Abstract
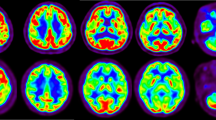
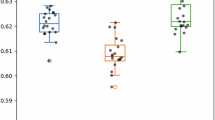
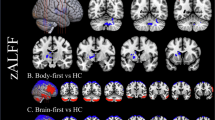
Parkinson’s disease (PD) is the second most common neurological disorder, but its diagnosis remains challenging. Cerebral glucose metabolism has emerged as a promising biomarker for PD based on previous studies. While these studies have established a PD-related pattern of metabolic activity of glucose in the brain, cerebral oxygen metabolism is less explored, and there is no well-established PD-related pattern of cerebral oxygen metabolism. This study investigates cerebral oxygen extraction fraction (OEF) as a measure of cerebral oxygen metabolism to monitor disease progression in early-stage PD. OEF was measured noninvasively using magnetic resonance imaging with the QSM + qBOLD technique in 50 PD patients and 30 healthy controls. Whole-brain and region-of-interest analyses were conducted, focusing on key regions within the basal ganglia. Results revealed significantly elevated OEF in the basal ganglia of PD patients compared to controls. Moreover, OEF showed a positive correlation with Unified Parkinson’s Disease Rating Scale Part III scores, indicating an association between increased oxygen extraction and motor impairment severity in early PD. These findings support the potential of cerebral OEF as an early biomarker of motor symptom severity. Therefore, it can enhance our understanding of metabolic dysfunction in the basal ganglia during the early stages of PD.
Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303 (2021).
Beach, T. G. & Adler, C. H. Importance of low diagnostic accuracy for early parkinson’s disease. Mov. Disord. 33, 1551–1554 (2018).
Meles, S. K., Teune, L. K., de Jong, B. M., Dierckx, R. A. & Leenders, K. L. Metabolic imaging in Parkinson disease. J. Nucl. Med. 58, 23–28 (2017).
Wu, P. et al. Metabolic brain network in the Chinese patients with parkinson’s disease based on 18F-FDG PET imaging. Parkinsonism Relat. Disord. 19, 622–627 (2013).
Wolfson, L. I., Leenders, K. L., Brown, L. L. & Jones, T. Alterations of regional cerebral blood flow and oxygen metabolism in parkinson’s disease. Neurology 35, 1399–1399 (1985).
Lin, T. P. et al. Metabolic correlates of subthalamic nucleus activity in parkinson’s disease. Brain 131, 1373–1380 (2008).
Powers, W. J. et al. Cerebral mitochondrial metabolism in early parkinson’s disease. J. Cereb. Blood Flow. Metabolism. 28, 1754–1760 (2008).
Matthews, D. C. et al. FDG PET parkinson’s disease-related pattern as a biomarker for clinical trials in early stage disease. NeuroImage: Clin. 20, 572–579 (2018).
Teune, L. K. et al. Parkinson’s disease-related perfusion and glucose metabolic brain patterns identified with PCASL-MRI and FDG-PET imaging. NeuroImage: Clin. 5, 240–244 (2014).
Teune, L. K. et al. Validation of parkinsonian disease-related metabolic brain patterns. Mov. Disord. 28, 547–551 (2013).
Borghammer, P. et al. Cerebral oxygen metabolism in patients with early parkinson’s disease. J. Neurol. Sci. 313, 123–128 (2012).
Jiang, D. & Lu, H. Cerebral oxygen extraction fraction MRI: techniques and applications. Magn. Reson. Med. 88, 575–600 (2022).
Buxton, R. B. Quantifying CBF with arterial spin labeling. J. Magn. Reson. Imaging. 22, 723–726 (2005).
Lu, H. et al. Calibration and validation of TRUST MRI for the Estimation of cerebral blood oxygenation. Magn. Reson. Med. 67, 42–49 (2012).
Zhang, J. et al. Quantitative susceptibility mapping-based cerebral metabolic rate of oxygen mapping with minimum local variance. Magn. Reson. Med. 79, 172–179 (2018).
He, X., Yablonskiy, D. A. & Quantitative, B. O. L. D. Mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn. Reson. Medicine: Official J. Int. Soc. Magn. Reson. Med. 57, 115–126 (2007).
Xu, F., Ge, Y. & Lu, H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn. Reson. Med. 62, 141–148 (2009).
Yan, S. et al. Spatiotemporal patterns of brain iron-oxygen metabolism in patients with parkinson’s disease. Eur. Radiol. 34, 3074–3083 (2024).
Melzer, T. R. et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in parkinson’s disease. Brain 134, 845–855 (2011).
Pelizzari, L. et al. Cerebral blood flow and cerebrovascular reactivity correlate with severity of motor symptoms in parkinson’s disease. Ther. Adv. Neurol. Disord. 12, 1756286419838354 (2019).
Yang, K. et al. White matter changes in parkinson’s disease. Npj Parkinson’s Disease. 9, 150 (2023).
Cho, J., Ma, Y., Spincemaille, P., Pike, G. B. & Wang, Y. Cerebral oxygen extraction fraction: comparison of dual-gas challenge calibrated BOLD with CBF and challenge‐free gradient echo QSM + qBOLD. Magn. Reson. Med. 85, 953–961 (2021).
Hughes, A. J., Daniel, S. E., Kilford, L. & Lees, A. J. Accuracy of clinical diagnosis of idiopathic parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 55, 181–184 (1992).
Goetz, C. G. et al. Movement disorder Society-sponsored revision of the unified parkinson’s disease rating scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov. Disorders: Official J. Mov. Disorder Soc. 23, 2129–2170 (2008).
Hoehn, M. M. & Yahr, M. D. Parkinsonism: onset, progression, and mortality. Neurology 17, 427–427 (1967).
Arevalo-Rodriguez, I. et al. Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. (2021).
Liu, T. et al. Morphology enabled dipole inversion (MEDI) from a single-angle acquisition: comparison with COSMOS in human brain imaging. Magn. Reson. Med. 66, 777–783 (2011).
Liu, T. et al. A novel background field removal method for MRI using projection onto dipole fields. NMR Biomed. 24, 1129–1136 (2011).
Cho, J. et al. Cerebral oxygen extraction fraction (OEF): comparison of challenge-free gradient echo QSM + qBOLD (QQ) with 15O PET in healthy adults. J. Cereb. Blood Flow. Metabolism. 41, 1658–1668 (2021).
Cho, J. et al. QQ-NET – using deep learning to solve quantitative susceptibility mapping and quantitative blood oxygen level dependent magnitude (QSM + qBOLD or QQ) based oxygen extraction fraction (OEF) mapping. Magn. Reson. Med. 87, 1583–1594 (2022).
Cho, J. et al. Cluster analysis of time evolution (CAT) for quantitative susceptibility mapping (QSM) and quantitative blood oxygen level-dependent magnitude (qBOLD)-based oxygen extraction fraction (OEF) and cerebral metabolic rate of oxygen (CMRO2) mapping. Magn. Reson. Med. 83, 844–857 (2020).
Cho, J., Spincemaille, P., Nguyen, T. D., Gupta, A. & Wang, Y. Temporal clustering, tissue composition, and total variation for mapping oxygen extraction fraction using QSM and quantitative BOLD. Magn. Reson. Med. 86, 2635–2646 (2021).
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. Fsl Neuroimage 62, 782–790 (2012).
Yushkevich, P. A., Gao, Y. & Gerig, G. In 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 3342–3345 (IEEE, 2016).
Tang, C. C., Poston, K. L., Dhawan, V. & Eidelberg, D. Abnormalities in metabolic network activity precede the onset of motor symptoms in parkinson’s disease. J. Neurosci. 30, 1049–1056 (2010).
Tang, B. L. Glucose, glycolysis, and neurodegenerative diseases. J. Cell. Physiol. 235, 7653–7662 (2020).
Kitamura, S. et al. Cerebral blood flow and oxygen metabolism in patients with parkinson’s disease. No Shinkei = Brain Nerve. 40, 979–985 (1988).
Wang, M. et al. White matter microstructural alterations and brain metabolism distributions in parkinson’s disease. Brain Imaging Behav. 2025, 1–11 (2025).
Hyder, F. et al. Uniform distributions of glucose oxidation and oxygen extraction in Gray matter of normal human brain: no evidence of regional differences of aerobic Glycolysis. J. Cereb. Blood Flow. Metabol. 36, 903–916 (2016).
Ko, J. H., Lerner, R. P. & Eidelberg, D. Effects of Levodopa on regional cerebral metabolism and blood flow. Mov. Disord. 30, 54–63 (2015).
Funding
This research was supported by a grant from the Korea Dementia Research Project through the Korea Dementia Research Center (KDRC), funded by the Ministry of Health & Welfare and the Ministry of Science and ICT, Republic of Korea (grant number: RS-2024-00334574). This work was partially supported by grants from the National Research Foundation of Korea of the Korean government (RS-2025-02216928).
Author information
Authors and Affiliations
Contributions
H.E.C. processed and conducted statistical analysis on the MRI data and wrote the first draft of the manuscript. D.L. performed the registration of MR images. H.L. and J.L. were responsible for acquiring MRI data and UPDRS-III scores. J.C. implemented the QQ-CCTV code. H.C. conceptualized the study and interpreted the results. All authors contributed to writing the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study protocol was approved by the institutional review board of the Pusan National University Yangsan Hospital (Yangsan, Republic of Korea).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Candan, H.E., Lee, D., Lee, H. et al. Elevated cerebral oxygen extraction fraction in Parkinson’s disease correlates with motor impairment severity. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36435-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36435-z