Abstract
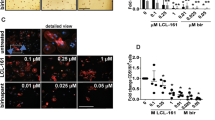
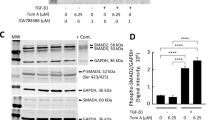
Abnormal scar formation is a clinical challenge with limited therapeutic options. Mechanical stimulation is implicated in abnormal scarring. Accordingly, the present study investigated the role of LIMK2, a component of the Rho/ROCK/LIMK/cofilin pathway, in cell differentiation and apoptosis in response to mechanical stimulation and proliferation in human dermal fibroblasts (HDFs). In normal HDFs, expression of α-smooth muscle actin (α-SMA), a marker of differentiation into myofibroblasts, significantly increased with mechanical stimulation; however, this change was not observed when LIMK2 was inactivated. Mechanical stimulation increased expression of the anti-apoptotic protein Bcl-2 and decreased that of the pro-apoptotic protein BAX in controls, but these effects were not observed with LIMK2 inactivation. Moreover, fibroblast proliferation was inhibited with LIMK2 inactivation. These findings suggest that LIMK2 inactivation suppresses mechanical stimulation-induced myofibroblast differentiation and resistance to apoptosis, and also inhibits HDF proliferation, highlighting LIMK2 as a potential therapeutic target for the prevention and treatment of abnormal scars.
Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Huang, J. et al. Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. Semin. Cell Dev. Biol. 128, 137–144 (2022).
Grinnell, F. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 124, 401–404 (1994).
Zhu, M. et al. A cyclical magneto-responsive massage dressing for wound healing. Small 20, 2400644 (2024).
Jeon, Y. R. et al. Antifibrotic effects of high-mobility group box 1 protein inhibitor (Glycyrrhizin) on keloid fibroblasts and keloid spheroids through reduction of autophagy and induction of apoptosis. Int. J. Mol. Sci. 20, 4134 (2019).
Lee, C.-C. et al. An updated review of the immunological mechanisms of keloid scars. Front. Immunol. 14, 1117630 (2023).
Nakazono-Kusaba, A., Takahashi-Yanaga, F., Morimoto, S., Furue, M. & Sasaguri, T. Staurosporine-induced cleavage of α-smooth muscle actin during myofibroblast apoptosis. J. Investig. Dermatol. 119, 1008–1013 (2002).
Hernández-Bule, M. L., Toledano-Macías, E., Pérez-González, L. A., Martínez-Pascual, M. A. & Fernández-Guarino, M. Anti-fibrotic effects of rf electric currents. Int. J. Mol. Sci. 24, 10986 (2023).
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C. & Brown, R. A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363 (2002).
Zhao, X.-H. et al. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J. Cell Sci. 120, 1801–1809 (2007).
Kuroda, K. et al. Altered actin dynamics is possibly implicated in the inhibition of mechanical stimulation-induced dermal fibroblast differentiation into myofibroblasts. Exp. Dermatol. 32, 2012–2022 (2023).
Huang, C. et al. Biological effects of cellular stretch on human dermal fibroblasts. J. Plast. Reconstr. Aesthet. Surg. 66, e351–e361 (2013).
Messadi, D. V. et al. Expression of apoptosis-associated genes by human dermal scar fibroblasts. Wound Repair Reg. 7, 511–517 (1999).
Kaneko-Kawano, T. & Suzuki, K. Mechanical stress regulates gene expression via Rho/Rho-kinase signaling pathway. JPFSM 4, 53–61 (2015).
Maekawa, M. et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285, 895–898 (1999).
Guan, G., Cannon, R. D., Coates, D. E. & Mei, L. Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components. Genes (Basel) 14, 272 (2023).
Rees, R. W. et al. Y-27632, a Rho-kinase inhibitor, inhibits proliferation and adrenergic contraction of prostatic smooth muscle cells. J. Urol. 170, 2517–2522 (2003).
Abe, H. et al. The Rho-kinase inhibitor HA-1077 suppresses proliferation/migration and induces apoptosis of urothelial cancer cells. BMC Cancer 14, 412 (2014).
Yang, X. et al. The Rho-kinase inhibitor inhibits proliferation and metastasis of small cell lung cancer. Biomed. Pharmacother. 66, 221–227 (2012).
Sumi, T., Matsumoto, K. & Nakamura, T. Specific Activation of LIM kinase 2 via Phosphorylation of Threonine 505 by ROCK, a Rho-dependent Protein Kinase *. J. Biol. Chem. 276, 670–676 (2001).
Gong, C. et al. LIM kinase 2 activates cardiac fibroblasts and exacerbates postinfarction left ventricular remodeling via crosstalk between the canonical and non-canonical Wnt pathways. Pharmacol. Res. 208, 107347 (2024).
Liu, L. et al. Asporin inhibits collagen matrix-mediated intercellular mechanocommunications between fibroblasts during keloid progression. FASEB J. 35, e21705 (2021).
Brissett, A. E. & Sherris, D. A. Scar contractures, hypertrophic scars, and keloids. Facial Plast. Surg. 17, 263–272 (2001).
Nobe, K. et al. Rho A and the Rho kinase pathway regulate fibroblast contraction: Enhanced contraction in constitutively active Rho A fibroblast cells. Biochem. Biophys. Res. Commun. 399, 292–299 (2010).
Fujiwara, T. et al. Direct contact of fibroblasts with neuronal processes promotes differentiation to myofibroblasts and induces contraction of collagen matrix in vitro. Wound Repair Regen. 21, 588–594 (2013).
Maharam, E. et al. Rho/Rock signal transduction pathway is required for MSC tenogenic differentiation. Bone Res. 3, 15015 (2015).
Lee, E. et al. Transplantation of cyclic stretched fibroblasts accelerates the wound-healing process in streptozotocin-induced diabetic mice. Cell Transplant 23, 285–301 (2014).
Hippenstiel, S. et al. Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 283, L830-838 (2002).
Shibata, R. et al. Rho-kinase inhibition reduces neointima formation after vascular injury by enhancing Bax expression and apoptosis. J. Cardiovasc. Pharmacol. 42, S43 (2003).
Del Re, D. P., Miyamoto, S. & Brown, J. H. RhoA/Rho Kinase Up-regulate Bax to Activate a Mitochondrial Death Pathway and Induce Cardiomyocyte Apoptosis*. J. Biol. Chem. 282, 8069–8078 (2007).
Chua, S. et al. Cyclic stretching boosts microRNA-499 to regulate Bcl-2 via microRNA-208a in atrial fibroblasts. J. Cell Mol. Med. 25, 3113–3123 (2021).
Wlodkowic, D., Telford, W., Skommer, J. & Darzynkiewicz, Z. Apoptosis and beyond: Cytometry in studies of programmed cell death. Methods Cell Biol. 103, 55–98 (2011).
Mardilovich, K. et al. LIM kinase inhibitors disrupt mitotic microtubule organization and impair tumor cell proliferation. Oncotarget 6, 38469–38486 (2015).
Li, R. et al. LIM kinase inhibition reduces breast cancer growth and invasiveness but systemic inhibition does not reduce metastasis in mice. Clin Exp Metastasis 30, 483–495 (2013).
Mardilovich, K. et al. Elevated LIM kinase 1 in nonmetastatic prostate cancer reflects its role in facilitating androgen receptor nuclear translocation. Mol. Cancer Ther. 14, 246–258 (2015).
Rybin, M. J. et al. A dual aurora and lim kinase inhibitor reduces glioblastoma proliferation and invasion. Bioorg. Med. Chem. Lett. 61, 128614 (2022).
Crane, A. M. & Bhattacharya, S. K. The Use of Bromodeoxyuridine Incorporation Assays to Assess Corneal Stem Cell Proliferation. In Corneal Regenerative Medicine: Methods and Protocols (eds Wright, B. & Connon, C. J.) 65–70 (Humana Press, 2013).
Huang, C., Akaishi, S. & Ogawa, R. Mechanosignaling pathways in cutaneous scarring. Arch. Dermatol. Res. 304, 589–597 (2012).
Chamseddin, B. H. & Le, L. Q. Management of cutaneous neurofibroma: current therapy and future directions. Neurooncol. Adv. 2, i107–i116 (2019).
Wang, Z.-C. et al. The roles of inflammation in keloid and hypertrophic scars. Front. Immunol. 11, 603187 (2020).
Limandjaja, G. C., Niessen, F. B., Scheper, R. J. & Gibbs, S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp. Dermatol. 30, 146–161 (2021).
Nishimura, K., Blume, P., Ohgi, S. & Sumpio, B. E. Effect of different frequencies of tensile strain on human dermal fibroblast proliferation and survival. Wound Repair Regen. 15, 646–656 (2007).
Santos, G. L., Hartmann, S., Zimmermann, W.-H., Ridley, A. & Lutz, S. Inhibition of Rho-associated kinases suppresses cardiac myofibroblast function in engineered connective and heart muscle tissues. J. Mol. Cell Cardiol. 134, 13–28 (2019).
Li, J. et al. Long-term explant culture: An improved method for consistently harvesting homogeneous populations of keloid fibroblasts. Bioengineered 13, 1565–1574 (2022).
Zhang, Q. et al. Collagen gel contraction assays: From modelling wound healing to quantifying cellular interactions with three-dimensional extracellular matrices. Eur. J. Cell Biol. 101, 151253 (2022).
Grada, A., Otero-Vinas, M., Prieto-Castrillo, F., Obagi, Z. & Falanga, V. Research techniques made simple: analysis of collective cell migration using the wound healing assay. J. Invest. Dermatol. 137, e11–e16 (2017).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers 18K19615, 20H03848, 22K09881, 23H03066 and 24K12848 and The Osaka Medical Research Foundation for Intractable Diseases Grant Numbers 29-1-18 and 30-2-35.
Author information
Authors and Affiliations
Contributions
The first and second authors equally contributed to this work. M.I., K.Ku., S.M. and T.K. designed, performed and interpreted all the experiments. K.Ku., K.Ka. and S.M. developed AAV vectors and performed experiments with the vectors. M.I., N.O., R.N. and T.O. performed mechanical stimulation experiments. K.Ka. and T.K. supervised and interpreted the data. M.I., K.Ku. and T.K. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ishii, M., Kuroda, K., Otani, N. et al. LIMK2 inactivation suppresses mechanical stimulation-induced dermal fibroblast differentiation and resistance to apoptosis. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37610-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37610-y