Abstract
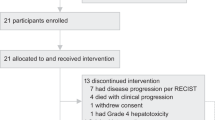
Clear cell renal cell carcinoma (ccRCC) remains a challenging malignancy to treat, with immune checkpoint inhibitors (ICIs) revolutionizing patient management. This pilot study, evaluated the efficacy and safety of combination therapy comprising camrelizumab, an anti-PD-1 antibody, and autologous cytokine-induced killer (CIK) cell therapy in patients with refractory ccRCC. Twenty-one patients with refractory ccRCC were randomly assigned to receive either camrelizumab monotherapy (control group, n = 12) or camrelizumab combined with CIK cell re-transfusion (trial group, n = 9). Due to early termination (21 of 60 planned patients), all endpoints were exploratory. The objective response rate (ORR) was numerically higher in the combination group (55.6% vs. 41.7%; odds ratio 1.75, 95% confidence interval [CI]: 0.32–9.51), but not statistically significant. Median progression-free survival (PFS) was 28.5 vs. 8.67 months (hazard ratio [HR] 0.40, 95% CI: 0.12–1.34), and median overall survival (OS) was not reached vs. 57.47 months (HR 0.48, 95% CI: 0.09–2.53). One patient in the trial group achieved a complete metabolic response (CMR). The combination was well-tolerated without new safety signals. Exploratory analysis suggested that higher baseline PD-1 expression on CD8+ T cells might be associated with a better response, and the frequency of PD-1 positive cells tended to decrease after camrelizumab administration. The addition of CIK cell therapy to anti-PD-1 antibody showed signals of potential benefit in refractory ccRCC with a tolerable safety profile. This pilot study suggests the combination approach appears feasible and warrants investigation in larger trials in pretreated ccRCC patients.
Registry: ClinicalTrials.gov, TRN: NCT03987698, Registration date: 17 June 2019.
Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article or its Supplementary Information.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Young, M. et al. Renal cell carcinoma. Lancet (London England). 404, 476–491. https://doi.org/10.1016/s0140-6736(24)00917-6 (2024).
Bukavina, L. et al. Epidemiology of renal cell carcinoma: 2022 update. Eur. Urol. 82, 529–542. https://doi.org/10.1016/j.eururo.2022.08.019 (2022).
Motzer, R. J. et al. Sunitinib versus interferon Alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 356, 115–124. https://doi.org/10.1056/NEJMoa065044 (2007).
Powles, T. et al. Pembrolizumab plus axitinib versus Sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 21, 1563–1573. https://doi.org/10.1016/s1470-2045(20)30436-8 (2020).
Plimack, E. R. et al. Pembrolizumab plus axitinib versus Sunitinib as First-line treatment of advanced renal cell carcinoma: 43-month Follow-up of the phase 3 KEYNOTE-426 study. Eur. Urol. 84, 449–454. https://doi.org/10.1016/j.eururo.2023.06.006 (2023).
Vuky, J. & Motzer, R. J. Cytokine therapy in renal cell cancer. Urol. Oncol. 5, 249–257. https://doi.org/10.1016/s1078-1439(00)00068-5 (2000).
Xu, W. et al. Heterogeneity in tertiary lymphoid structures predicts distinct prognosis and immune microenvironment characterizations of clear cell renal cell carcinoma. J. Immunother. Cancer. 11 https://doi.org/10.1136/jitc-2023-006667 (2023).
Braun, D. A. et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer cell. 39, 632–648e638. https://doi.org/10.1016/j.ccell.2021.02.013 (2021).
Hamid, O. & Carvajal, R. D. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin. Biol. Ther. 13, 847–861. https://doi.org/10.1517/14712598.2013.770836 (2013).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 Blockade. Proc. Natl. Acad. Sci. U.S.A. 99, 12293–12297. https://doi.org/10.1073/pnas.192461099 (2002).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced Renal-Cell carcinoma. N. Engl. J. Med. 373, 1803–1813. https://doi.org/10.1056/NEJMoa1510665 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 checkmate 025 trial. Cancer 126, 4156–4167. https://doi.org/10.1002/cncr.33033 (2020).
Motzer, R. et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 384, 1289–1300. https://doi.org/10.1056/NEJMoa2035716 (2021).
Powles, T. et al. Nivolumab plus Cabozantinib versus Sunitinib for first-line treatment of advanced renal cell carcinoma: extended follow-up from the phase III randomised checkmate 9ER trial. ESMO Open. 9, 102994. https://doi.org/10.1016/j.esmoop.2024.102994 (2024).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus Sunitinib in advanced Renal-Cell carcinoma. N. Engl. J. Med. 378, 1277–1290. https://doi.org/10.1056/NEJMoa1712126 (2018).
Schmidt-Wolf, I. G. et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp. Hematol. 21, 1673–1679 (1993).
Jiang, Y., Qiu, J., Ye, N. & Xu, Y. Current status of cytokine-induced killer cells and combination regimens in breast cancer. Front. Immunol. 16, 1476644. https://doi.org/10.3389/fimmu.2025.1476644 (2025).
Meng, Y. et al. Cell-based immunotherapy with cytokine-induced killer (CIK) cells: from Preparation and testing to clinical application. Hum. Vaccin Immunother.. 13, 1–9. https://doi.org/10.1080/21645515.2017.1285987 (2017).
Zhang, Q. et al. The dual-functional capability of cytokine-induced killer cells and application in tumor immunology. Hum. Immunol. 76, 385–391. https://doi.org/10.1016/j.humimm.2014.09.021 (2015).
Gao, X. et al. Cytokine-Induced killer cells as Pharmacological tools for cancer immunotherapy. Front. Immunol. 8, 774. https://doi.org/10.3389/fimmu.2017.00774 (2017).
Wang, Z. et al. Association of myeloid-derived suppressor cells and efficacy of cytokine-induced killer cell immunotherapy in metastatic renal cell carcinoma patients. J. Immunother. 37, 43–50. https://doi.org/10.1097/cji.0000000000000005 (2014).
Liu, L. et al. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin. Cancer Res. 18, 1751–1759. https://doi.org/10.1158/1078-0432.Ccr-11-2442 (2012).
Han, Y. et al. Autologous cytokine-induced killer (CIK) cells enhance the clinical response to PD-1 blocking antibodies in patients with advanced non-small cell lung cancer: A preliminary study. Thorac. Cancer. 12, 145–152. https://doi.org/10.1111/1759-7714.13731 (2021).
Zhou, L. et al. A phase IB trial of autologous Cytokine-Induced killer cells in combination with Sintilimab, monoclonal antibody against programmed cell Death-1, plus chemotherapy in patients with advanced Non-Small-Cell lung cancer. Clin. Lung Cancer. 23, 709–719. https://doi.org/10.1016/j.cllc.2022.07.009 (2022).
Motzer, R. J. et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J. Clin. Oncol. 22, 454–463. https://doi.org/10.1200/jco.2004.06.132 (2004).
Liu, L. et al. Randomized, multicenter, open-label trial of autologous cytokine-induced killer cell immunotherapy plus chemotherapy for squamous non-small-cell lung cancer: NCT01631357. Signal. Transduct. Target. Ther. 5, 244. https://doi.org/10.1038/s41392-020-00337-x (2020).
Hong, B. et al. Differential expression of PD-L1 between sporadic and VHL-Associated hereditary Clear-Cell renal cell carcinoma and its correlation with clinicopathological features. Clin. Genitourin. Cancer. 17, 97–104e101. https://doi.org/10.1016/j.clgc.2018.11.001 (2019).
Shen, M. et al. Association between PD-L1 expression and the prognosis and clinicopathologic features of renal cell carcinoma: A systematic review and Meta-Analysis. Urol. Int. 104, 533–541. https://doi.org/10.1159/000506296 (2020).
Donskov, F. et al. Molecular analysis and favorable clinical outcomes in real-world patients with metastatic renal cell carcinoma. Acta Oncol. 61, 1268–1277. https://doi.org/10.1080/0284186x.2022.2119100 (2022).
Yeong, J. et al. PD-L1 expression is an unfavourable prognostic indicator in Asian renal cell carcinomas. J. Clin. Pathol. 73, 463–469. https://doi.org/10.1136/jclinpath-2019-206092 (2020).
Sobottka, B. et al. Integrated analysis of immunotherapy treated clear cell renal cell carcinomas: an exploratory study. J. Immunother. 45, 35–42. https://doi.org/10.1097/cji.0000000000000387 (2022).
Maiorano, B. A. et al. Significance of PD-L1 in metastatic urothelial carcinoma treated with immune checkpoint inhibitors: A systematic review and Meta-Analysis. JAMA Netw. Open. 7, e241215. https://doi.org/10.1001/jamanetworkopen.2024.1215 (2024).
Velcheti, V. et al. 5-Year Real-World outcomes with frontline pembrolizumab monotherapy in PD-L1 Expression ≥ 50% advanced NSCLC. Clin. Lung Cancer. 25, 502–508e503. https://doi.org/10.1016/j.cllc.2024.05.002 (2024).
Zhu, Q. et al. PD-L1 expression patterns in tumour cells and their association with CD8(+) tumour infiltrating lymphocytes in clear cell renal cell carcinoma. J. Cancer. 10, 1154–1161. https://doi.org/10.7150/jca.29052 (2019).
Lee, K. S., Yun, S., Lee, K., Moon, S. & Choe, G. Clinicopathological implications of the expression of vascular endothelial growth factor and programmed death ligand 1 in clear-cell renal cell carcinoma. Hum. Pathol. 99, 88–97. https://doi.org/10.1016/j.humpath.2020.03.013 (2020).
Budimir, N., Thomas, G. D., Dolina, J. S. & Salek-Ardakani, S. Reversing T-cell exhaustion in cancer: lessons learned from PD-1/PD-L1 immune checkpoint Blockade. Cancer Immunol. Res. 10, 146–153. https://doi.org/10.1158/2326-6066.Cir-21-0515 (2022).
Carlisle, J. W. et al. Clinical outcome following checkpoint therapy in renal cell carcinoma is associated with a burst of activated CD8 T cells in blood. J. Immunother. Cancer. 10 https://doi.org/10.1136/jitc-2022-004803 (2022).
Liu, S., Sun, Q. & Ren, X. Novel strategies for cancer immunotherapy: counter-immunoediting therapy. J. Hematol. Oncol. 16 https://doi.org/10.1186/s13045-023-01430-8 (2023).
Tomita, Y. et al. Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup analysis from the checkmate 025 study. Jpn J. Clin. Oncol. 47, 639–646. https://doi.org/10.1093/jjco/hyx049 (2017).
Nishimura, R. et al. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood 112, 2563–2574. https://doi.org/10.1182/blood-2007-06-092817 (2008).
Cook, S. et al. Immune-Related adverse events and survival among patients with metastatic NSCLC treated with immune checkpoint inhibitors. JAMA Netw. Open. 7, e2352302. https://doi.org/10.1001/jamanetworkopen.2023.52302 (2024).
Infantino, V., Santarsiero, A., Convertini, P., Todisco, S. & Iacobazzi, V. Cancer cell metabolism in hypoxia: role of HIF-1 as key regulator and therapeutic target. Int. J. Mol. Sci. 22 https://doi.org/10.3390/ijms22115703 (2021).
Apte, R. S., Chen, D. S. & Ferrara, N. VEGF in signaling and disease: beyond discovery and development. Cell 176, 1248–1264. https://doi.org/10.1016/j.cell.2019.01.021 (2019).
Qian, C., Liu, C., Liu, W., Zhou, R. & Zhao, L. Targeting vascular normalization: a promising strategy to improve immune-vascular crosstalk in cancer immunotherapy. Front. Immunol. 14, 1291530. https://doi.org/10.3389/fimmu.2023.1291530 (2023).
Mellman, I., Chen, D. S., Powles, T. & Turley, S. J. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity 56, 2188–2205. https://doi.org/10.1016/j.immuni.2023.09.011 (2023).
Tian, L. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254. https://doi.org/10.1038/nature21724 (2017).
Tu, J. et al. Nintedanib enhances the efficacy of PD-L1 Blockade by upregulating MHC-I and PD-L1 expression in tumor cells. Theranostics 12, 747–766. https://doi.org/10.7150/thno.65828 (2022).
Allen, E. et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 9 https://doi.org/10.1126/scitranslmed.aak9679 (2017).
Choueiri, T. K. et al. Lenvatinib plus pembrolizumab versus Sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 24, 228–238. https://doi.org/10.1016/s1470-2045(23)00049-9 (2023).
Motzer, R. J. et al. NCCN Guidelines® insights: kidney Cancer, version 2.2024. J. Natl. Compr. Canc Netw. 22, 4–16. https://doi.org/10.6004/jnccn.2024.0008 (2024).
Acknowledgements
We are grateful to our coworkers for their contribution to the clinical management of the patients.
Funding
This work was funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009 A), The Science & Technology Development Fund of Tianjin Education Commission for Higher Education (2021KJ203), National Natural Science Foundation of China (82372779, 82373283, and 82302913), National Natural Science Foundation (NSFC) Cultivation Program of Tianjin Medical University Cancer Institute & Hospital (230103) and Doctor Startup Fund of Tianjin Medical University Cancer Institute and Hospital (B2414). This investigator-initiated trial funded by Tianjin Medical University Cancer Institute and Hospital. Drug supply: Camrelizumab was provided by Jiangsu Hengrui Medicine Co., Ltd. at no cost. Funder role: No involvement in study design, data collection, analysis, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
S. Li and J. Qin wrote the main manuscript text. Q. Sun and H. Zhao conducted quality control and visualization. Y. Xiong, Y. Wang, Y. Han, J. Zhang, W. Zhang, M. Shen, F. Yang, B. Ren, and L. Zhou conducted data investigation and curation. R. Li, Z. Hui, X. Tian, S. Cao, and W. Du contributed to data interpretation. W. Yu performed flow cytometry assessments. L. Liu, X. Zhang, and X.Ren conceptualized the study, reviewed, and revised the whole manuscript. All authors agreed on all aspects of the work and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Tianjin Medical University Cancer Hospital and Institute (No. E2017232). All patients provided written informed consent for their involvement and for the publication of the results.
Consent for publication
The authors affirm that participant provided informed consent for publication of the images in Fig. 5A, B and C and Figure S10.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Qin, J., Sun, Q. et al. Randomized pilot study of camrelizumab with or without autologous cytokine-induced killer cells in refractory clear cell renal cell carcinoma. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38881-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38881-1