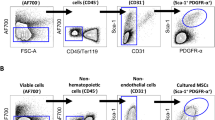
Abstract
Mesenchymal stem cells (MSCs), particularly those derived from bone marrow (BMMSCs), hold substantial promise for bone regeneration in the maxillofacial region, especially after surgical resections with bone involvement. However, their use in patients with resections undergoing oral cancer treatment poses potential risks due to the effects of MSCs in modulating cancer cell behavior. This study aimed to assess the effect of circulatory BMMSCs on proliferation, migration, invasion, tumor growth, and metastasis of oral squamous cell carcinoma (OSCC) cells. Human BMMSCs were isolated, characterized, and their conditioned medium (CM) was tested on OSCC cell lines (Ca1 and OSCC1). Further, BMMSCs were transduced with lentiviral particles to express firefly luciferase for live cell tracking in vivo to study their biodistribution and homing capability to xenograft tongue tumors. In vitro assays revealed that BMMSC-CM did not significantly alter OSCC proliferation or invasion in 3D organotypic assays, while significantly reducing their migration in 2D scratch wound assay. In vivo, bioluminescent imaging and histological analyses indicated that human BMMSCs predominantly localized to the lungs without homing to other organs or to the human xenograft tongue tumors. Moreover, circulatory BMMSCs did not influence tumor size, nor did they promote lung metastasis in xenografted mice under these conditions. These findings suggest that circulating BMMSCs did not exacerbate OSCC progression, supporting their potential use in regenerative applications for patients post-OSCC resection.
Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Kangari, P., Talaei-Khozani, T., Razeghian-Jahromi, I. & Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 11(1), 492 (2020).
Khorsand, A. et al. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J. Oral Implantol. 39(4), 433–443 (2013).
Wu, X. et al. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 11(1), 345 (2020).
Joshi, S. et al. Enhanced drug delivery system using mesenchymal stem cells and membrane-coated nanoparticles. Molecules 28(5), 2130 (2023).
Dongre, H. N. et al. Targeted next-generation sequencing of cancer-related genes in a Norwegian patient cohort with head and neck squamous cell carcinoma reveals novel actionable mutations and correlations with pathological parameters. Front. Oncol. 11, 734134 (2021).
Mendenhall, W. M. et al. Current role of radiotherapy in the management of oral cavity squamous cell carcinoma. Craniomaxillofac. Trauma Reconstr. 14(1), 79–83 (2020).
Bartold, M., Gronthos, S., Haynes, D. & Ivanovski, S. Mesenchymal stem cells and biologic factors leading to bone formation. J. Clin. Periodontol. 46(S21), 12–32 (2019).
Sybil, D., Jain, V., Mohanty, S. & Husain, S. A. Oral stem cells in intraoral bone formation. J. Oral Biosci. 62(1), 36–43 (2020).
Galland, S. & Stamenkovic, I. Mesenchymal stromal cells in cancer: a review of their immunomodulatory functions and dual effects on tumor progression. J. Pathol. 250(5), 555–572 (2020).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 74(3), 229–263 (2024).
Gjerde, C. et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 9(1), 213 (2018).
Mohamed-Ahmed, S. et al. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 9(1), 168 (2018).
Biddle, A. et al. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 71(15), 5317–5326 (2011).
Sapkota, D. et al. S100A14 inhibits proliferation of oral carcinoma derived cells through G1-arrest. Oral Oncol. 48(3), 219–225 (2012).
Costea, D. E., Kulasekara, K., Neppelberg, E., Johannessen, A. C. & Vintermyr, O. K. Species-specific fibroblasts required for triggering invasiveness of partially transformed oral keratinocytes. Am. J. Pathol. 168(6), 1889–1897 (2006).
Sapkota, D. et al. S100A16 promotes differentiation and contributes to a less aggressive tumor phenotype in oral squamous cell carcinoma. BMC Cancer 15, 631 (2015).
Dongre, H. et al. Establishment of a novel cancer cell line derived from vulvar carcinoma associated with lichen sclerosus exhibiting a fibroblast-dependent tumorigenic potential. Exp. Cell Res. 386(1), 111684 (2020).
Liu, C. et al. Bone marrow mesenchymal stem cells promote head and neck cancer progression through periostin-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer Sci. 109(3), 688–698 (2018).
Bruna, F., Plaza, A., Arango, M., Espinoza, I. & Conget, P. Systemically administered allogeneic mesenchymal stem cells do not aggravate the progression of precancerous lesions: A new biosafety insight. Stem Cell Res. Ther. 9(1), 137 (2018).
Zurmukhtashvili, M. et al. Mesenchymal stem cell transplantation attenuates growth of chemotherapy treated oral squamous cell carcinoma in an animal model. J. Oral Pathol. Med. 49(7), 655–664 (2020).
Yang, T., Tang, S., Peng, S. & Ding, G. The effects of mesenchymal stem cells on oral cancer and possible therapy regime. Front. Genet. 13, 949770 (2022).
Reagan, M. R. & Kaplan, D. L. Concise review: Mesenchymal stem cell tumor-homing: Detection methods in disease model systems. Stem Cells. 29(6), 920–927 (2011).
Kalimuthu, S. et al. Migration of mesenchymal stem cells to tumor xenograft models and in vitro drug delivery by doxorubicin. Int. J. Med. Sci. 15(10), 1051–1061 (2018).
Salo, S. et al. Human bone marrow mesenchymal stem cells induce collagen production and tongue cancer invasion. PLoS ONE 8(10), e77692 (2013).
Raj, A. T., Kheur, S., Bhonde, R., Gupta, A. A. & Patil, S. Assessing the effect of human mesenchymal stem cell-derived conditioned media on human cancer cell lines: A systematic review. Tissue Cell. 71, 101505 (2021).
Bagheri, R. et al. Conditioned media derived from mesenchymal stem cells induces apoptosis and decreases cell viability and proliferation in squamous carcinoma cell lines. Gene 782, 145542 (2021).
Li, Y. et al. Pre-metastatic niche: From revealing the molecular and cellular mechanisms to the clinical applications in breast cancer metastasis. Theranostics. 13(7), 2301–2318 (2023).
Sanchez-Diaz, M. et al. Biodistribution of mesenchymal stromal cells after administration in animal models and humans: A systematic review. J. Clin. Med. 10(13), 2925 (2021).
Eggenhofer, E. et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 3, 297 (2012).
Zhuang, W. Z. et al. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 28(1), 28 (2021).
Acknowledgements
The authors would like to acknowledge the Flow Cytometry Core Facility, Department of Clinical Science, University of Bergen for their assistance in acquisition.
Funding
Open access funding provided by University of Bergen. The authors declare that financial support was received from the Research Council of Norway through its Centers of Excellence funding scheme, (DEC and HND, Grant No. 22325 to Center of Excellency for Cancer Biomarkers CCBIO) and The Western Norway Regional Health Authority (DEC, Helse Vest Grant Nos 912260/2019 and F-13105/2024). The authors also acknowledge the Trond Mohn Foundation (KBEM, OsteoStem Grant No. TMS2021TMT08) for financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization, DS, HP, KM, DEC and HND; data curation, DS, HP, IEH, DK, LL, SMA and HND.; formal analysis, DS, HP and HND; funding acquisition, HR, KM, DEC and HND; investigation, DS, HP, IEH, DK, LL, SMA, DEC and HND.; methodology, DS, HP, DEC and HND.; project administration, HR, KM, DEC and HND.; supervision, HP, DEC and HND.; validation, DS, HP and HND; visualization, DS, HP and DEC; writing—original draft, DS and HND; writing—review & editing, DS, HP, HR, SMA, KM, DEC and HND. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Appropriate informed consent was obtained from all donors and the study was approved by the Regional Committee for Medical Research Ethics (REK), Norway (2013–1248/REK-sør-øst C and 2016-1266/REK-nord).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siyam, D., Parajuli, H., El herch, I. et al. Human bone marrow derived mesenchymal stem cells do not promote oral cancer cell growth in vitro and metastasis in vivo. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38370-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38370-5