Abstract
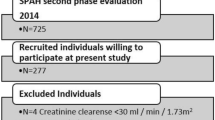
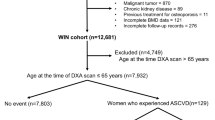
Osteoporosis is associated with cardiovascular disease, but the relationship between bone mineral density (BMD) and cardiac structure and function remains incompletely understood. This cross-sectional study analyzed baseline data from 1233 participants (median age 60 years; 59% female) in the Osteoarthritis and Cardiovascular Health Cohort. BMD was categorized as normal (T-score ≥ − 1.0, n = 364), osteopenia (− 2.5 < T-score < − 1.0, n = 404), or osteoporosis (T-score ≤ − 2.5, n = 465). Cardiac parameters were measured via echocardiography. Multivariable linear regression adjusted for age, sex, and cardiovascular risk factors. Osteoporosis was associated with higher LAVI (median: 29 vs. 26 mL/m2, P < 0.001) and RWT (0.38 vs. 0.37, P = 0.047) compared to normal BMD. After adjustment, T-score was inversely associated with LAVI (β = − 0.358, P = 0.043) and RWT (β = − 0.003, P = 0.013). Subgroup analyses showed stronger effects in women (LAVI β = − 0.737, P = 0.001) and participants < 50 years (LAVI β = − 0.909, P = 0.022). No significant associations were observed for left ventricular mass index, ejection fraction, or diastolic function metrics. Lower BMD, measured by tibial ultrasound, is independently associated with left atrial enlargement and increased ventricular wall thickness, particularly in women and younger adults. These findings suggest shared pathophysiological mechanisms between bone loss and cardiac remodeling, warranting more in-depth research.
Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. De-identified individual participant data, statistical code, and supporting documentation will be made available via Haoran Wang (washingtonhr@163.com) upon request. Data access will be granted following approval by the study’s Data Access Committee.
References
Salari, N. et al. Global prevalence of osteoporosis among the world older adults: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 16, 669. https://doi.org/10.1186/s13018-021-02821-8 (2021).
Yang, Y. & Huang, Y. Association between bone mineral density and cardiovascular disease in older adults. Front Publ. Health. 11, 1103403. https://doi.org/10.3389/fpubh.2023.1103403 (2023).
Fiechter, M. et al. Association between vertebral bone mineral density, myocardial perfusion, and long-term cardiovascular outcomes: A sex-specific analysis. J. Nucl. Cardiol. 27(3), 726–736. https://doi.org/10.1007/s12350-019-01802-z (2020).
Bellasi, A. & Raggi, P. Bone metabolism and cardiovascular disease: An overlooked association?. Atherosclerosis 335, 87–88. https://doi.org/10.1016/j.atherosclerosis.2021.09.009 (2021).
Qu, X. et al. Bone mineral density and all-cause, cardiovascular and stroke mortality: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 166(2), 385–393. https://doi.org/10.1016/j.ijcard.2011.10.114 (2013).
Bhatta, L. et al. Bone mineral density and risk of cardiovascular disease in men and women: The HUNT study. Eur. J. Epidemiol. 36(11), 1169–1177. https://doi.org/10.1007/s10654-021-00803-y (2021).
Guan, X. Q. et al. Low bone mineral density is associated with global coronary atherosclerotic plaque burden in stable angina patients. Clin. Interv. Aging. 13, 1475–1483. https://doi.org/10.2147/CIA.S168445 (2018).
Azeez, T. A. Osteoporosis and cardiovascular disease: A review. Mol. Biol. Rep. 50(2), 1753–1763. https://doi.org/10.1007/s11033-022-08088-4 (2023).
Yan, L. et al. Age- and gender-related differences in bone mineral status and biochemical markers of bone metabolism in Northern Chinese men and women. Bone 30(2), 412–415. https://doi.org/10.1016/s8756-3282(01)00676-7 (2002).
Lins Vieira, N. F., da Silva Nascimento, J., do Nascimento, C. Q., Barros Neto, J. A. & Oliveira Dos Santo, A. C. S. Association between bone mineral density and nutritional status, body composition and bone metabolism in older adults. J Nutr Health Aging. 25(1), 71-76. https://doi.org/10.1007/s12603-020-1452-y (2021).
Merz, A. A. & Cheng, S. Sex differences in cardiovascular ageing. Heart 102(11), 825–831. https://doi.org/10.1136/heartjnl-2015-308769 (2016).
Wang, H. et al. Association between knee cartilage degradation and cardiac remodeling: A cross-sectional analysis in a community-based cohort. Cardiac Res. 1(1), 38–49. https://doi.org/10.1097/re9.0000000000000004 (2025).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 61(4), 344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008 (2008).
Hans, D. & Baim, S. Quantitative ultrasound (QUS) in the management of osteoporosis and assessment of fracture risk. J. Clin. Densitom. 20(3), 322–333. https://doi.org/10.1016/j.jocd.2017.06.018 (2017).
Luo, W. et al. Osteoporosis diagnostic model using a multichannel convolutional neural network based on quantitative ultrasound radiofrequency signal. Ultrasound Med. Biol. 48(8), 1590–1601. https://doi.org/10.1016/j.ultrasmedbio.2022.04.005 (2022).
Chinese Society of Osteoporosis and Bone Mineral Research. Guidelines for the Diagnosis and Treatment of Primary Osteoporosis (2022). Chinese General Practice. 26(14), 1671–1691. https://doi.org/10.12114/j.issn.1007-9572.2023.0121 (2023).
Foldes, A. J., Rimon, A., Keinan, D. D. & Popovtzer, M. M. Quantitative ultrasound of the tibia: A novel approach for assessment of bone status. Bone 17(4), 363–367. https://doi.org/10.1016/s8756-3282(95)00244-8 (1995).
Prevrhal, S. et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporos Int. 12(1), 28–34. https://doi.org/10.1007/s001980170154 (2001).
Olszynski, W. P. et al. Multisite quantitative ultrasound for the prediction of fractures over 5 years of follow-up: The Canadian Multicentre Osteoporosis Study. J. Bone Miner. Res. 28(9), 2027–2034. https://doi.org/10.1002/jbmr.1931 (2013).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28(1), 1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003 (2015).
Chinese Society of Cardiology, Chinese College of Cardiovascular Physicians, Chinese Society of Heart Failure Physicians, Editorial Board of Chinese Journal of Cardiology. Update on the diagnosis and treatment of heart failure in China: Guidelines 2024. Chinese J. Cardiol. 52(3), 235–275 (2024).
Bibbins-Domingo, K., Brubaker, L. & Curfman, G. The 2024 revision to the declaration of Helsinki: Modern ethics for medical research. JAMA 333(1), 30–31. https://doi.org/10.1001/jama.2024.22530 (2025).
Iantomasi, T. et al. Oxidative stress and inflammation in osteoporosis: Molecular mechanisms involved and the relationship with microRNAs. Int. J. Mol. Sci. 24(4), 3772. https://doi.org/10.3390/ijms24043772 (2023).
Tiidus, P. M. Estrogen and gender effects on muscle damage, inflammation, and oxidative stress. Can J. Appl. Physiol. 25(4), 274–287. https://doi.org/10.1139/h00-022 (2000).
Viña, J., Gambini, J., García-García, F. J., Rodriguez-Mañas, L. & Borrás, C. Role of oestrogens on oxidative stress and inflammation in ageing. Horm. Mol. Biol. Clin. Investig. 16(2), 65–72. https://doi.org/10.1515/hmbci-2013-0039 (2013).
Boopalan, T., Arumugam, A., Parada, J., Saltzstein, E. & Lakshmanaswamy, R. Receptor activator for nuclear factor-κB ligand signaling promotes progesterone-mediated estrogen-induced mammary carcinogenesis. Cancer Sci. 106(1), 25–33. https://doi.org/10.1111/cas.12571 (2015).
Sivasinprasasn, S. et al. DPP-4 inhibitor and estrogen share similar efficacy against cardiac ischemic-reperfusion injury in obese-insulin resistant and estrogen-deprived female rats. Sci. Rep. 7, 44306. https://doi.org/10.1038/srep44306 (2017).
Martiniakova, M. et al. Current knowledge of bone-derived factor osteocalcin: Its role in the management and treatment of diabetes mellitus, osteoporosis, osteopetrosis and inflammatory joint diseases. J. Mol. Med. (Berl). 102(4), 435–452. https://doi.org/10.1007/s00109-024-02418-8 (2024).
Sharma, V. C., Vidyasagar, S., Sukumar, C. A., Krishna, B. N. & Shree, S. Association between serum osteocalcin and atherosclerosis in Type-2 diabetes mellitus: A cross-sectional study. BMC Endocr. Disord. 23(1), 269. https://doi.org/10.1186/s12902-023-01462-8 (2023).
Seidu, S., Kunutsor, S. K. & Khunti, K. Association of circulating osteocalcin with cardiovascular disease and intermediate cardiovascular phenotypes: Systematic review and meta-analysis. Scand Cardiovasc. J. 53(6), 286–295. https://doi.org/10.1080/14017431.2019.1655166 (2019).
Zhang, X. L. et al. Low serum osteocalcin levels are correlated with left ventricular systolic dysfunction and cardiac death in Chinese men. Acta Pharmacol Sin. 40(4), 486–491. https://doi.org/10.1038/s41401-018-0080-0 (2019).
Mori, T. et al. Enhanced cardiac inflammation and fibrosis in ovariectomized hypertensive rats: A possible mechanism of diastolic dysfunction in postmenopausal women. Hypertens Res. 34(4), 496–502. https://doi.org/10.1038/hr.2010.261 (2011).
Apaijai, N. et al. Estrogen deprivation aggravates cardiac hypertrophy in nonobese type 2 diabetic Goto-Kakizaki (GK) rats. Biosci Rep. 37(5), BSR20170886. https://doi.org/10.1042/BSR20170886 (2017).
Veronese, N. et al. Relationship between low bone mineral density and fractures with incident cardiovascular disease: A systematic review and meta-analysis. J. Bone Miner. Res. 32(5), 1126–1135. https://doi.org/10.1002/jbmr.3089 (2017).
Magnus, J. H. & Broussard, D. L. Relationship between bone mineral density and myocardial infarction in US adults. Osteoporos Int. 16(12), 2053–2062. https://doi.org/10.1007/s00198-005-1999-9 (2005).
Frysz, M. et al. Bone mineral density is positively related to carotid intima-media thickness: Findings from a population-based study in adolescents and premenopausal women. J. Bone Miner. Res. 31(12), 2139–2148. https://doi.org/10.1002/jbmr.2903 (2016).
Li, X. S. et al. Bone mineral density is negatively associated with arterial stiffness in men with hypertension. J. Clin. Hypertens (Greenwich). 18(11), 1106–1111. https://doi.org/10.1111/jch.12848 (2016).
Tangen, J. et al. Left atrial volume assessed by echocardiography identifies patients with high risk of adverse outcome after acute myocardial infarction. Echo Res. Pract. 11(1), 24. https://doi.org/10.1186/s44156-024-00060-1 (2024).
Poulsen, M. K. et al. Left atrial volume index: Relation to long-term clinical outcome in type 2 diabetes. J. Am. Coll. Cardiol. 62(25), 2416–2421. https://doi.org/10.1016/j.jacc.2013.08.1622 (2013).
Biton, Y. et al. Relative wall thickness and the risk for ventricular tachyarrhythmias in patients with left ventricular dysfunction. J. Am. Coll. Cardiol. 67(3), 303–312. https://doi.org/10.1016/j.jacc.2015.10.076 (2016).
Li, L., Shigematsu, Y., Hamada, M. & Hiwada, K. Relative wall thickness is an independent predictor of left ventricular systolic and diastolic dysfunctions in essential hypertension. Hypertens Res. 24(5), 493–499. https://doi.org/10.1291/hypres.24.493 (2001).
Acknowledgements
The authors thank the staff and participants of the “Longitudinal Investigation of Osteoarthritis and Cardiovascular Health Status” cohort study for their contributions. Haoran Wang (washingtonhr@163.com) and Qiaotao Xie (18639998899@126.com) confirms compliance with authorship criteria and acknowledges receipt of written permissions for all contributions and disclosures.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81803318), the Henan Provincial Science and Technology Research Project (232300420069, 232102310231, 232300420289), and the Henan Ministry of Education (22B320003). The funders had no role in study design, data collection, analysis, interpretation, manuscript preparation, or the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
H.W. contributed to conception, data analysis, manuscript drafting/revision, statistical expertise, and funding acquisition; Q.W. participated in study design, data analysis, and manuscript editing from an orthopedic perspective; B.H., J.B., and J.C. were responsible for data collection/assembly; N.W., D.L., and J.W. provided data organization and logistical/administrative support; Q.X. involved in study design and manuscript review. H.W. and Q.X. confirm responsibility for the work’s integrity. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethical approval was obtained from the Institutional Review Board of Luohe Central Hospital on April 13th, 2023 (approval number 2023010). All participants provided written informed consent, and procedures adhered to the 2000 revision of the Helsinki Declaration.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, H., Wang, Q., He, B. et al. Osteoporosis and cardiac remodeling in middle-aged and older adults: a cross-sectional study. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37360-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37360-x