biomaterials and Vascularized Tissues in Immunocompetent Mice. ACS Biomater Sci Eng 8(4), 1656–1666 (2022).
Ren X, Shao Z, Fan W, Wang Z, Chen K, Yu X: Untargeted metabolomics reveals the effect of lovastatin on steroid-induced necrosis of the femoral head in rabbits. J Orthop Surg Res 15(1), 497 (2020).
RenLim YW, Kim YS, Lee JW, Kwon SY: Stem cell implantation for osteonecrosis of the femoral head. Exp. Mol. Med. 45(11), e61 (2013).
Jiang L-Y, Yu X, Pang Q-J: Research in the precaution of recombinant human erythropoietin to steroid-induced osteonecrosis of the rat femoral head. J. Int. Med. Res. 45(4), 1324–1331 (2017).
Özmen E, İzol Özmen H, Atasoy S, Dursun M, Bilgiç B, Salduz A: The effects of prophylactic tadalafil use on VEGF expression in the rabbit model of steroid-induced femoral head avascular necrosis. Acta. Orthop. Traumatol. Turc. 57(5), 237–242 (2023).
Ding H, Wang Y, Lu Y: [Treatment of osteonecrosis of the femoral head with vascularized bone grafting]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 35(3), 381–386 (2021).
Apte RS, Chen DS, Ferrara N: VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 176(6), 1248–1264 (2019).
Tian ZJ, Liu BY, Zhang YT, Chen XZ, Qiao GY, Wang S, Ma ZL: MiR-145 silencing promotes steroid-induced avascular necrosis of the femoral head repair via upregulating VEGF. Eur. Rev. Med. Pharmacol. Sci. 21(17), 3763–3769 (2017).
Mazidi M, Rokni H, Sahebkar AH, Mohammadi A, Ghayour-Mobarhan M, Ferns GA: Simvastatin Treatment Does Not Affect Serum Vitamin D Concentrations in Patients with Dyslipidemia: A Randomized Double-blind Placebo-controlled Cross-over Trial. Int. J. Prev. Med. 7, 80. (2016).
Takenaka M, Hirade K, Tanabe K, Akamatsu S, Dohi S, Matsuno H, Kozawa O: Simvastatin stimulates VEGF release via p44/p42 MAP kinase in vascular smooth muscle cells. Biochem Biophys Res Commun 301(1), 198–203 (2003).
Wang S-F, Ji Q-H, Qiao X-F, Zhao P, Xue Y, Li Y-B: Efficacy of artificial femoral head replacement for femoral head avascular necrosis. Med. 98(17), e15411 (2019).
Author information
Authors and Affiliations
Contributions
Qing Yang: Methodology, Data curation, Validation. Yi Yang: Methodology. Gang Li: Methodology, supervision. Biao Li: Conceptualization and review. All authors agree to be accountable for all aspects of this work, ensuring its integrity and accuracy.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Q., Yang, Y., Li, B. et al. Pravastatin combined with fibrin sealant-embedded BMSCs enhances recovery in steroid-induced avascular necrosis of the femoral head. Sci Rep 16, 5074 (2026). https://doi.org/10.1038/s41598-026-35663-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-026-35663-7