Abstract
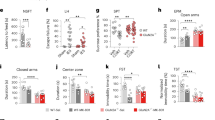
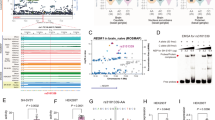
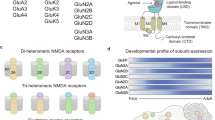
The NEGR1 gene has been implicated in several psychiatric disorders, and increased NMDA receptor binding density has been demonstrated in vitro in hippocampal slices from Negr1-deficient mice. In this study, we expanded on these findings by investigating the behavioural response to NMDA receptor antagonism, expression of NMDA receptor subunits, and kynurenine pathway metabolites in a Negr1-deficient mouse model. Male and female wild-type and Negr1-deficient mice received daily injections of MK-801, a non-competitive NMDA receptor antagonist, until behavioural tolerance developed in the open field test (after 9 days in males and 5 days in females). In drug-naive animals, acute MK-801 administration (0.2 mg/kg) elicited a stronger motor response in Negr1-deficient males compared to wild-type controls. However, with repeated dosing, Negr1-deficient males exhibited a blunted behavioural response and attenuated progression of rapid behavioural tolerance during every-second-day MK-801 administration, suggesting altered receptor sensitivity. Gene expression analysis revealed sex- and brain region-specific changes in NMDA receptor subunit expression. Additionally, kynurenine pathway metabolites showed genotype- and sex-dependent alterations. These findings suggest that NEGR1 protein modulates NMDA receptor function and tryptophan metabolism in a sex-dependent manner, highlighting the importance of considering both genetic background and sex in models of glutamatergic dysfunction relevant to neuropsychiatric disorders.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available upon reasonable request to the corresponding author.
References
Rakofsky, J. & Rapaport, M. Mood disorders. Continuum (Minneap Minn). 24, 804–827 (2018).
World Health Organization. Mental disorders [Fact sheet]. (2022). https://www.who.int/news-room/fact-sheets/detail/mental-disorders
Carboni, L. et al. Depression–associated gene Negr1–Fgfr2 pathway is altered by antidepressant treatment. Cells 9, 1818. https://doi.org/10.3390/cells9081818 (2020).
Singh, K. et al. Neuronal growth and behavioral alterations in mice deficient for the psychiatric disease–associated Negr1 gene. Front. Mol. Neurosci. 11, 30. https://doi.org/10.3389/fnmol.2018.00030 (2018).
Singh, K. et al. Neural cell adhesion molecule Negr1 deficiency in mouse results in structural brain endophenotypes and behavioral deviations related to psychiatric disorders. Sci. Rep. 9, 5457. https://doi.org/10.1038/s41598-019-41849-7 (2019).
Vanaveski, T. et al. Promoter–specific expression and genomic structure of iglon family genes in mouse. Front. Neurosci. 11, 38. https://doi.org/10.3389/fnins.2017.00038 (2017).
Howard, D. M. et al. Genome–wide meta–analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352 (2019).
Kaare, M. et al. Depression–associated Negr1 gene–deficiency induces alterations in the monoaminergic neurotransmission enhancing time–dependent sensitization to amphetamine in male mice. Brain Sci. 12, 1696. https://doi.org/10.3390/brainsci12121696 (2022).
Karis, K. et al. Altered expression profile of iglon family of neural cell adhesion molecules in the dorsolateral prefrontal cortex of schizophrenic patients. Front. Mol. Neurosci. 11, 8. https://doi.org/10.3389/fnmol.2018.00008 (2018).
Levey, D. F. et al. Bi–ancestral depression GWAS in the million veteran program and meta–analysis in > 1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 24, 954–963 (2021).
Noh, K. et al. Negr1 controls adult hippocampal neurogenesis and affective behaviors. Mol. Psychiatry. 24, 1189–1205 (2019).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
Su, F. et al. Neuronal growth regulator 1 (NEGR1) promotes the synaptic targeting of glutamic acid decarboxylase 65 (GAD65). J Neurochem. 169(1):e16279. (2025). https://doi.org/10.1111/jnc.16279. PMID: 39676071.
Goldschmidt Merrion, H. et al. Dynamic extracellular interactions with AMPA receptors. BioRxiv 2025 Jul 11:2025.07.11.664166. https://doi.org/10.1101/2025.07.11.664166
Baez, M. V., Cercato, M. C. & Jerusalinsky, D. A. NMDA receptor subunits change after synaptic plasticity induction and learning and memory acquisition. Neural Plast. 5093048; (2018). https://doi.org/10.1155/2018/5093048 (2018).
Hunt, D. L. & Castillo, P. E. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr. Opin. Neurobiol. 22, 496–508 (2012).
Kemp, J. A. & McKernan, R. M. NMDA receptor pathways as drug targets. Nat. Neurosci. 5, 1039–1042 (2002).
Yamamoto, H. et al. Specific roles of NMDA receptor subunits in mental disorders. Curr. Mol. Med. 15, 193–205 (2015).
Myint, A. M. & Halaris, A. Imbalances in kynurenines as potential biomarkers in the diagnosis and treatment of psychiatric disorders. Front. Psychiatry. 13, 913303. https://doi.org/10.3389/fpsyt.2022.913303 (2022).
Yovanno, R. A. et al. Excitatory and inhibitory D–serine binding to the NMDA receptor. eLife 11, e77645; (2022). https://doi.org/10.7554/eLife.77645
Janus, A. et al. MK–801 and cognitive functions: investigating the behavioral effects of a non–competitive NMDA receptor antagonist. Psychopharmacology 240, 2435–2457 (2023).
Latysheva, N. V. & Rayevsky, K. S. Chronic neonatal N–methyl–D–aspartate receptor Blockade induces learning deficits and transient hypoactivity in young rats. Prog Neuropsychopharmacol. Biol. Psychiatry. 27, 787–794 (2003).
Wong, E. H. et al. The anticonvulsant MK–801 is a potent N–methyl–D–aspartate antagonist. Proc. Natl Acad. Sci. USA 83, 7104–7108 (1986).
Coyle, J. T. & Balu, D. T. The role of Serine racemase in the pathophysiology of brain disorders. Adv. Pharmacol. 82, 35–56 (2018).
Labrie, V. et al. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum. Mol. Genet. 18, 3227–3243 (2009).
Wolosker, H. et al. Serine racemase: a glial enzyme synthesizing D–serine to regulate glutamate–N–methyl–D–aspartate neurotransmission. Proc. Natl. Acad. Sci. USA. 96, 13409–13414 (1999).
Birch, P. J. et al. Kynurenic acid antagonises responses to NMDA via an action at the strychnine–insensitive Glycine receptor. Eur. J. Pharmacol. 154, 85–87 (1988).
Danysz, W. & Parsons, C. G. Glycine and N–methyl–D–aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol. Rev. 50, 597–664 (1998).
de Carvalho, L. P. et al. The endogenous agonist quinolinic acid and the non–endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochem Int. 28, 445–452 (1996).
Lugo–Huitrón, R. et al. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013 (104024). https://doi.org/10.1155/2013/104024 (2013).
Savitz, J. The kynurenine pathway: a finger in every pie. Mol. Psychiatry. 25, 131–147 (2020).
Dauvermann, M. R. et al. Glutamatergic regulation of cognition and functional brain connectivity: insights from pharmacological, genetic and translational schizophrenia research. Br. J. Pharmacol. 174, 3136–3160 (2017).
Kuuskmäe, C. et al. Kynurenine pathway dynamics in patients with schizophrenia spectrum disorders across the disease trajectory. Psychiatry Res. 328, 115423. https://doi.org/10.1016/j.psychres.2023.115423 (2023).
Goto, Y. et al. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol. Psychiatry. 67, 199–207 (2010).
Morris, R. G. et al. Hippocampal synaptic plasticity and NMDA receptors: a role in information storage? Philos. Trans. R Soc. Lond. B Biol. Sci. 329, 187–204 (1990).
Perkins, M. N. & Stone, T. W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 247, 184–187 (1982).
Deutschenbaur, L. et al. Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog Neuropsychopharmacol. Biol. Psychiatry. 64, 325–333 (2016).
Braidy, N. et al. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotoxicol Res. 16, 77–86 (2009).
Ostapiuk, A. & Urbanska, E. M. Kynurenic acid in neurodegenerative disorders–unique neuroprotection or double–edged sword? CNS Neurosci. Ther. 28, 19–35 (2022).
Maitre, M., Taleb, O., Jeltsch-David, H., Klein, C. & Mensah-Nyagan, A. G. Xanthurenic acid: A role in brain intercellular signaling. J. Neurochem. 168 (9), 2303–2315. https://doi.org/10.1111/jnc.16099 (2024).
Pocivavsek, A., Schwarcz, R. & Erhardt, S. Neuroactive kynurenines as Pharmacological targets: new experimental tools and exciting therapeutic opportunities. Pharmacol. Rev. 76 (6), 978–1008. https://doi.org/10.1124/pharmrev.124.000239 (2024).
Cross–Disorder Group of the Psychiatric Genomics Consortium. Genomic Relationships, novel Loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469–1482. https://doi.org/10.1016/j.cell.2019.11.020 (2019). .e11.
Cohen, S. M. et al. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res. 167, 98–107 (2015).
Su, F. et al. Neuronal growth regulator 1 (NEGR1) promotes the synaptic targeting of glutamic acid decarboxylase 65 (GAD65). J. Neurochem. 169, e16279. https://doi.org/10.1111/jnc.16279 (2025).
Gandal, M. J. et al. GABAB–mediated rescue of altered excitatory–inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR–hypofunction. Transl Psychiatry. 2, e142. https://doi.org/10.1038/tp.2012.69 (2012).
Rosenmund, C. et al. Synaptic NMDA receptor channels have a low open probability. J. Neurosci. 15, 2788–2795 (1995).
Hardingham, G. E. et al. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut–off and cell death pathways. Nat. Neurosci. 5, 405–414 (2002).
Liu, Y. et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 27, 2846–2857 (2007).
Tovar, K. R. & Westbrook, G. L. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J. Neurosci. 19, 4180–4188 (1999).
Stanika, R. I. et al. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc. Natl. Acad. Sci. USA. 106, 9854–9859 (2009).
Zhou, X. et al. Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N–methyl–D–aspartate receptor function and neuronal excitotoxicity. J. Biol. Chem. 288, 24151–24159 (2013).
Wang, G. et al. GluN2A: A promising target for developing novel antidepressants. Int. J. Neuropsychopharmacol. 27, pyae037. https://doi.org/10.1093/ijnp/pyae037 (2024).
Brim, B. L. et al. Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav. Brain Res. 238, 211–226 (2013).
Cui, Y. et al. Forebrain NR2B overexpression facilitating the prefrontal cortex long–term potentiation and enhancing working memory function in mice. PLoS One. 6, e20312. https://doi.org/10.1371/journal.pone.0020312 (2011).
Sun, Y. Y. et al. Surface expression of hippocampal NMDA GluN2B receptors regulated by fear conditioning determines its contribution to memory consolidation in adult rats. Sci. Rep. 6, 30743. https://doi.org/10.1038/srep30743 (2016).
Philips, M. A. et al. Myg1–deficient mice display alterations in stress–induced responses and reduction of sex–dependent behavioural differences. Behav. Brain Res. 207, 182–195 (2010).
Kaare, M. et al. High–Fat diet induces pre–diabetes and distinct sex–specific metabolic alterations in Negr1–deficient mice. Biomedicines 9, 1148. https://doi.org/10.3390/biomedicines9091148 (2021).
Holter, K. M. et al. Use of quantitative electroencephalography to inform Age– and Sex–Related differences in NMDA receptor function following MK–801 administration. Pharmaceuticals 17, 237. https://doi.org/10.3390/ph17020237 (2024).
Kniffin, A. R. & Briand, L. A. Sex differences in glutamate transmission and plasticity in reward related regions. Front. Behav. Neurosci. 18, 1455478. https://doi.org/10.3389/fnbeh.2024.1455478 (2024).
Neuhäusel, T. S. & Gerevich, Z. Sex–specific effects of subchronic NMDA receptor antagonist MK–801 treatment on hippocampal gamma oscillations. Front. Neurosci. 18, 1425323. https://doi.org/10.3389/fnins.2024.1425323 (2024).
Lee, A. W. et al. Functional inactivation of the genome–wide association study obesity gene neuronal growth regulator 1 in mice causes a body mass phenotype. PLoS One. 7, e41537. https://doi.org/10.1371/journal.pone.0041537 (2012).
Schalkwyk, L. C. et al. Interpretation of knockout experiments: the congenic footprint. Genes Brain Behav. 6 (3), 299–303. https://doi.org/10.1111/j.1601-183X.2007.00304.x (2007).
Varul, J. et al. Dopamine System, NMDA receptor and EGF family expressions in brain structures of Bl6 and 129Sv strains displaying different behavioral adaptation. Brain Sci. 11, 725. https://doi.org/10.3390/brainsci11060725( (2021).
Funding
This research was funded by the investigation grant PRG2544 from the Estonian Research Council (E.V.).
Author information
Authors and Affiliations
Contributions
Conceptualisation: M-A.P., E.V.; Methodology: C.K., K.M., K.K., M.K., M.J., N.M., G.I., E.L., M-A.P; Analysis: C.K, K.M., K.K., M-A.P.; Writing—original draft preparation: C.K., M-A.P., E.V.; Writing—review and editing: C.K., K.M., K.K., M.K., M.J., N.M., G.I., E.L., M-A.P, E.V.; Prepared figures: C.K, M-A.P. Funding acquisition: M-A.P., E.V. All authors critically revised the manuscript for intellectual content and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declaration
All animal procedures were approved by the local animal ethics committee (Permit No. 150, 27 September 2019) and conducted in accordance with institutional and national guidelines for the care and use of animals. Euthanasia was performed by trained and experienced personnel. Rapid decapitation was used as the method of euthanasia, which is incompatible with life and therefore constitutes confirmation of death prior to disposal of the remains. Full compliance with the ARRIVE guidelines is detailed in the ARRIVE checklist provided at the end of the Supplementary Information.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuuskmäe, C., Mikheim, K., Mohammadrahimi, N. et al. Negr1 deficiency alters glutamate signalling and kynurenine pathway in a mouse model of psychiatric disorders. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35968-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35968-7