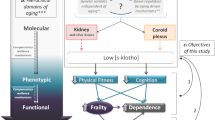
Abstract
Increasing aging populations globally have made maintaining a healthy life expectancy for older adults a critical issue. Older adults are particularly susceptible to frailty, physical and mental vulnerability caused by age-related decline in physiological and psychological resilience. Preventing its onset is essential for extending a healthy lifespan. Declines in antioxidant capacity contributes to frailty; therefore, administration of antioxidants may help prevent or treat this condition. Silicon (Si)-based agent reacts with water to produce hydrogen, which selectively scavenges deleterious reactive oxygen species. Oral Si-based agent administration can effectively alleviate symptoms in various disease models associated with oxidative stress, including Parkinson’s disease, ulcerative colitis, facial nerve palsy, and small intestinal ischemia–reperfusion injury. We investigated whether a Si-based agent could prevent or ameliorate frailty associated with aging using klotho mice, a premature aging model, and 105-week-old C57BL/6J mice. In klotho mice, the Si-based agent significantly alleviated age-related physical changes, including kyphosis and deterioration of coat conditions, and frailty-related somatic symptoms, including decreased spontaneous activity and motor function. Moreover, multicriteria frailty classification revealed fewer frail and pre-frail phenotypes in Si-based agent–treated klotho mice than in untreated mice. In aged C57BL/6J mice, the Si-based agent mitigated oxidative stress, suppressed motor performance and body weight decline, and reduced early mortality associated with age-related deterioration. Si-based agent may represent a potential strategy for combating frailty.
Similar content being viewed by others
Data availability
All relevant data are included within this paper.
References
Arai, H. Implication of frailty in elderly care. Japanese J. Geriatr. 51, 497–501 (2014).
Rockwood, K. & Howlett, S. E. Fifteen years of progress in Understanding frailty and health in aging. BMC Med. 16, 220. https://doi.org/10.1186/s12916-018-1223-3 (2018).
Fang, J. et al. Combining motivational and exercise intervention components to reverse pre-frailty and promote self-efficacy among community-dwelling pre-frail older adults: a randomized controlled trial. BMC Geriatr. 24, 896. https://doi.org/10.1186/s12877-024-05464-6 (2024).
Travers, J. et al. Building resilience and reversing frailty: a randomised controlled trial of a primary care intervention for older adults. Age Ageing https://doi.org/10.1093/ageing/afad012 (2023).
Yakabi, A. et al. A longitudinal study of frailty reversibility through a multi-component dementia prevention program. J. Phys. Ther. Sci. 37, 256–261. https://doi.org/10.1589/jpts.37.256 (2025).
Álvarez-Satta, M. et al. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging (Albany NY). 12, 9982–9999. https://doi.org/10.18632/aging.103295 (2020).
Tembo, M. C. et al. Total antioxidant capacity and frailty in older men. Am. J. Mens Health. 14, 1557988320946592. https://doi.org/10.1177/1557988320946592 (2020).
Soysal, P. et al. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 99, 66–72. https://doi.org/10.1016/j.maturitas.2017.01.006 (2017).
Kameda, M., Teruya, T., Yanagida, M. & Kondoh, H. Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proc. Natl. Acad. Sci. U S A. 117, 9483–9489. https://doi.org/10.1073/pnas.1920795117 (2020).
Viña, J., Borras, C. & Gomez-Cabrera, M. C. A free radical theory of frailty. Free Radic Biol. Med. 124, 358–363. https://doi.org/10.1016/j.freeradbiomed.2018.06.028 (2018).
Kim, J. et al. Au nanozyme-driven antioxidation for preventing frailty. Colloids Surf. B Biointerfaces. 189, 110839. https://doi.org/10.1016/j.colsurfb.2020.110839 (2020).
Wang, D. et al. Correction to: Antioxidant apigenin relieves Age-Related muscle atrophy by inhibiting oxidative stress and hyperactive mitophagy and apoptosis in skeletal muscle of mice. J. Gerontol. Biol. Sci. Med. Sci. 77, 1173. https://doi.org/10.1093/gerona/glac086 (2022).
El Assar, M. & Angulo, J. Rodríguez-Mañas, L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic Biol. Med. 149, 72–77. https://doi.org/10.1016/j.freeradbiomed.2019.08.011 (2020).
Kobayashi, Y., Matsuda, S., Imamura, K. & Kobayashi, H. Hydrogen generation by reaction of Si nanopowder with neutral water. J. Nanopart. Res. 19, 176. https://doi.org/10.1007/s11051-017-3873-z (2017).
Kobayashi, Y. et al. Renoprotective and neuroprotective effects of enteric hydrogen generation from Si-based agent. Sci. Rep. 10, 5859. https://doi.org/10.1038/s41598-020-62755-9 (2020).
Koyama, Y. et al. A new therapy against ulcerative colitis via the intestine and brain using the Si-based agent. Sci. Rep. 12, 9634. https://doi.org/10.1038/s41598-022-13655-7 (2022).
Koyama, Y. et al. Therapeutic strategy for facial paralysis based on the combined application of Si-based agent and methylcobalamin. Biochem. Biophys. Rep. 32, 101388. https://doi.org/10.1016/j.bbrep.2022.101388 (2022).
Shimada, M., Koyama, Y., Kobayashi, Y., Kobayashi, H. & Shimada, S. Effect of the new silicon-based agent on the symptoms of interstitial pneumonitis. Sci. Rep. 13, 5707. https://doi.org/10.1038/s41598-023-32745-8 (2023).
Shimada, M. et al. Si-based agent alleviated small bowel ischemia-reperfusion injury through antioxidant effects. Sci. Rep. 14, 4141. https://doi.org/10.1038/s41598-024-54542-7 (2024).
Harada, S. et al. Efficacy of the silicon based agent for age related decline in vestibular function. Sci. Rep. 15, 29790. https://doi.org/10.1038/s41598-025-14302-7 (2025).
Kuro-o, M. et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51. https://doi.org/10.1038/36285 (1997).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833. https://doi.org/10.1126/science.1112766 (2005).
Sahu, A. et al. Age-related declines in α-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 9, 4859. https://doi.org/10.1038/s41467-018-07253-3 (2018).
Baumann, C. W., Kwak, D. & Thompson, L. V. Assessing onset, prevalence and survival in mice using a frailty phenotype. Aging (Albany NY). 10, 4042–4053. https://doi.org/10.18632/aging.101692 (2018).
Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. Biol. Sci. Med. Sci. 56, M146–M156. https://doi.org/10.1093/gerona/56.3.M146 (2001).
Saio, V., Kharbuli, Z., Ratnakaran, N., Marquez, M. & Chakraborty, A. Amelioration of age-dependent increase in oxidative stress markers in male mice by extract of Potentilla fulgens. Redox Rep. 21, 130–138 (2016).
Lim, J., Luderer, U. & Lipshultz, L. I. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Aging Cell. 10, 278–291 (2011).
Nakatsukasa, T. et al. Pathological analysis of age-related bladder dysfunction. Kawasaki Med. J. 49, 57–67 (2023).
Yamamoto, M. et al. Regulation of oxidative stress by the anti-aging hormone Klotho. J. Biol. Chem. 280, 38029–38034. https://doi.org/10.1074/jbc.M509039200 (2005).
Hsieh, C. C., Kuro-o, M., Rosenblatt, K. P., Brobey, R. & Papaconstantinou, J. The ASK1-Signalosome regulates p38 MAPK activity in response to levels of endogenous oxidative stress in the Klotho mouse models of aging. Aging (Albany NY). 2, 597–611. https://doi.org/10.18632/aging.100194 (2010).
Li-Zhen, L., Chen, Z. C., Wang, S. S., Liu, W. B. & Zhuang, X. D. Klotho deficiency causes cardiac ageing by impairing autophagic and activating apoptotic activity. Eur. J. Pharmacol. 911, 174559. https://doi.org/10.1016/j.ejphar.2021.174559 (2021).
Nguyen, B. T. et al. Ginsenoside re attenuates memory impairments in aged Klotho deficient mice via interactive modulations of angiotensin II AT1 receptor, Nrf2 and GPx-1 gene. Free Radic Biol. Med. 189, 2–19. https://doi.org/10.1016/j.freeradbiomed.2022.07.003 (2022).
Moos, W. H. et al. Klotho Pathways, myelination Disorders, neurodegenerative Diseases, and epigenetic drugs. Biores Open. Access. 9, 94–105. https://doi.org/10.1089/biores.2020.0004 (2020).
Liguori, I. et al. Oxidative stress, aging, and diseases. Clin. Interv Aging. 13, 757–772. https://doi.org/10.2147/CIA.S158513 (2018).
Sahu, A. et al. Age-related declines in α-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 9, 4859. https://doi.org/10.1038/s41467-018-07253-3 (2018).
Nagai, T. et al. Cognition impairment in the genetic model of aging Klotho gene mutant mice: a role of oxidative stress. FASEB J. 17, 50–52. https://doi.org/10.1096/fj.02-0448fje (2003).
Shin, E. J. et al. Melatonin attenuates memory impairment induced by Klotho gene deficiency via interactive signaling between MT2 receptor, ERK, and Nrf2-related antioxidant potential. Int. J. Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyu105 (2014).
Nguyen, B. T. et al. Theanine attenuates memory impairments induced by Klotho gene depletion in mice. Food Funct. 10, 325–332. https://doi.org/10.1039/c8fo01577e (2019).
Bose, C. et al. Iron chelation prevents Age-Related skeletal muscle sarcopenia in Klotho gene mutant Mice, a genetic model of aging. J. Cachexia Sarcopenia Muscle. 16, e13678. https://doi.org/10.1002/jcsm.13678 (2025).
Ohsawa, I. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 13, 688–694. https://doi.org/10.1038/nm1577 (2007).
Bean, L. A. et al. Klotho deficiency promotes skeletal muscle weakness and is associated with impaired motor unit connectivity. Int. J. Mol. Sci. 26, 7986. https://doi.org/10.3390/ijms26167986 (2025).
Yuan, N. et al. Hearing analysis in heterozygous and homozygous Klotho gene-deficient mice. J. Otol. 13, 131–134. https://doi.org/10.1016/j.joto.2018.04.001 (2018).
Crawley, J. N. What’s Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice 2nd edn. (Wiley, 2007).
Whitehead, J. C. et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J. Gerontol. Biol. Sci. Med. Sci. 69, 621–632. https://doi.org/10.1093/gerona/glt136 (2014).
Kane, A. E. et al. Animal models of frailty: current applications in clinical research. Clin. Interv Aging. 11, 1519–1529. https://doi.org/10.2147/CIA.S105714 (2016).
Kane, A. E. et al. Impact of longevity interventions on a validated mouse clinical frailty index. J. Gerontol. Biol. Sci. Med. Sci. 71, 333–339. https://doi.org/10.1093/gerona/glu315 (2016).
Graber, T. G., Ferguson-Stegall, L., Liu, H. & Thompson, L. V. Voluntary aerobic exercise reverses frailty in old mice. J. Gerontol. Biol. Sci. Med. Sci. 70, 1045–1058. https://doi.org/10.1093/gerona/glu163 (2015).
Pajski, M. L. et al. Endurance exercise preserves physical function in adult and older male C57BL/6 mice: high intensity interval training (HIIT). Front. Aging. 5, 1356954. https://doi.org/10.3389/fragi.2024.1356954 (2024).
Bronikowski, A. M. et al. Lifelong voluntary exercise in the mouse prevents age-related alterations in gene expression in the heart. Physiol. Genomics. 12, 129–138. https://doi.org/10.1152/physiolgenomics.00082.2002 (2003).
Navarro, A., Gomez, C., López-Cepero, J. M. & Boveris, A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R505–511. https://doi.org/10.1152/ajpregu.00208.2003 (2004).
Garcia-Valles, R. et al. Life-long spontaneous exercise does not prolong lifespan but improves health span in mice. Longev. Healthspan. 2, 14. https://doi.org/10.1186/2046-2395-2-14 (2013).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256. https://doi.org/10.1038/s41591-018-0092-9 (2018).
Martin-Montalvo, A. et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192. https://doi.org/10.1038/ncomms3192 (2013).
Kane, A. E. et al. Long-term NMN treatment increases lifespan and healthspan in mice in a sex dependent manner. BioRxiv https://doi.org/10.1101/2024.06.21.599604 (2024).
Mills, K. F. et al. Long-Term administration of nicotinamide mononucleotide mitigates Age-Associated physiological decline in mice. Cell. Metab. 24, 795–806. https://doi.org/10.1016/j.cmet.2016.09.013 (2016).
Xun, Z. M. et al. Effects of long-term hydrogen intervention on the physiological function of rats. Sci. Rep. 10, 18509. https://doi.org/10.1038/s41598-020-75492-w (2020).
Conti, V. et al. Antioxidant supplementation in the treatment of aging-associated diseases. Front. Pharmacol. 7, 24. https://doi.org/10.3389/fphar.2016.00024 (2016).
Meulmeester, F. L. et al. Antioxidant supplementation in oxidative stress-related diseases: what have we learned from studies on alpha-tocopherol?. Antioxidants 11, 2322. https://doi.org/10.3390/antiox11122322 (2022).
Shen, H. W. et al. Humanized Transgenic mouse models for drug metabolism and Pharmacokinetic research. Curr. Drug Metab. 12, 997–1006. https://doi.org/10.2174/138920011798062265 (2011).
Lanske, B. & Razzaque, M. S. Premature aging in Klotho mutant mice: cause or consequence? Ageing Res. Rev. 6, 73–79. https://doi.org/10.1016/j.arr.2007.02.002 (2007).
Nabeshima, Y. Klotho deficient mouse: an in vivo model for human aging. Drug Discov Today Dis. Models. 1, 223–227. https://doi.org/10.1016/j.ddmod.2004.11.023 (2004).
Harada, S. et al. A mouse model of autoimmune inner ear disease without endolymphatic hydrops. Biochim. Biophys. Acta Mol. Basis Dis. 1870, 167198. https://doi.org/10.1016/j.bbadis.2024.167198 (2024).
Acknowledgements
This research was supported by the Center of Innovation Program (COI Program, Grant Number JPMJCE1310, JST Japan. We would like to thank Editage (www.editage.com) for English language editing, Nagahama Bio Science Laboratory belong to Oriental Yeast Co., Ltd. for the serum biochemical analyses, and the Center for Medical Research and Education, Graduate School for Medicine, Osaka University, for technical support.
Funding
This work was supported by Center of Innovation Program (COI Program) Grant Number JPMJCE1310, JST Japan.
Author information
Authors and Affiliations
Contributions
Yo.K. designed the study, analyzed the data, and wrote the paper. Yu.K. and H.K. developed the method for fabrication of Si-based agent. S.S. supervised this study and provided intellectual directions. All authors discussed the findings and commented on this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Koyama, Y., Kobayashi, Y., Kobayashi, H. et al. Efficacy of silicon-based agent against aging-related frailty. Sci Rep (2026). https://doi.org/10.1038/s41598-026-39711-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-39711-0