Abstract
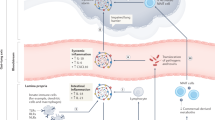
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection first emerged in Wuhan, Hubei Province, China, in December 2019 and spread rapidly to other provinces and other countries. Angiotensin I converting enzyme 2 (ACE2) is the receptor for SARS-CoV and has been suggested to be also the receptor for SARS-CoV-2. Paradoxically, ACE2 expression in the lung protects mice from SARS-CoV spike protein induced lung injury by attenuating the renin-angiotensin system. In the intestine, ACE2 also suppresses intestinal inflammation by maintaining amino acid homeostasis, antimicrobial peptide expression and ecology of the gut microbiome. We performed analysis of single cell-RNA sequencing data from control subjects and those with colitis or inflammatory bowel disease (6 controls, 6 colitis cases, 2 ulcerative colitis cases and 3 Crohn’s disease cases). The single cell-RNA sequencing data was also used to conduct co-expression analysis and GO enrichment analysis. Multiplex immunofluorescence (mIF) was performed to assess the expression of ACE2, IFNA4, and RSAD2 on colon specimens obtained from patients, including non‑diseased (control) tissue, ulcerative colitis (UC) tissue and Crohn’s disease (CD) tissue. We revealed that ACE2 exhibited specific and high expression levels in colonocytes. Furthermore, genes implicated in viral infection and anti-infection immunity were also found to be highly expressed in colonocytes. Additionally, we conducted an analysis of genes co-expressed with ACE2 within colonocytes, and total of 3420 and 2136 genes were identified as being positively and negatively correlated with ACE2 expression. Concurrently, through Gene Ontology (GO) enrichment analysis, it was observed that genes positively associated with ACE2 expression were significantly enriched in pathways related to viral infection, organismal immunity, and energy metabolism. Accordingly, mIF showed a significant increase in IFNA4 and RSAD2 expression in the colonic epithelial ACE2⁺ cells of UC and CD patients relative to controls. Integrated data from single cell-RNA sequencing and patient’s mIF highlighted the expression profile of ACE2 in colonic epithelial cells, suggesting the possible involvement of ACE2 in the intestinal tract of patients with SARS-CoV-2 pneumonia in enteroviral infection, immunity and energy metabolism functions.
Similar content being viewed by others
Data availability
The data generated in this study have been deposited in two public repositories. The processed gene expression and raw sequencing data are available under controlled access from the Genome Sequence Archive (GSA) of the Beijing Institute of Genomics, Chinese Academy of Sciences under accession number HRA000072 (application guidance: http://bigd.big.ac.cn/gsa-human). The processed data from the pooling dataset are publicly available in the Gene Expression Omnibus (GEO) under accession number GSE121380.
References
Porcari, S. et al. Prevalence of irritable bowel syndrome and functional dyspepsia after acute gastroenteritis: Systematic review and meta-analysis. Gut 73(9), 1431–1440 (2024).
Mehta, D. et al. Gastrointestinal manifestations and outcomes of COVID-19: A comprehensive systematic review and meta-analysis. Cureus 15(10), e47028 (2023).
Tripathi, K. et al. COVID-19 and outcomes in patients with inflammatory bowel disease: Systematic review and meta-analysis. Inflamm. Bowel Dis. 28(8), 1265–1279 (2022).
Du, L. et al. Cleavage of spike protein of SARS coronavirus by protease factor Xa is associated with viral infectivity. Biochem. Biophys. Res. Commun. 359(1), 174–179 (2007).
Investigators, E.-P. et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 366(14), 1287–1297 (2012).
Kuba, K. et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 11(8), 875–879 (2005).
Chan, J. F. et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 9(1), 221–236 (2020).
Marshall, R. P. The pulmonary renin-angiotensin system. Curr. Pharm. Des. 9(9), 715–722 (2003).
Clements, R. T. et al. Treatment of pulmonary hypertension with angiotensin II receptor blocker and neprilysin inhibitor sacubitril/valsartan. Circ. Heart Fail 12(11), e005819 (2019).
Hubers, S. A. & Brown, N. J. Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation 133(11), 1115–1124 (2016).
Huang, B. et al. Mucosal profiling of pediatric-onset colitis and IBD reveals common pathogenics and therapeutic pathways. Cell 179(5), 1160–1176 (2019).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36(5), 411–420 (2018).
Hashimoto, T. et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487(7408), 477–481 (2012).
Vuille-dit-Bille, R. N. et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids 47(4), 693–705 (2015).
Ye, R. & Liu, Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp. Mol. Pathol. 113, 104350 (2019).
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020.
Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur. Respi.r J. 2020; 55(1).
Tonew, E., Indulen, M. K. & Dzeguze, D. R. Antiviral action of dipyridamole and its derivatives against influenza virus A. Acta Virol. 26(3), 125–129 (1982).
Fata-Hartley, C. L. & Palmenberg, A. C. Dipyridamole reversibly inhibits mengovirus RNA replication. J. Virol. 79(17), 11062–11070 (2005).
Tenser, R. B., Gaydos, A. & Hay, K. A. Inhibition of herpes simplex virus reactivation by dipyridamole. Antimicrob. Agents Chemother. 45(12), 3657–3659 (2001).
Szebeni, J. et al. Dipyridamole potentiates the inhibition by 3’-azido-3’-deoxythymidine and other dideoxynucleosides of human immunodeficiency virus replication in monocyte-macrophages. Proc. Natl. Acad. Sci. U S A 86(10), 3842–3846 (1989).
Kozhukharova, M. S., Slepushkin, A. N., Radeva, Kh. T., Lavrukhina, L. A. & Demidova, S. A. Evaluation of dipyridamole efficacy as an agent for preventing acute respiratory viral diseases. Vopr. Virusol. 32(3), 294–297 (1987).
Kuzmov, K., Galabov, A. S., Radeva, K., Kozhukharova, M. & Milanov, K. Epidemiological trial of the prophylactic effectiveness of the interferon inducer dipyridamole with respect to influenza and acute respiratory diseases. Zh Mikrobiol. Epidemiol. Immunobiol. 6, 26–30 (1985).
Hu XQ, wANG XL. Treatment of viral upper respiratory tract infection in children with dipyridamole. Chinese Journal of Hospital Pharmacy 1995; (9).
Roberts, C. Universal precautions: Improving the knowledge of trained nurses. Br. J. Nurs. 9(1), 43–47 (2000).
Wu, K. et al. Ace2 mutation disrupts amino acid absorption, impairs growth, and alters microbiota dynamics in zebrafish. Commun. Biol. 8(1), 1226 (2025).
Acknowledgements
The authors are quite grateful for the available data used in this study provided by Zhang Lab.
Funding
Natural Science Foundation of China (91742109, 31770978, 31722003, 31770925, 31370847, 81770552), National Key Research and Development Program (2016YFC0900102), National Science and Technology Major Project (2018ZX10302205), Guangdong Provincial Key Laboratory of Research in Structural Birth Defect Disease (2019B030301004), Guangzhou Women and Children’s Medical Center Fund (5001-3001032) and National Health and Medical Research Council of Australia (1037321, 1105209, 1143976, and 1080321 to A.M.L.) funded this study. Guangdong Province Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong Joint Funding Programme) (2025A0505080010). Guangzhou Science and Technology fund (2024A04J3928).
Author information
Authors and Affiliations
Contributions
Y.Z., Q.T. and W.X. conceived of and supervised the project. Y.Q., H.C. and Q.T. performed the bioinformatic analysis. Y.H. performed the experiment with assistance from H.C., J. L.. Y.Z. wrote the manuscript with significant assistance from Q.T., W.X. and M.L. All authors read, discussed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qi, Y., Huang, Y., Chen, H. et al. ACE2 expression by colonic epithelial cells is associated with viral infection, immunity, and energy metabolism. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36052-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36052-w