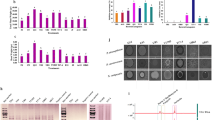
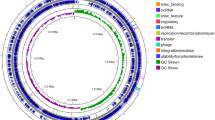
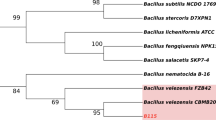
Abstract
Biological agents offer an alternative to chemical pesticides in managing plant diseases, as they help decrease environmental contamination and reduce selective pressure for resistant pathogens. In this study, bacteria isolated from the larval food of stingless bees were tested for in vitro antibacterial activity against Xanthomonas citri pv. glycines, the cause of soybean bacterial pustule. A strain identified as Bacillus velezensis showed notable growth inhibition in agar diffusion tests. Fractionation of the bacterial culture supernatant revealed that antimicrobial activity was concentrated in the metabolic fraction, especially in the ethyl acetate extract, which exhibited a concentration-dependent inhibition (CDI) of 0.015 mg/mL against X. citri pv. glycines. A phytotoxicity test using soybean seeds indicated no immediate effects on germination under experimental conditions. LC–MS analysis of the bioactive fraction tentatively identified 15 metabolites, mainly diketopiperazines, although other molecular features remained unknown. Whole-genome sequencing showed a genome size of about 4.0 Mb and 13 predicted biosynthetic gene clusters, including those likely involved in lipopeptide and polyketide production; however, these compounds were not detected in the analyzed extract. These findings demonstrate in vitro antibacterial activity linked to metabolites produced by a newly characterized Bacillus velezensis strain mandacaium and outline its biosynthetic potential at the genomic level. The results lay the groundwork for future in planta research to assess its role in sustainable plant disease management.
Similar content being viewed by others
Data availability
Genomic data of Bacillus velezensis strain mandacaium are available at NCBI under accession number SAMN41589789. All other data are provided within the paper.
References
Tudi, M. et al. Agriculture Development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public. Health. 18, 1112 (2021).
Aktar, W., Sengupta, D. & Chowdhury, A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol. 2, 1–12 (2009).
Sabarwal, A., Kumar, K. & Singh, R. P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 63, 103–114 (2018).
Rani, L. et al. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 283, 124657 (2021).
Agboola, A. R., Okonkwo, C. O., Agwupuye, E. I. & Mbeh, G. Biopesticides and conventional pesticides: comparative review of mechanism of action and future perspectives. AROC Agric. 1, 14–32 (2022).
KLIEBENSTEIN, D. J. Secondary metabolites and plant/environment interactions: a view through Arabidopsis Thaliana tinged glasses. Plant. Cell. Environ. 27, 675–684 (2004).
Khursheed, A. et al. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 173, 105854 (2022).
Kumar, S., Singh, A. & Biopesticides Present status and the future prospects. J Biofertil. Biopestic 06, 129 (2015).
Thakur, N., Kaur, S., Tomar, P., Thakur, S. & Yadav, A. N. Microbial biopesticides: Current status and advancement for sustainable agriculture and environment. in New and Future Developments in Microbial Biotechnology and Bioengineering 243–282Elsevier, (2020). https://doi.org/10.1016/B978-0-12-820526-6.00016-6
Essiedu, J. A., Adepoju, F. O. & Ivantsova, M. N. Benefits and limitations in using biopesticides: A review. in 080002 (2020). https://doi.org/10.1063/5.0032223
Baharudin, M. M. A. A. et al. Antimicrobial activities of Bacillus velezensis strains isolated from stingless bee products against methicillin-resistant Staphylococcus aureus. PLoS One. 16, e0251514 (2021).
Liang, X. et al. Identification and genomic insights into a strain of Bacillus velezensis with phytopathogen-inhibiting and plant growth-promoting properties. Microbiol. Res. 285, 127745 (2024).
Grady, E. N. et al. Characterization and complete genome analysis of the surfactin-producing, plant-protecting bacterium Bacillus velezensis 9D-6. BMC Microbiol. 19, 5 (2019).
Rabbee, M. F., Hwang, B. S. & Baek, K. H. Bacillus velezensis: A beneficial biocontrol agent or facultative phytopathogen for sustainable agriculture. Agronomy 13, 840 (2023).
Tang, T. et al. Bacillus velezensis LT1: a potential biocontrol agent for Southern blight on Coptis chinensis. Front Microbiol 15, 1337655 (2024).
Jia, J. et al. 2,5-Diketopiperazines: A review of Source, Synthesis, Bioactivity, Structure, and MS fragmentation. Curr. Med. Chem. 30, 1060–1085 (2023).
Jang, S. et al. History of a model plant growth-promoting rhizobacterium, Bacillus velezensis GB03: from isolation to commercialization. Front Plant. Sci 14, 1230957 https://doi.org/10.3389/fpls.2023.1230957 (2023).
Fan, B. et al. Bacillus velezensis FZB42 in 2018: the Gram-Positive model strain for plant growth promotion and biocontrol. Front Microbiol 9, 2491 https://doi.org/10.3389/fmicb.2018.02491 (2018).
Violatti, M. R. & Tebaldi, N. D. Detecção de Xanthomonas axonopodis pv. glycines Em Sementes de Soja. Summa Phytopathol. 42, 268–270 (2016).
Hong, S. J. et al. Influence of disease severity of bacterial pustule caused by Xanthomonas axonopodis pv. glycines on soybean yield. Res. Plant. Disease. 17, 317–325 (2011).
Wang, S. et al. Bacterial pustule disease in soybean: Occurrence, Host–Pathogen interactions and management. Plant. Pathol. 74, 1941–1957 (2025).
Kang, I. J. et al. Characterization of Xanthomonas citri pv. glycines population genetics and virulence in a National survey of bacterial pustule disease in Korea. Plant. Pathol. J. 37, 652–661 (2021).
Cardoso-Sichieri, R. et al. Genome-Wide association studies and QTL mapping reveal a new locus associated with resistance to bacterial pustule caused by Xanthomonas citri pv. glycines in soybean. Plants 13, 2484 (2024).
LEMES, E. CASTRO, L. ; & ASSIS, R. Doenças da soja: melhoramento genético e técnicas de manejo. Campinas: Millenium (2015).
Wrather, J. A. et al. Soybean disease loss estimates for the top ten soybean-producing counries in 1998. Can. J. Plant Pathol. 23, 115–121 (2001).
Leite, J. C. et al. Cadeia produtiva Da soja: Armazenamento e Logística. UNICIÊNCIAS 26, 31–36 (2022).
Zhao, R., Kang, I. J. & Lee, S. Current status and future prospects in genomic research and breeding for resistance to Xanthomonas citri pv. glycines in soybean. Agronomy 13, 490 (2023).
Chen, Z. et al. The antagonistic mechanism of Bacillus velezensis ZW10 against rice blast disease: evaluation of ZW10 as a potential biopesticide. PLoS One. 16, e0256807 (2021).
Wang, C., Ye, X., Ng, T. B. & Zhang, W. Study on the biocontrol potential of antifungal peptides produced by Bacillus velezensis against Fusarium Solani that infects the passion fruit Passiflora Edulis. J. Agric. Food Chem. 69, 2051–2061 (2021).
Chen, M. et al. Biocontrol of tomato bacterial wilt by the new strain Bacillus velezensis FJAT-46737 and its lipopeptides. BMC Microbiol. 20, 160 (2020).
Kim, J. et al. Plant protective and growth promoting effects of seed endophytes in soybean plants. Plant. Pathol. J. 39, 513–521 (2023).
Santos, A. C. C. et al. Bacteria, yeasts, and fungi associated with larval food of Brazilian native stingless bees. Sci. Rep. 13, 5147 (2023).
Hartfelder, K. & Engels, W. The composition of larval food in stingless bees: evaluating nutritional balance by chemosystematic methods. Insectes Soc. 36, 1–14 (1989).
Costa, N. M. G. et al. Screening of microorganisms isolated from stingless bees’ larval food in the biocontrol of Meloidogyne incognita. J Nematol 57, 1, 20250028 https://doi.org/10.2478/jofnem-2025-0028 (2025).
Santos, A. C. C. et al. Antimicrobial activity of supernatants produced by bacteria isolated from Brazilian stingless bee’s larval food. BMC Microbiol. 22, 127 (2022).
Feliatra, F., Batubara, U. M., Nurulita, Y., Lukistyowati, I. & Setiaji, J. The potentials of secondary metabolites from Bacillus cereus SN7 and vagococcus fluvialis CT21 against fish pathogenic bacteria. Microb. Pathog. 158, 105062 (2021).
Wang, S. et al. Bacillus velezensis BM21, a potential and efficient biocontrol agent in control of corn stalk rot caused by fusarium graminearum. Egypt. J. Biol. Pest Control. 30, 9 (2020).
Li, X., Munir, S., Xu, Y., Wang, Y. & He, Y. Combined mass spectrometry-guided genome mining and virtual screening for acaricidal activity in secondary metabolites of Bacillus velezensis W1. RSC Adv. 11, 25441–25449 (2021).
Yang, B. et al. A review on the screening methods for the discovery of natural antimicrobial peptides. J. Pharm. Anal. 15, 101046 (2025).
Wang, L. et al. Genomic and metabolomic insights into the antimicrobial compounds and plant growth-promoting potential of Bacillus velezensis Q-426. BMC Genom. 24, 589 (2023).
Adeniji, A. A., Loots, D. T. & Babalola, O. O. Bacillus velezensis: phylogeny, useful applications, and avenues for exploitation. Appl. Microbiol. Biotechnol. 103, 3669–3682 (2019).
Rabbee, M. et al. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules 24, 1046 (2019).
Letertre, M. P. M., Giraudeau, P. & de Tullio, P. Nuclear magnetic resonance spectroscopy in clinical metabolomics and personalized medicine: current challenges and perspectives. Front Mol. Biosci 8, 698337 https://doi.org/10.3389/fmolb.2021.698337 (2021).
Singh, S. et al. Targeted isolation of antibiofilm compounds from halophytic endophyte Bacillus velezensis 7NPB-3B using LC-HR-MS-Based metabolomics. Microorganisms 12, 413 (2024).
Ramarathnam, R. et al. Molecular and biochemical detection of fengycin- and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of Canola and wheat. Can. J. Microbiol. 53, 901–911 (2007).
Gu, Q. et al. Bacillomycin D produced by Bacillus amyloliquefaciens is involved in the antagonistic interaction with the Plant-Pathogenic fungus fusarium graminearum. Appl Environ. Microbiol 83, e01075–17 https://doi.org/10.1128/AEM.01075-17 (2017).
Wu, L. et al. Difficidin and Bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci. Rep. 5, 12975 (2015).
Chen, X. H. et al. Difficidin and Bacilysin produced by plant-associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. J. Biotechnol. 140, 38–44 (2009).
Yu, C. et al. Profiling of antimicrobial metabolites synthesized by the endophytic and genetically amenable biocontrol strain Bacillus velezensis DMW1. Microbiol Spectr 11, e00038–23 https://doi.org/10.1128/spectrum.00038-23 (2023).
Alenezi, F. N. et al. Bacillus velezensis: A treasure house of bioactive compounds of Medicinal, biocontrol and environmental importance. Forests 12, 1714 (2021).
Ye, M. et al. Characteristics and application of a novel species of Bacillus: Bacillus velezensis. ACS Chem. Biol. 13, 500–505 (2018).
Richa Metabolite-based bioformulation: next generation of biofertilizers. in Metabolomics, Proteomics and Gene Editing Approaches in Biofertilizer Industry 53–81 (Springer Nature Singapore, Singapore, doi:https://doi.org/10.1007/978-981-97-2910-4_4. (2024).
O’Brien, J. & Wright, G. D. An ecological perspective of microbial secondary metabolism. Current Opinion in Biotechnology vol. 22 552–558 Preprint at (2011). https://doi.org/10.1016/j.copbio.2011.03.010
Lima, D. S. D. Identificação e caracterização dos metabólitos secundários ativos secretados pela bactéria >i/i< que causam inibição no crescimento de fungos filamentosos fitopatogênicos. (Universidade de São Paulo, São Paulo, 2019). doi:10.11606/D.87.2019.tde-25072019-095221.
Fenibo, E. O., Ijoma, G. N. & Matambo, T. Biopesticides in Sustainable Agriculture: Current Status and Future Prospects. in New and Future Development in Biopesticide Research: Biotechnological Exploration 1–53Springer Nature Singapore, Singapore, (2022). https://doi.org/10.1007/978-981-16-3989-0_1
Dhakal, R. & Biopesticides A key to sustainable agriculture. Int. J. Pure Appl. Biosci. 7, 391–396 (2019).
Chowdhury, S. P., Hartmann, A., Gao, X. & Borriss, R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42 – a review. Front Microbiol 6, 780 https://doi.org/10.3389/fmicb.2015.00780(2015).
Junior, S. et al. AVALIAÇÃO DE MÉTODOS PARA OBTENÇÃO DE PROTEÍNAS RECOMBINANTES. (2017).
Bittar, V. P. et al. Bioactive compounds from the leaves of maytenus Ilicifolia Mart. Ex reissek: Inhibition of LDL oxidation, glycation, lipid peroxidation, target enzymes, and microbial growth. J. Ethnopharmacol. 319, 117315 (2024).
Ahameethunisa, A. R. & Hopper, W. Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria. BMC Complement. Altern. Med. 10, 6 (2010).
Cassán, F. et al. Azospirillum Brasilense Az39 and Bradyrhizobium Japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea Mays L.) and soybean (Glycine max L). Eur. J. Soil. Biol. 45, 28–35 (2009).
Moser, J. C. et al. Role of K + and Ca2 + Channels in the Vasodilator Effects of Plectranthus barbatus (Brazilian Boldo) in Hypertensive Rats. Cardiovasc Ther 1–11 (2023). (2023).
Brandão, L. T. D., Côrtes, M. V. & de Filippi, C. B. M. C. C. de & Silva-Lobo, V. L. Protocolo de Extração de DNA Genômico Para Os Principais Fungos Fitopatogênicos do Arroz. Boletim De Pesquisa E Desenvolvimento 15, 01–15 (2019).
Silva, R. et al. Etiology and prevalence of macadamia diseases in Brazil. Australas. Plant Pathol. 53, 159–174 (2024).
Santos, A. R. et al. PANNOTATOR: an automated tool for annotation of pan-genomes. Genet. Mol. Res. 12, 2982–2989 (2013).
Carver, T., Harris, S. R., Berriman, M., Parkhill, J. & McQuillan, J. A. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 28, 464–469 (2012).
Jain, C., Rodriguez-R, L. M., Phillippy, A. M., Konstantinidis, K. T. & Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 9, 5114 (2018).
Blin, K. et al. AntiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 51, W46–W50 (2023).
Anjos, W. F., Lanes, G. C., Azevedo, V. A. & Santos, A. R. GENPPI: standalone software for creating protein interaction networks from genomes. BMC Bioinform. 22, 596 (2021).
Acknowledgements
We express our deep appreciation for the assistance provided by Dr. Luiz Ricardo Goulart Filho, who tragically became one of the millions of victims of COVID-19. And the UFU, FAPEMIG, and CNPQ for making this project possible.
Funding
This project was funded by the Research Support Foundation of the State of Minas Gerais (FAPEMIG APQ-02766-17, APQ-00269-22) and the National Council for Scientific and Technological Development (CNPq, grant number: 403193/2022-2), as well as FAPEMIG (grant number: CBB-APQ-03613-17) for INCT-TeraNano.
Author information
Authors and Affiliations
Contributions
JLC, ACCS, RCC, and CUV were responsible for the conception and design of the study, data collection and analysis, as well as manuscript writing. NSC, CAC, and ARS contributed to data analysis, critical revision of intellectual content, and approval of the final version of the manuscript. NDT, CUV, AMB, and TSR provided study supervision, conducted critical revision of intellectual content, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Correa, J.L., Santos, A.C.C., Cerqueira, R.C. et al. In vitro effects of Bacillus velezensis strain Mandacaium against Xanthomonas citri pv. glycines: genomic and metabolomic insights. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36508-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36508-z