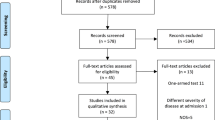
Abstract
During severe and critical COVID-19, therapeutic options remain scarce. Among interventions, the use of interleukin-6 receptor inhibitor (IL-6Ri) is especially controversial due to persistent uncertainty about their efficacy and safety. To compare the occurrence of secondary infections, digestive and hematological complication function of the administration of IL-6Ri we conducted a multicentric retrospective French observational study. All severe or critical COVID-19 requiring hospital admission were included. Among 2587 patients requiring hospital admission, 1603 had a severe COVID-19 and 984 a critical one requiring ICU admission. 224 received at least one dose of tocilizumab or sarilumab. Incidence of secondary infection was 29.5% in the IL-6Ri group vs. 19.5% without IL-6Ri (p = 0.0004) in the whole population. This result remained consistent after adjustment, without multiple imputation (MI) and after MI (adjusted OR: 1.47 [1.25; 1.72]; p < 0.0001)). Incidence of hematological or digestive complication were similar between groups. Mortality of patients admitted in ward was higher in the IL-6Ri group (18.7% vs 10.5%, p = 0.0155). No difference in 28 days, ICU, hospital of 90 days mortality was noticed among ICU patients.
Clinical trial registration: This study was registred on ClinicalTrial.gov: NCT05017441.
Similar content being viewed by others
Data availability
Datasets used/analyzed in this study are accessible upon reasonable request to the corresponding author.
References
Abani, O. et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet 397, 1637–1645. https://doi.org/10.1016/S0140-6736(21)00676-0 (2021).
Tomazini, B. M. et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA 324, 1307–1316. https://doi.org/10.1001/jama.2020.17021 (2020).
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al (2020) Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 324: 1330–1341 https://doi.org/10.1001/jama.2020.17023
Schultze, J. L. & Aschenbrenner, A. C. COVID-19 and the human innate immune system. Cell 184, 1671–1692. https://doi.org/10.1016/j.cell.2021.02.029 (2021).
Sabaka, P. et al. Role of interleukin 6 as a predictive factor for a severe course of Covid-19: retrospective data analysis of patients from a long-term care facility during Covid-19 outbreak. BMC Infect Dis 21, 308. https://doi.org/10.1186/s12879-021-05945-8 (2021).
Chen, X. et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis 71, 1937–1942. https://doi.org/10.1093/cid/ciaa449 (2020).
Plocque, A. et al. Should we interfere with the interleukin-6 receptor during COVID-19: what do we know so far?. Drugs 83, 1–36. https://doi.org/10.1007/s40265-022-01803-2 (2023).
Ghosn L, Assi R, Evrenoglou T, et al (2023) Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst Rev 6:CD013881. https://doi.org/10.1002/14651858.CD013881.pub2
Yu, S.-Y. et al. Clinical efficacy and safety of interleukin-6 receptor antagonists (tocilizumab and sarilumab) in patients with COVID-19: a systematic review and meta-analysis. Emerg Microbes Infect 11, 1154–1165. https://doi.org/10.1080/22221751.2022.2059405 (2022).
Albuquerque, A. M. et al. Mortality rates among hospitalized patients With COVID-19 infection treated with tocilizumab and corticosteroids: A Bayesian reanalysis of a previous meta-analysis. JAMA Netw Open 5, e220548. https://doi.org/10.1001/jamanetworkopen.2022.0548 (2022).
Garbers, C., Heink, S., Korn, T. & Rose-John, S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov 17, 395–412. https://doi.org/10.1038/nrd.2018.45 (2018).
Kang, S., Tanaka, T., Narazaki, M. & Kishimoto, T. Targeting interleukin-6 signaling in clinic. Immunity 50, 1007–1023. https://doi.org/10.1016/j.immuni.2019.03.026 (2019).
Taniguchi, K. et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 519, 57–62. https://doi.org/10.1038/nature14228 (2015).
Plocque, A. & Philippart, F. Authors’ reply to chia siang kow et al.: comment on: ‘should we interfere with the interleukin-6 receptor during COVID-19: What Do We Know So Far?. Drugs 83, 647–648. https://doi.org/10.1007/s40265-023-01870-z (2023).
Kow, C. S., Ramachandram, D. S. & Hasan, S. S. Comment on: ‘should we interfere with the interleukin-6 receptor during COVID-19: what do we know?’. Drugs 83, 645–646. https://doi.org/10.1007/s40265-023-01869-6 (2023).
Tleyjeh, I. M. et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis, first update. Clin Microbiol Infect 27, 1076–1082. https://doi.org/10.1016/j.cmi.2021.04.019 (2021).
Avni, T. et al. Tocilizumab in the treatment of COVID-19-a meta-analysis. QJM 114, 577–586. https://doi.org/10.1093/qjmed/hcab142 (2021).
Aziz, M. et al. Efficacy of tocilizumab in COVID-19: A systematic review and meta-analysis. J Med Virol 93, 1620–1630. https://doi.org/10.1002/jmv.26509 (2021).
Bhimraj A, Morgan RL, Shumaker AH, et al (2022) Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis ciac724. https://doi.org/10.1093/cid/ciac724
Torres, A. et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J 50, 1700582. https://doi.org/10.1183/13993003.00582-2017 (2017).
Leone, M. et al. Brief summary of French guidelines for the prevention, diagnosis and treatment of hospital-acquired pneumonia in ICU. Ann. Intensive Care 8, 104. https://doi.org/10.1186/s13613-018-0444-0 (2018).
Kalil, A. C. et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases Society of America and the American Thoracic Society. Clin Infect Dis 63, e61–e111. https://doi.org/10.1093/cid/ciw353 (2016).
Calandra T, Cohen J, International Sepsis Forum Definition of Infection in the ICU Consensus Conference 2005 The international sepsis forum consensus conference on definitions of infection in the intensive care unit Crit Care Med 33 1538 1548
Tabah, A. et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med 38, 1930–1945. https://doi.org/10.1007/s00134-012-2695-9 (2012).
Laine, L. et al. ACG clinical guideline: upper gastrointestinal and ulcer bleeding. Am. J. Gastroenterol. 116, 899–917. https://doi.org/10.14309/ajg.0000000000001245 (2021).
Gralnek, I. M. et al. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 54, 1094–1120. https://doi.org/10.1055/a-1939-4887 (2022).
Sengupta, N. & Cifu, A. S. Management of patients with acute lower gastrointestinal tract bleeding. JAMA 320, 86–87. https://doi.org/10.1001/jama.2018.5684 (2018).
Stolarski, A. E., Kim, J., Zhang, Q. & Remick, D. G. Cytokine drizzle-the rationale for abandoning “cytokine storm”. Shock 56, 667–672. https://doi.org/10.1097/SHK.0000000000001769 (2021).
Leisman, D. E. et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 8, 1233–1244. https://doi.org/10.1016/S2213-2600(20)30404-5 (2020).
Chalmers, J. D. et al. Management of hospitalised adults with coronavirus disease-19 (COVID-19): A European Respiratory Society living guideline. Eur. Respir. J. https://doi.org/10.1183/13993003.00048-2021 (2021).
Rose-John, S., Winthrop, K. & Calabrese, L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol 13, 399–409. https://doi.org/10.1038/nrrheum.2017.83 (2017).
Rossi, J.-F. et al. Optimisation of anti-interleukin-6 therapy: Precision medicine through mathematical modelling. Front Immunol 13, 919489. https://doi.org/10.3389/fimmu.2022.919489 (2022).
Ripa, M. et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect 27, 451–457. https://doi.org/10.1016/j.cmi.2020.10.021 (2021).
Kimmig, L. M. et al. IL-6 inhibition in critically Ill COVID-19 patients is associated with increased secondary infections. Front Med (Lausanne) 7, 583897. https://doi.org/10.3389/fmed.2020.583897 (2020).
Selvaraj, V. et al. Tocilizumab in hospitalized patients with COVID-19: A meta analysis of randomized controlled trials. Lung 199, 239–248. https://doi.org/10.1007/s00408-021-00451-9 (2021).
Chen, C.-X. et al. Systematic review and meta-analysis of tocilizumab in persons with coronavirus disease-2019 (COVID-19). Leukemia 35, 1661–1670. https://doi.org/10.1038/s41375-021-01264-8 (2021).
Khan, F. A. et al. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 76, 907–919. https://doi.org/10.1136/thoraxjnl-2020-215266 (2021).
Koeckerling, D. & Barker, J. Accelerating the evolution of severe acute respiratory syndrome coronavirus 2: A risk of combining dexamethasone and tocilizumab for severe coronavirus disease 2019. J Infect Dis 224, 934–937. https://doi.org/10.1093/infdis/jiab328 (2021).
Rosas, I. O. et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 384, 1503–1516. https://doi.org/10.1056/NEJMoa2028700 (2021).
Ramos, R. et al. COVID-19 associated infections in the ICU setting: A retrospective analysis in a tertiary-care hospital. Enferm Infecc Microbiol Clin 41, 278–283. https://doi.org/10.1016/j.eimc.2021.10.014 (2023).
Vazquez Guillamet, M. C. et al. Interleukin-6 trajectory and secondary infections in mechanically ventilated patients with coronavirus disease 2019 acute respiratory distress syndrome treated with interleukin-6 receptor blocker. Crit Care Explor 3, e0343. https://doi.org/10.1097/CCE.0000000000000343 (2021).
Buetti, N. et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med 47, 180–187. https://doi.org/10.1007/s00134-021-06346-w (2021).
Liu, H. et al. Infectious complications after tocilizumab in patients with COVID: A real-world experience. J Community Hosp Intern Med Perspect 13, 11–19. https://doi.org/10.55729/2000-9666.1141 (2023).
Guaraldi, G. et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2, e474–e484. https://doi.org/10.1016/S2665-9913(20)30173-9 (2020).
Stone, J. H. et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 383, 2333–2344. https://doi.org/10.1056/NEJMoa2028836 (2020).
Acknowledgements
This work was supported by the Groupe Hospitalier Paris Saint‑Joseph All data generated or analyzed during this study are included in this study or its supplementary material files. Further inquiries can be directed to the corresponding author
Funding
This work was supported by the Groupe Hospitalier Paris Saint‑Joseph.
Author information
Authors and Affiliations
Contributions
Conceptualization: CL, TFB, AP, MT, FP. Methodology: MC, AF, GC, FP. Inclusions, review and editing: CL, TFB, AP, FP, LS, DM, OV, FB, LW, AP, SL, NR, PRB, MT, NJ, CB, JD. First draft: CL, TFB, FP. Secondary statistical analysis MY.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was carried out in accordance with the Declaration of Helsinki and French law. The TOCSIN study was approved by our institutional review board (GERM; IRB n°00012157). Patients were informed about the study and their non-opposition to the collection and use of non-identifying data for research purposes was obtained. The processing of patient data complied with the reference methodology 004 of the French Commission Nationale de l’Informatique et des Libertés (French National Data Protection Agency).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior publication: This work was submitted on MedRxiv: https://doi.org/10.1101/2025.03.04.25323313.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lefèvre, C., Funck-Brentano, T., Cachanado, M. et al. Benefit and risk associated with interleukin-6 receptor inhibitor administration during severe COVID-19: a retrospective multicentric study. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36864-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36864-w