Abstract
Carbonyl sulfide (COS) is gaining interest as a proxy for gross primary productivity (GPP). Thinning of the Hyytiälä (Finland) forest in the winter of 2019–2020 altered the response of COS fluxes to environmental conditions in the summer of 2021. For the first time, extended periods of ecosystem-scale COS emissions were observed in a boreal forest. The warm and dry conditions in the summer of 2021 reduced the COS uptake by the canopy and elevated soil abiotic COS production. However, the reduction in canopy uptake and the increase in soil production do not fully explain the observed ecosystem-level emissions. The analysis suggests an unidentified, homogeneously distributed COS source in the eddy covariance footprint area, potentially from the photodegradation of forest floor litter and cutting residue from thinning. Such a source in a boreal forest stand warrants further source apportionment studies to effectively use COS as a proxy for GPP.
Similar content being viewed by others
Introduction
Carbonyl sulfide (COS) is an atmospheric tracer for ecosystem carbon and water fluxes because the diffusive pathway for COS is similar to that for carbon dioxide (CO2)1,2,3,4,5. COS, along with CO2, is taken up through the stomata to the chloroplast surface, where COS is irreversibly hydrolysed by carbonic anhydrase (CA)6,7,8. While CO2 is emitted back to the atmosphere during plant respiration, COS emission by leaves is not observed under ambient conditions7,9,10. The key assumption behind using COS as a proxy for ecosystem-level gross primary production (GPP) is that photosynthesising plants are a major terrestrial sink of COS.
In the boreal forest ecosystem, canopy uptake is typically the largest COS flux, even at night, followed by soil uptake11. The incomplete closure of stomata and the light-independent nature of CA lead to the nighttime uptake of COS12,13,14, which correlates with stomatal conductance11,15. During the day, stomatal conductance linearly increases with light intensity in a limited range until it plateaus, and COS uptake could be used to derive canopy and stomatal conductance, given that soil fluxes could be distinguished from separate measurements13,16,17.
In order to accurately use COS as a GPP proxy, all the non-stomatal sinks and sources have to be quantified. The low seasonal variation of COS soil fluxes in the boreal forest makes it easier to characterise the canopy uptake and account for ecosystem budgets and GPP estimates18. Soil COS chamber measurements at Hyytiälä forest station have shown an average flux of ~ −3 pmol m−2 s−1 during the growing season, with weak seasonal changes18. Soil uptake is linked to the presence of CA in microbial communities19,20, necessitating optimal soil temperature and moisture conditions for the maximal absorption of COS by soil18,21,22,23,24. The contribution of soil to the global COS sink is estimated to be 26–33%1,25 but is lower in boreal forests (10–20%)5,18.
Wetlands and anoxic soils are considered terrestrial sources of COS20,26,27,28, while oxic soils are generally inferred to be COS sinks from measurements18,19,29 and modelling studies24,30,31. However, exposure to high temperatures (above 15 °C) and high insolation in dry conditions can result in COS emissions from oxic soils14,21,32,33,34. Soil COS emissions are primarily linked to the oxic and abiotic degradation of organic matter within the soil34, and in the case of anoxic soils, redox reactions are important21,26. Furthermore, emission of COS due to exposure to UV radiation was shown to vary with the amount of soil organic matter34,35. Agriculturally managed temperate mountain grasslands turned into a net source of COS shortly after grass cutting, and the soil contribution could not explain the net ecosystem level emissions36. Commane et al.37 observed unexpected net COS emission over a mid-latitude forest site during the warmest summer weeks.
To better understand and quantify the source and sink processes in various ecosystems, and thereby improve the accuracy of GPP estimates, COS measurements in a variety of ecosystems at longer temporal scales are needed1,38,39,40. The bottom-up global estimate of COS is not closed, suggesting an unaccounted source of COS in the tropics41. Inverse modelling studies have also shown a missing source in the tropics and a missing sink in the high latitudes42,43,44. Parameterising seasonal and interannual variation of the boreal forest COS uptake in the Simple Biosphere Model Version 4 (SiB4) was able to match the missing COS sink to a certain limit in the high latitude, but the regional variation remains unknown5. A recent measurement has found boreal wetlands to be a sink for COS45, which was previously reported as a source4,26,27, underscoring the complexity of ecosystem processes in the global COS budget.
COS fluxes at the ecosystem level can be studied using the eddy covariance (EC) method at high temporal resolution. Several EC flux measurements of COS and attempts to estimate GPP from them have been reported in the literature5,13,37,46,47. The most extensive EC measurements of COS flux from a boreal forest were conducted at the Hyytiälä forest station, spanning over 32 months across five years from 2013 to 20175. This multi-year dataset showed that monthly median COS uptake varied by up to 26%. The main controlling bioclimatic factors of COS fluxes at the ecosystem level were photosynthetically active radiation (PAR), vapour pressure deficit (VPD), air temperature (Ta), and leaf area index (LAI).
In this study, we measured and analysed three more years (2020–2022) of COS flux measurements at the Hyytiälä forest station following a thinning conducted in 2019–2020 that removed ~ 40% basal area. Building on the well-studied COS dynamics of Hyytiälä forest station, we leveraged this unique ecosystem manipulation in the boreal forest to better understand the role of different ecosystem components in COS fluxes. Thinning altered the microclimate of the forest floor and created a large amount of cutting residue that was left on the site48. The post-thinning COS fluxes exhibited anomalous behaviour, and in the summer of 2021, we observed continuous and long-lasting ecosystem-level COS emissions from the boreal forest. Here, we quantify the emission of COS, identify the various potential sources of COS and understand the drivers that controlled the anomalous COS emission in the boreal forest ecosystem.
Results and discussions
Anomalous COS emissions and meteorological conditions
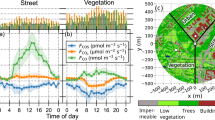
Thinning reduced forest canopy COS uptake, determined from the net ecosystem COS fluxes (FCOS) measured by EC. FCOS was ~19 % lower in 2020 (−19.6 ± 13.4 pmol m−2 s−1; mean ± standard deviation) and nearly 24 % lower in 2022 (−18.4 ± 13.1 pmol m−2 s−1) compared to the pre-thinning five-year (2013–17) average of −24.2 ± 13.3 pmol m−2 s−1. However, FCOS was about 75 % lower in 2021 (−6.0 ± 14.5 pmol m−2 s−1) due to anomalous COS emissions that were observed from June to mid-August and peaked in June and July (Fig. 1). Consequently, this study focuses on these months to understand and isolate the contributing conditions. The seasonal variation (FCOS) remained consistent in the post-thinning years, with maximum uptake in summer months and minimal uptake in winter months (Supplementary Fig. 1).
Mean diel variation of photosynthetically active radiation (PAR), air temperature average over heights of 16.8 m and 33.6 m (Ta), vapour pressure deficit (VPD), soil temperature (Ts) of horizon B1 (9–14 cm depth) and FCOS for each month. The black dashed line denotes the average, and the shaded region represents the interquartile range from 2013 to 2017. The blue circle, red cross and yellow triangle denote the post-thinning years 2020, 2021 and 2022, respectively.
Examining the environmental variables, June–July 2021 stands out as warmer and drier compared to other years (Fig. 1 and Supplementary Fig. 2). The monthly diel variation of Ta, VPD and soil temperature (Ts) shows that they stayed well above their interquartile range (IQR) from previous years. June 2020 was also warmer and drier than usual, but July and August returned to previous IQR values (Supplementary Fig. 2). PAR remained within average limits during the study period (Fig. 1 and Supplementary Fig. 2) and it peaked during June and July with values ~ 1700 μmol m−2s−1. Ta ranged from −24.7 °C to 30.9 °C during the 3 years of study, and the highest temperatures were measured during June 2021. Similarly, the highest VPD (3.18 kPa) and Ts (16.2 °C) were also measured in the same month. The forest thinning, which happened in January–March of 2020, opened the forest canopy crown, drastically increasing the light availability at the forest floor48. The surface layer soil temperature Ts,humus increased on average by 2 °C in the post-thinning summer months. The precipitation in June and July 2021 was lower than in previous years (Supplementary Fig. 2f), which, in combination with the higher Ts, led to a reduced soil water content (SWC) at the site (Supplementary Fig. 2e). In previous years, SWC peaked in April-May and the minimum occurred in August-September. In 2021, SWC was generally low between June and August, with the minimum in July (Supplementary Fig. 2e). The mean summer SWC was 0.20 ± 0.03 m3m−3 (mean ± standard deviation) during the study period, but the values dropped below 0.14 m3m−3 for a short period in the last 2 weeks of July 2021. However, the water potential at the mineral soil surface dropped to −0.5 MPa on 28 July 2021, which could be considered a mild drought49. In Hyytiälä, the forest generally acts as a sink for COS, with interannual variability dependent on environmental conditions. A reduction in uptake was reported in July 2014, when Ta and VPD were higher5. Similar COS emissions were observed in summer over Harvard Forest under high VPD and Ta conditions37.
To understand the ecosystem-level responses and isolate the meteorological influence on FCOS during June and July, FCOS is presented as a function of PAR, Ta and VPD (Fig. 2). The relationships between FCOS and environmental variables during 2020 and 2022 were similar to the pre-thinning years5, showing a decrease of COS uptake with an increase in Ta and VPD, and remained relatively stable under high PAR (>1200 μmol m−2s−1). In 2021, the responses were different, the uptake was lower and net emissions were observed at PAR > 1300 μmol m−2s−1, Ta > 24 °C and VPD > 1.8 kPa. A quadratic function was fitted to the FCOS responses (Supplementary Fig. 3), to determine if the meteorological responses differed significantly between years (p < 0.001). The functions were fitted separately for each year and then combined for all post-thinning years. The fitting coefficients for the quadratic functions are different for 2021 when compared to other years (Supplementary Table 1). The regression metrics (Supplementary Table 1) show that the year-specific model fits were able to explain the variance very well (R2: PAR = 0.94, Ta = 0.93 and VPD = 0.91) compared to one fit for all years (R2: PAR = 0.10, Ta = 0.44 and VPD = 0.39). The improved fit highlights that the interannual variation of the flux responses was significant (p < 0.001).
Binned median correlation between daytime FCOS and environmental variables (a PAR, b Ta and c VPD) during June–July for each year. The half-hourly flux data is sorted equally into ten decile bins, and the shaded regions represent the interquartile range. The number of data points in each bin is indicated in the corresponding panel. The blue circle, red cross, and yellow triangle denote the post-thinning years 2020, 2021 and 2022, respectively.
Reduction in canopy uptake during extreme conditions
The changes in the meteorological conditions could alter the canopy conductance (GC) and therefore the FCOS. The GC plotted against each season’s low, medium, and high percentiles of PAR from 2020 to 2022 (Fig. 3a) shows that the GC was slightly lower (~12 − 17%) in the 2021 summer. The COS uptake is proportional to the stomatal conductance11, which was observed for all the years, including 2021. The slope of the line fitted against FCOS and GC is similar in all the years (Fig. 3b), with only the intercept being positive in 2021. The intercept shows the FCOS when the canopy conductance is zero, i.e., the contribution from forest floor and soil. The positive intercept points towards an emission source, which offset the non-canopy sinks in 2021.
Sapflow upscaled canopy conductance (GC) and its relation with PAR and FCOS during June–July of each year. a Box-whisker plot for daytime GC in low (10–250 μmol m−2s−1), medium (250–750 μmol m−2s−1) and high (>750 μmol m−2s−1) PAR conditions. The blue, red and yellow-coloured boxes denote the years 2020, 2021 and 2022, respectively. The solid diamonds, circles and squares in each box represent the mean values for 2020, 2021 and 2022, respectively. b Binned median correlation between daytime FCOS and GC (solid lines). The half-hourly data is sorted equally into ten decile bins, and the number of data points in each bin is given. Linear regression between daytime FCOS and GC (dashed lines). The blue circle, red cross and yellow triangle with solid lines denote the years 2020, 2021 and 2022, respectively. The shaded region represents the interquartile range.
The influence of meteorological conditions on the reduced COS uptake can also be explored through a meteorologically-driven ecosystem COS flux model. The parameterisation for the daily scale net COS forest sink presented by Vesala et al.5 could be used to quantify the changes in the canopy uptake. The parameterisation considers PAR, Ta, VPD and LAI to estimate COS uptake, reflecting its sensitivity to stomatal conductance (R2 = 0.73, p < 0.001). The parameterisation scheme could closely follow the observed fluxes on a daily scale and effectively capture seasonal variations. In this study, the parameterisation was applied to the Hyytiälä forest stand, accounting for the thinning-related drop in LAI (Supplementary Fig. 4). The average parameterised canopy uptake for summer months was estimated to be −12.4 ± 4.1, −11.2 ± 2.8 and −13.7 ± 3.4 pmol m−2 s−1, for 2020, 2021 and 2022, respectively. The parameterisation generally underestimated the uptake compared to the EC measurements (2020: 34% and 2022: 20%), possibly due to the underrepresentation of forest floor vegetation in the LAI. However, in 2021, the parameterisation overestimated the COS uptake by a factor of two compared to EC measurements. The parameterisation fails to capture the seasonal pattern observed in 2021 (Supplementary Fig. 5). The changes in parameterised fluxes were observed each year, owing to changes in stomatal conductance and, thus, the daily canopy uptake. The average parameterised fluxes for the June-July months demonstrated a small reduction in canopy uptake in 2021 (~1 pmol m−2 s−1) compared to 2020 and 2022.
The parameterisation and the changes in the GC both showed that the uptake of COS has undergone slight changes (~5% and ~ 10%, respectively) over the years, which did not explain the observed emissions. When the parameterised COS fluxes are compared with the measured EC daily mean for the 2021 summer (Supplementary Table 2), there was an unaccounted ~ 8 pmol m−2 s−1 COS flux in the budget of this ecosystem.
Changes in soil fluxes
In the absence of direct soil chamber measurements in the post-thinning years (2020–22), a depth-resolved diffusion-reaction model (COS Soil Model, COSSM) was used to simulate soil COS fluxes from meteorological and soil physical inputs30. The thinning opened the canopy and warmed the forest floor compared to the pre-thinning years (pre-thinning Ts,humus: 12.3 ± 2.6 °C; post-thinning Ts,humus: 13.5 ± 2.2, 15.5 ± 2.7, 13.7 ± 2.6 °C during 2020, 2021, 2022, respectively) which can significantly influence the soil COS dynamics. The average modelled net soil COS fluxes for June-July were −2.3 ± 0.8, −1.7 ± 1.4 and −2.0 ± 1.0 pmol m−2 s−1, respectively, for 2020, 2021 and 2022. The higher temperature during the 2021 summer led to an increase in the soil COS production rates, thereby reducing the net soil fluxes (Supplementary Fig. 6). The modelled net soil fluxes were still low compared to total ecosystem-level fluxes, which were in the range of −3.7 ± 18.4 pmol m−2 s−1(FCOS, June-July: 2021). It could not account for the total COS emission observed in 2021.
The changes in the soil COS fluxes in response to the warmer forest floor alone cannot explain the observed emissions. The COSSM used the soil chamber dataset of Hyytiälä from the growing season of 201518 for fitting the model. The soil chamber measurements during 2015 were on bare soil without a moss or litter layer, and the temperature throughout the measurement was lower than 18.0 °C. Aslan et al.48 showed that in Hyytiälä, light penetration onto the forest floor was much lower in the pre-thinning years (~0.2) compared to post-thinning (~0.4). These factors could contribute to the uncertainty in model-predicted soil COS fluxes. Nevertheless, an increase in soil COS production is most likely in the post-thinning years. At high temperatures and radiation exposure, soils have been found to emit COS14,32,33. Soil dryness could also enhance abiotic COS production14,34. Wheat field soil shifted from consumption to production in the dark at high (>25 °C) temperatures, and sterilised soil shifts from uptake to emissions in the dark measured at a temperature of 19 °C33. Sulfur-abundant agricultural soils emitted COS at ~ 20 °C in dark conditions34. Bryophytes turned from a sink of COS in the dark to a source in light conditions50. Even though other ecosystems have demonstrated COS soil production, it is unlikely that the soil alone could explain the ecosystem-level emissions at Hyytiälä.
Spielmann et al.36 have shown that COS emissions from mountain grasslands after harvest hint towards possible COS sources from dead plant matter degradation. Meredith et al.34 found that the microbial cycling of sulfur-containing amino acids, which are COS precursors, was the underlying cause of COS soil fluxes in soil samples from 58 sites. Their study further found that the increase in temperature could explain the apparent light effect, and maximum emissions were observed in boreal peatland soils. The exposure of litter to UV radiation has been found to increase the photodegradation by direct photolysis and also facilitate the microbial degradation by making the organic compounds bioavailable51,52. Solar radiation can also cause thermal and photodegradation of COS precursors analogous to the processes observed in marine ecosystems53,54. Differentiating the direct photolysis and microbial activity is difficult, but UV exposure has consistently enhanced COS emissions or decreased uptake by soil and litter samples, particularly those with high organic matter content55. COS photoproduction from the soil and litter layer was omitted in the COSSM soil model, which could contribute to the observed anomalous emissions.
Nighttime fluxes
The light independence of plant CA enzyme and incomplete closure of leaf stomata lead to nighttime canopy uptake of COS11,14. Soil chamber measurements from Hyytiälä have shown that nighttime soil uptake was −2.7 pmol m−2 s−1 during the measurement in the 2015 growing season18. The forest was a nighttime COS sink (−12.4 ± 10.6 pmol m−2 s−1) during the 2013–2017 measurement period. The mean nighttime uptake during 2021 (−3.2 ± 14.4 pmol m−2 s−1) was less than other years (2020: −10.7 ± 11.7 pmol m−2 s−1 and 2022: −9.0 ± 14.4 pmol m−2 s−1), demonstrating reduced ecosystem uptake. Kooijmans et al.11 have shown that the nighttime uptake of COS is controlled by stomatal uptake and reduces with an increase in Ta and VPD. The relation of nighttime FCOS to Ta and VPD (Supplementary Fig. 7) shows this dependence across all years, i.e., reduction of COS uptake as Ta and VPD increase. Even though the median COS nighttime uptake in 2021 was smaller than in other years, uptake still happened at night. The higher average nighttime Ta reduced the stomatal conductance, which would partially explain the reduction in nighttime COS uptake. However, in similar Ta and VPD ranges, the COS uptake was lower in 2021. COS emissions from litter due to the reminiscent heat on the forest floor could not be ruled out, as small emissions were observed at higher temperature conditions at night. Therefore, the emission mechanism could have a thermal component along with a photolytic component that was more active in the daytime.
COS emission from a disturbed forest stand
The thinning in 2020 disturbed the ecosystem, reduced the GPP by 20% compared to the long-term mean and made it a net source of CO248. The forest stand was able to recover to long-term mean values by 202248. The recovery of the forest was reflected in the response of GPP estimated by partitioning the NEE (GPPNEE), as shown in Fig. 4a. Further, we compare the response of GPP from COS (GPPCOS) in the post-thinning years with the pre-thinning years (Fig. 4b). The responses were noisier than GPPNEE, and the recovery of the forest from the thinning was not visible. The GPPCOS during the year 2022 was lower than in 2020, which is clearly not the case in GPPNEE. Generally, GPPCOS overestimates the GPP compared to GPPNEE3,47, but in 2021, GPPCOS deviated from the normal response of the ecosystem and clearly underestimates GPPNEE.
a Binned median correlation between daytime GPPNEE and environmental variables (PAR, Ta and VPD) during June-July for each year. The half-hourly data is sorted equally into ten decile bins, and the number of data points in each bin is given. b same as a but for GPPCOS. The black square, blue circle, red cross and yellow triangle denote the years 2013–17, 2020, 2021 and 2022, respectively. The shaded region represents the interquartile range.
The disturbance in the boreal forest stand has disrupted the COS budget with an unidentified and potentially significant emission source. In order to estimate the emission in the 2021 summer, the COS uptake by the forest has to be quantified indirectly. The COS uptake by the forest stand can be quantified by inverting the COS-GPP relation (FCOS,GPP)2. Assuming that the forest was the major sink, the difference between the FCOS and COS uptake thus gives the COS emissions (equation (11), Supplementary Fig. 8, Fems:COS,GPP). The mean estimated COS emissions Fems:COS,GPP thus estimated were −6.0 ± 14.4, 23.3 ± 17.5 and 2.7 ± 14.4 pmol m−2 s−1, respectively, in 2020, 2021 and 2022 for the June-July months. This partitioning method for COS had its uncertainties associated with using GPPNEE, LRU and nighttime COS uptake. The emission in 2020 was negative since FCOS,GPP was lower than FCOS, i.e., there was an extra COS sink in 2020 that could not be accounted for by the GPP in that year. Another method to estimate COS uptake is to assume a linear relation between GC and FCOS and use the slope during 2020 and 2022 to estimate the COS uptake in 2021 FCOS,GC. The difference between FCOS,GC and FCOS (equation (12), Supplementary Fig. 8) would give us an estimate of COS emission (Fems:COS,GC: −0.8 ± 12.9, 12.2 ± 14.3 and 0.8 ± 12.5 pmol m−2 s−1 for the years 2020, 2021 and 2022, respectively). FCOS,GC considers canopy-level conductance upscaled from a few tree samples in the forest stand and completely ignores the subcanopy vegetation and soil uptake of COS fluxes (Supplementary Fig. 8). However, FCOS,GC appear closer to FCOS in 2020 and 2022 (Supplementary Table 2). Even though both estimation methods have their own uncertainties, they showed COS emission in 2021 that overwhelmed the average uptake of COS in June–July. The COS atmospheric concentrations (Supplementary Fig. 9) were high during June-July of 2021 (396.6 ± 28.9) compared to the other two years (2020: 281.7 ± 39.4 and 2022: 307.1 ± 37.6). The leaf and soil COS uptake are concentration-dependent39; therefore, the estimated emissions could be underestimated.
Despite the aforementioned uncertainties, the main drivers for Fems:COS,GPP and Fems:COS,GC could be clearly identified (Supplementary Fig. 10). The correlation was significant only in the 2021 summer, as the Fems:COS,GPP and Fems:COS,GC are close to zero in 2020 and 2022. The univariate analysis against PAR, Ta, VPD, Ts,humus and surface layer soil water content (SWChumus), shows that Ta and Ts,humus and PAR primarily drive the emissions in both daily and weekly scales. Various combinations of the drivers, avoiding intercorrelation, were used as drivers in the multivariate analysis. A combination of PAR + Ta and PAR + Ts,humus was able to best explain the emissions. It is likely that the emissions were driven by a photothermal component, and we are unsure if it is the temperature that drives the effect of radiation or vice versa.
The nature of the emission sources, whether they are occurring in hotspots or spread evenly through the footprint of the EC measurements, could be inferred from a flux-weighted map of positive FCOS (Supplementary Fig. 11) and weighted mean polar plots (Supplementary Fig. 12). Both analyses point towards the unknown COS source distributed heterogeneously within the footprint area. Thinning in the Hyytiälä forest stand has opened up the canopy and doubled the amount of radiation reaching the forest floor (PARff in pre-thinning years: 100.2 ± 112.9 μmol m−2s−1 to 242.8 ± 245.8 μmol m−2s−1 in 2020). The forest floor vegetation recovered steadily in 2021 and 2022, as demonstrated by the forest floor GPP48. The forest floor (Rff) and ecosystem (Reco) respiration fluxes during June were higher in 2021 compared to 2020 and 2022, which was evident from the nighttime NEE fluxes (Supplementary Fig. 13) and modelled respiration (Supplementary Fig. 14). The total respiration for the entire growing season in 2021 was lower than in 2020 or 202248, but the warmer June-July months have seemingly spiked the ecosystem respiration in 2021 (Supplementary Fig. 14). A similar pattern can be found in the forest floor NEE (Supplementary Fig. 14).
The presence of S-containing amino acids (SAAs) in soil was found to be a potential precursor of COS34,56. These SAAs could be introduced into the soil through the excess litter left after the thinning. The amount of leaf litter of various species (Supplementary Table 4) over the years and the estimate of S content57,58,59,60 in them can give a rough estimate of available S in the soil. The yearly S-content from litter was 0.41 ± 0.1, 0.46 ± 0.1 and 0.44 ± 0.1 g S m−2 in 2020, 2021 and 2022, respectively, which may drive the observed COS emissions.
The COS emission during the senescing period of wheat soil was associated with the mobilisation of SAA and its metabolism14. It should be noted that the surface moss layer in Hyytiälä was considered a COS sink18, due to the consumption of COS by CA enzymes in mosses. The thinning resulted in ~120 g C m−2 decomposable fine-cutting residue, including leaves and needles. Aslan et al.48 estimated that ~50% of the residue already decomposed in 2020–2022 post-thinning years, assuming an exponential decomposition rate61. An initial delay in the decomposition is possible, as the cutting residue was relatively intact and was not incorporated into the litter layer, as it was not compacted by snow right after the thinning in winter 2020. The cutting residue was partially degraded over the following winter and was more susceptible to photothermal degradation over the summer of 2021. The decomposition rate depends on environmental variables such as soil temperature, which was above the seasonal averages, especially in the summer of 2021. The combined effect of the delay in the decomposition of pine needles and the higher temperature possibly resulted in elevated decomposition rates in the 2021 summer. Consequently, the elevated decomposition likely contributed to COS emission from the forest floor.
The source of the COS emission observed in summer 2021 can only be speculated at this point. The global COS budget remains unbalanced, and we have demonstrated that the boreal forest could be a source when disturbed. A complete understanding of the COS sources and sinks is necessary for its effective use as a GPP tracer. The continuous observations from the forest floor in a disturbed forest stand could advance our understanding of COS fluxes. Identifying and quantifying this newly observed yet unknown source is required to close the COS budget, reducing a key uncertainty in using COS as a tracer for global photosynthesis. This improvement will help refine carbon cycle models and enhance our ability to monitor and predict ecosystem responses to climate change, with implications for science and policy.
Methods
Site description and thinning
The flux measurements from the SMEAR II station in Hyytiälä, Finland (61°51’N, 24°17’E; 181 m a.s.l.) were used in this study. The forest stand is dominated by 50–60-year-old Scots pine (Pinus sylvestris L.) and consists of other tree species, including Norway spruce (Picea abies (L.) Karst.) and deciduous trees (e.g., Betula sp., Populus tremula, and Sorbus aucuparia). Understorey vegetation is characterised by dominant shrub species, notably lingonberry (Vaccinium vitis-idaea L.) and bilberry (Vaccinium myrtillus L.), and prominent mosses such as Schreber’s big red stem moss (Pleurozium schreberi (Brid.) Mitt.)62. In 2018, the basal area was 30.7 m2 ha−1, basal area weighted mean canopy height was 19.9 m, one-sided leaf area index (LAI) 3.9 m2 m−2, and stem density 1141 stems ha−1. The stand was thinned in two phases, i.e., understorey removal and main thinning in March–April 2019 and January–March 2020 (Supplementary Fig. 15), respectively, reducing the basal area by ~ 40%, and foliage by ~ 45%, stem density by ~ 60%48,63. Post-thinning measurements indicated a basal area of 18.0 m2 ha−1, LAI of 2.1 m2 m−2, and stem density of 465 stems ha−1. The species composition remained unchanged after the thinning, as the thinning ratio was almost uniform. Due to canopy opening, light availability almost doubled at the forest floor, and wind speed and daytime air temperature increased at the trunk space in 2020 compared to pre-thinning years48. The daytime duration varied greatly over the months, from ~19.5 h in summer to ~8.3 h in winter. All results were presented in Finnish winter time (UTC + 2). Daytime is defined as the solar elevation angle greater than 0 degrees, and nighttime is less than 0 degrees.
EC and other measurements
FCOS were measured during 2020–2022 at the height of 23 m, which consists of an ultrasonic anemometer (uSonic-3 Class A, METEK Meteorologische Messtechnik GmbH, Elmshorn, Germany) for measuring wind speed in three dimensions and sonic temperature; an Aerodyne quantum cascade laser spectrometer (QCLS; Aerodyne Research Inc., Billerica, MA, USA) for measuring COS, CO2, carbon monoxide (CO) and water vapour (H2O) mole fractions. The processing and gap-filling of the COS fluxes were described by Kohonen et al.64. The setup was similar to that reported by Vesala et al.5, which used the data from 2013–17 from Kohonen, K.-M. & Kooijmans, L.65.
The EC data were processed using the EddyUH software66 following the procedure recommended by Kohonen et al.64 for COS flux processing. Raw data were despiked so that the difference between subsequent 10 Hz data points was a maximum of 200 ppt for the COS mixing ratio and 5 m s−1 for the vertical wind velocity component (w). The coordinate system was set using a 2D-coordinate rotation, and linear detrending was used to separate the time series into mean and fluctuating components. The time lag of COS was determined by maximising the cross-covariance between CO2 mixing ratio and w. High-frequency spectral losses were corrected using the experimental approach67 according to Mammarella et al.68 so that the response time of CO2 was used for COS spectral correction. Low-frequency correction was done according to Rannik and Vesala69. Finally, only 60% of all the half-hourly fluxes that met the following criteria were accepted: the number of spikes in the 30-minute COS mixing ratio and w less than 100, the second wind rotation angle less than 10 degrees in absolute value, flux stationarity less than 0.3, kurtosis between 1 and 8, and skewness between −2 and 2 (Flag 0 and 1 in Supplementary Fig. 16).
The net ecosystem exchange of CO2 was separately measured from above the forest canopy (NEEeco) and at the forest floor (NEEff) using EC. The NEEeco was measured at a height of 27 m above the ground using a closed-path nondispersive infrared sensor (LI-7200, LI-COR Biosciences, USA) paired with an ultrasonic three-dimensional anemometer (HS-50, Gill Ltd, UK) measuring at 10 Hz. This setup is part of the Integrated Carbon Observation System (ICOS) Ecosystem Station network70,71 and the measurements are according to the ICOS recommendations72. (NEEff) was measured at 2.4 m height with an EC system that comprised of a closed-path nondispersive infrared sensor (LI-7200, LI-COR Biosciences, USA) along with a three-dimensional ultrasonic anemometer (model USA-1; METEK GmbH, Elmshorn, Germany). Gas sampling was conducted using an Eaton Synflex tube, with diameters of 6 mm (0.35 m length) and 12.5 mm (1.67 m length), at a flow rate of 12 LPM.
The temperature was measured at two heights, 16.8 m and 33.6 m, using Pt100 sensors inside ventilated radiation shields. Similarly, RH was measured at 16.8 and 35 m heights using Rotronic MP102H RH sensors. The average of the measurements at these two heights was used to represent the conditions at the flux measurement height of 23 m. Photosynthetically active radiation (PAR) was measured at 35 m height using a LI-COR LI-190SZ quantum sensor. Ts was measured at five locations in the following depths: organic layer(soil surface), A1 (2–5 cm), B1 horizon (9–14 cm depth), B2 horizon (22–29 cm) and C horizon (42–58 cm) of the forest floor using Philips KTY81-110 temperature sensors. Soil water content (SWC) was also measured using a Delta-T ML3 sensor at 4 locations at above mentioned depths. The all-sided leaf area index (LAI) was measured for a three-day interval using an array of four under-canopy PAR measurement sensors in four different plots by inverting the Beer-Lambert equation (Supplementary Fig. 4). The average of all the sensors was fitted to a bell curve using the scipy.optimize.curve_fit73 package, and the wintertime LAI was interpolated (October-March).
Sap flow density was measured with constant heat-dissipation probes74 in eight Scots pine and four silver birch trees. Sensors were installed at breast height on the northern side of the stem, with the reference and heated needles 10 cm apart. The sensors were covered with a reflective aluminium shelter to prevent temperature artefacts. Data were recorded every minute for trees on an RMD680 channel transmitter (Nokeval, Nokeval Oy, Nokia, Finland). Data were averaged over 15-minute periods, and sap flow density was then calculated according to the original calibration74. Maximum temperature difference under zero flow conditions was estimated according to Oishi et al.75 with the following thresholds: nighttime conditions (PAR < 50 μmol m−2 s−1), low vapour pressure deficit (<0.2 kPa) and a stable temperature difference (coefficient of variation < 0.5% over two hours period). When those conditions were not met, the maximum temperature difference under zero-flow conditions was linearly interpolated from neighbouring days.
Sapflow upscaled stomatal conductance
The sap flow measurements from twelve trees (eight Scots pine and four birches) distributed around the forest stand are used to estimate the transpiration and upscale to the ecosystem level. The diameter class technique for upscaling the sap flow observations is utilised, which is based on the assumption that sap flow rates depend only on the diameter at the breast height (DBH) of the tree76,77,78. The DBH distribution for different species of trees per hectare in the sampled area is given in Supplementary Fig. 17. An exponential function is fitted to the relationship between sap flow rates (Qtree) of the sampled trees and their DBH78,79 for Scots pine and birches separately. The coefficients from the exponential function fit are used to obtain the average sap flow for all given tree DBH classes. Further, sap flow for all the DBH classes was aggregated accordingly for sample areas (on a scale of 1 ha) and divided by the average sap flow for sample trees measured within the sample areas. This allowed us to obtain a specific scaling factor (Fs) for sap flow measurements at thinned and control areas, transforming the measurements performed on sample trees to an ecosystem scale. The stand transpiration (TSF) for sample areas was obtained equation (1).
The stomatal conductance upscaled from sapflow was calculated by dividing TSF by VPD (equation (2)).
Upscaling sap flow measurements from tree level to ecosystem level is challenging and brings uncertainty to achieved results78,80. The comparison of upscaled sap flow measurements, which on a daily or longer time scale represent transpiration with fluxes measured by the EC system, is affected by the footprint dimension and tree distribution within the fetch area. It has also been reported that the uncertainty of upscaled sap flow measurements could also result from the systematic underestimation of sap flux by the sensors (especially thermal dissipation probes, operated according to the constant power principle81,82, but also for the Tissue Heat Balance method78). Upscaling the sap flow measurements is more challenging for mixed forests than monocultures, which also causes uncertainties80. Nevertheless, it is a known and widely used method for assessing transpiration at the ecosystem level based on point sap flow measurement in the tree scale.
COS uptake parameterisation
The COS fluxes from the Hyytiälä forest stand were parameterised using the PAR, Ta, VPD, and all-sided LAI from the long time series data shown by Vesala et al.5. The LAI was reduced by almost 40% after the thinning in 2020. The parameterisation of COS flux uptake is given as equation (3).
The four functions for the parameterisation and the parameter values were taken from5, where FPAR is the function of stomatal response to PAR; FS is the phenology of biochemical reactions; FVPD gives the stomatal regulation, and FLAI is the function of foliage and canopy light penetration (Supplementary Fig. 18). The functions and parameterisation description can be found in Vesala et al.5. The parameterised COS fluxes are plotted against the measured EC COS fluxes in Supplementary Fig. 5.
Mechanistic soil COS flux model
We used a depth-resolved diffusion-reaction model (COS Soil Model, COSSM) to simulate soil COS fluxes from meteorological and soil physical inputs30. The model represents both abiotic COS source and enzymatic COS uptake activities and evolves the COS concentration profile prognostically to solve for soil-atmosphere COS fluxes. Here, key improvements have been made for simulating soil COS fluxes at Hyytiälä. Firstly, the model framework has been reimplemented in a differentiable manner using the JAX library in Python83, which facilitates parameter optimisation against soil chamber observations and improves computational efficiency through just-in-time compilation. Secondly, the vertical discretisation of soil layers now follows the Community Land Model version 5 (CLM5)84, with 10 layers of increasing thickness down to 1.5 m depth, fewer than the 25 layers in the original model30. The reduced total number of soil layers and tightly packed soil layers near the surface make simulations faster without aliasing the COS concentration profile.
We used chamber measurements of soil COS fluxes at Hyytiälä in 201518 to parameterise the model. Between the two soil chambers in 2015, we used data from soil chamber #1 (SC1) as the training set to optimise COS metabolic parameters and data from soil chamber #2 (SC2) as the test set to assess model generalisability. The parameters that were optimised include COS production capacity (Vmax,P), temperature sensitivity of COS production as indicated by Q10, carbonic anhydrase (CA) COS uptake capacity (Vmax,CA), Gibbs free energy, enthalphy change, and entropy change associated with enzyme activation/deactivation (ΔGa, ΔHd, and ΔSd), and the optimal soil moisture for COS uptake (θopt). The Michaelis constant for CA-mediated COS uptake (Km) is fixed at 0.039 mol m−3 following the experimental value for β-CA7,24. See Supplementary Table 5 for prior and optimised parameter values. The optimised model achieved a root-mean-square error (RMSE) of 1.12 pmol m−2 s−1 on the training set and an RMSE of 1.53 pmol m−2 s−1 on the test set for hourly flux measurements and better performance on daily fluxes (Supplementary Fig. 19). The reduced performance on SC2 data is not surprising given that the root-mean-square deviation between quasi-concurrent SC1 and SC2 flux measurements is 1.50 pmol m−2s−1, reflecting small-scale heterogeneity.
We then used meteorological and soil physical observations at Hyytiälä during 2013–2022 to drive the optimised model for half-hourly soil COS flux simulations. June–July soil COS fluxes for 2013–2017 and 2020–2022 are summarised in Supplementary Fig. 6.
GPP estimate from COS
The measured EC COS fluxes comprise both vegetation and soil flux components. There were no COS soil chamber measurements during the 2020–2022 period. Hence, average COS soil flux (−2.7 pmol m−2s−1) measured by Sun et al.18 from 2015 in Hyytiälä was used for this analysis. The soil contribution was subtracted from the total EC COS fluxes to estimate the vegetation contribution (FCOS,canopy) and the GPP from COS fluxes (GPPCOS) was estimated using the method described by Sandoval-Soto et al.2 (equation (4)).
where \({[C{O}_{2}]}_{a}\) and [COS]a are mixing ratio of CO2 and COS measured by the QCL spectrometer. The leaf relative uptake (LRU) was estimated as a function of PAR (equation (5))3.
GPP from partitioning of NEE
The GPPNEE was estimated by traditional NEE flux partitioning (equation (6)) :
where R was estimated from the nighttime NEE fluxes (equation (7)).
where Rc is the respiration at a reference temperature (T = 10 °C) and Q10 is the temperature sensitivity at which the R increases for every 10 °C and T is the driving temperature for the ecosystem. The mean of the soil temperature at 5 cm depth and air temperature at 16.8 m height was found to be a better driving temperature for the entire ecosystem respiration85,86, in the case of the forest floor respiration, only the soil temperature at 5 cm depth is used. The Q10 was 2.0 for the above-canopy fluxes and 2.5 for the forest floor, while Rc was estimated in a moving time window of 11 days (above-canopy fluxes) or 15 days (forest floor fluxes) and seasonal variability ranged from ~ 0.5 to 2.5 μmol m−2 s−1 for above canopy and ~ 0.2 to 1.2 μmol m−2 s−1 for the forest floor. A non-linear regression model for GPP (GPPNLR) is used when the NEE measurements are not available (equation (8)).
where α and Pmax are fitting parameters that were estimated in a moving time window, f(Ta) is an instantaneous temperature response that reduces the GPP to zero near freezing temperatures (equation (9)). The seasonal variability of Pmax was 0–25 μmol m−2 s−1 and 0–5 μmol m−2 s−1 at the canopy and the forest floor, respectively. α ranged from 0 to 0.04 and from 0 to 0.015 (dimensionless) at the canopy and the forest floor, respectively.
where the inflection point is at T0 = − 2 °C87.
Estimation of apparent COS emissions
The GPP-COS relation described by Sandoval-Soto et al.2 can be inverted to estimate the COS uptake by the ecosystem (equation (10)). The GPPNEE was found to follow the natural restoration of the forest stand post-thinning48 (Fig. 4). If there were no emissions in the canopy, the FCOS would have also shown increased uptake as the forest recovered. Therefore, GPPNEE is input in the GPP-COS equation to estimate the COS uptake of the ecosystem (FCOS,GPP) as if the emissions were absent. This method assumes that COS uptake is happening only during photosynthesis and does not consider the COS uptake during the nighttime.
The COS uptake thus estimated was used to find the emission as given in equation (11).
The uptake of COS during the day and night is a direct function of the ecosystem stomatal conductance (GC)11, which can also be used to estimate the COS uptake in the forest stand. Assuming the COS emissions were negligible in the summer of 2020 and 2022, the linear relation between GC and FCOS was estimated (FCOS = −0.21× GC - 12.4) and was used to estimate the canopy uptake of COS (FCOS,GC) in the 2021 summer (Supplementary Table 2). The COS emission was estimated as given in equation (12).
Data availability
The code used for analyses can be found in the following GitHub repository https://github.com/t-abin/COS_emission. The COS flux data used in this study can be found at https://doi.org/10.5281/zenodo.17052203. The COSSM model version for simulating soil COS fluxes, along with the required input data, is available at https://doi.org/10.5281/zenodo.17555492. The flux data from the 27m tower (NEE) used in this study can be found at https://doi.org/10.23729/b4575245-5612-4874-a33b-541b215b1a10, and the environmental data at https://doi.org/10.23729/23dd00b2-b9d7-467a-9cee-b4a122486039. Most recent versions of the fluxes and the environmental data can be downloaded from https://smear.avaa.csc.fi.
References
Berry, J. et al. A coupled model of the global cycles of carbonyl sulfide and CO2 a possible new window on the carbon cycle. J. Geophys. Res. Biogeosci. 118, 842–852 (2013).
Sandoval-Soto, L. et al. Global uptake of carbonyl sulfide (COS) by terrestrial vegetation: estimates corrected by deposition velocities normalized to the uptake of carbon dioxide (CO2). Biogeosciences 2, 125–132 (2005).
Kooijmans, L. M. J. et al. Influences of light and humidity on carbonyl sulfide-based estimates of photosynthesis. Proc. Natl. Acad. Sci. USA 116, 2470–2475 (2019).
Whelan, M. E. et al. Reviews and syntheses: carbonyl sulfide as a multi-scale tracer for carbon and water cycles. Biogeosciences 15, 3625–3657 (2018).
Vesala, T. et al. Long-term fluxes of carbonyl sulfide and their seasonality and interannual variability in a boreal forest. Atmos. Chem. Phys. 22, 2569–2584 (2022).
Protoschill-Krebs, G. & Kesselmeier, J. Enzymatic pathways for the consumption of carbonyl sulphide (COS) by higher plants*. Botanica Acta 105, 206–212 (1992).
Protoschill-Krebs, G., Wilhelm, C. & Kesselmeier, J. Consumption of carbonyl sulphide (COS) by higher plant carbonic anhydrase (CA). Atmos. Environ. 30, 3151–3156 (1996).
DiMario, R. J., Clayton, H., Mukherjee, A., Ludwig, M. & Moroney, J. V. Plant carbonic anhydrases: structures, locations, evolution, and physiological roles. Mol. Plant 10, 30–46 (2017).
Schenk, S., Kesselmeier, J. & Anders, E. How does the exchange of one oxygen atom with sulfur affect the catalytic cycle of carbonic anhydrase?. Chem. A Eur. J. 10, 3091–3105 (2004).
Stimler, K., Montzka, S. A., Berry, J. A., Rudich, Y. & Yakir, D. Relationships between carbonyl sulfide (cos) and co2 during leaf gas exchange. N. Phytol. 186, 869–878 (2010).
Kooijmans, L. M. J. et al. Canopy uptake dominates nighttime carbonyl sulfide fluxes in a boreal forest. Atmos. Chem. Phys. 17, 11453–11465 (2017).
Caird, M. A., Richards, J. H. & Donovan, L. A. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 143, 4–10 (2007).
Wehr, R. et al. Dynamics of canopy stomatal conductance, transpiration, and evaporation in a temperate deciduous forest, validated by carbonyl sulfide uptake. Biogeosciences 14, 389–401 (2017).
Maseyk, K. et al. Sources and sinks of carbonyl sulfide in an agricultural field in the southern great plains. Proc. Natl. Acad. Sci. USA 111, 9064–9069 (2014).
White, M. L. et al. Carbonyl sulfide exchange in a temperate loblolly pine forest grown under ambient and elevated CO2. Atmos. Chem. Phys. 10, 547–561 (2010).
Seibt, U., Kesselmeier, J., Sandoval-Soto, L., Kuhn, U. & Berry, J. A. A kinetic analysis of leaf uptake of cos and its relation to transpiration, photosynthesis and carbon isotope fractionation. Biogeosciences 7, 333–341 (2010).
Yang, F., Qubaja, R., Tatarinov, F., Rotenberg, E. & Yakir, D. Assessing canopy performance using carbonyl sulfide measurements. Glob. Change Biol. 24, 3486–3498 (2018).
Sun, W. et al. Soil fluxes of carbonyl sulfide (COS), carbon monoxide, and carbon dioxide in a boreal forest in southern finland. Atmos. Chem. Phys. 18, 1363–1378 (2018).
Sauze, J. et al. The interaction of soil phototrophs and fungi with pH and their impact on soil CO2,CO18O and OCS exchange. Soil Biol. Biochem. 115, 371–382 (2017).
Meredith, L. K. et al. Soil exchange rates of COS and CO18o differ with the diversity of microbial communities and their carbonic anhydrase enzymes. ISME J. 13, 290–300 (2019).
Whelan, M. E. et al. Carbonyl sulfide exchange in soils for better estimates of ecosystem carbon uptake. Atmos. Chem. Phys. 16, 3711–3726 (2016).
Van Diest, H. & Kesselmeier, J. Soil atmosphere exchange of carbonyl sulfide (COS) regulated by diffusivity depending on water-filled pore space. Biogeosciences 5, 475–483 (2008).
Kesselmeier, J., Teusch, N. & Kuhn, U. Controlling variables for the uptake of atmospheric carbonyl sulfide by soil. J. Geophys. Res. Atmos. 104, 11577–11584 (1999).
Ogée, J. et al. A new mechanistic framework to predict OCS fluxes from soils. Biogeosciences 13, 2221–2240 (2016).
Launois, T., Peylin, P., Belviso, S. & Poulter, B. A new model of the global biogeochemical cycle of carbonyl sulfide - part 2: use of carbonyl sulfide to constrain gross primary productivity in current vegetation models. Atmos. Chem. Phys. 15, 9285–9312 (2015).
Whelan, M. E., Min, D.-H. & Rhew, R. C. Salt marsh vegetation as a carbonyl sulfide (COS) source to the atmosphere. Atmos. Environ. 73, 131–137 (2013).
Li, X.,Liu,J. & Yang, J. Variation of H2S and COS emission fluxes from calamagrostis angustifolia wetlands in sanjiang plain, northeast china. Atmos. Environ. 40, 6303–6312 (2006).
Caron, F. & Kramer, J. R. Formation of volatile sulfides in freshwater environments. Sci. Total Environ. 153, 177–194 (1994).
Kaisermann, A., Jones, S. P., Wohl, S., Ogée, J. & Wingate, L. Nitrogen fertilization reduces the capacity of soils to take up atmospheric carbonyl sulphide. Soil Syt. 2, 62 (2018).
Sun, W., Maseyk, K., Lett, C. & Seibt, U. A soil diffusion-reaction model for surface COS flux: COSSM v1. Geosci. Model Dev. 8, 3055–3070 (2015).
Yi, Z. et al. Soil uptake of carbonyl sulfide in subtropical forests with different successional stages in south china. J. Geophys. Res. Atmos. 112, D08302 (2007).
Kitz, F. et al. In situ soil COS exchange of a temperate mountain grassland under simulated drought. Oecologia 183, 851–860 (2017).
Whelan, M. E. & Rhew, R. C. Carbonyl sulfide produced by abiotic thermal and photodegradation of soil organic matter from wheat field substrate. J. Geophys. Res. Biogeosci. 120, 54–62 (2015).
Meredith, L. K. et al. Coupled biological and abiotic mechanisms driving carbonyl sulfide production in soils. Soil Syt. 2, 37 (2018).
Kitz, F. et al. Soil carbonyl sulfide exchange in relation to microbial community composition: insights from a managed grassland soil amendment experiment. Soil Biol. Biochem. 135, 28–37 (2019).
Spielmann, F. M., Hammerle, A., Kitz, F., Gerdel, K. & Wohlfahrt, G. Seasonal dynamics of the COS and CO2 exchange of a managed temperate grassland. Biogeosciences 17, 4281–4295 (2020).
Commane, R. et al. Seasonal fluxes of carbonyl sulfide in a midlatitude forest. Proc. Natl. Acad. Sci. USA 112, 14162–14167 (2015).
Maignan, F. et al. Carbonyl sulfide: comparing a mechanistic representation of the vegetation uptake in a land surface model and the leaf relative uptake approach. Biogeosciences 18, 2917–2955 (2021).
Kooijmans, L. M. J. et al. Evaluation of carbonyl sulfide biosphere exchange in the simple biosphere model (sib4). Biogeosciences 18, 6547–6565 (2021).
Abadie, C. et al. Carbon and water fluxes of the boreal evergreen needleleaf forest biome constrained by assimilating ecosystem carbonyl sulfide flux observations. J. Geophys. Res. Biogeosci. 128, e2023JG007407 (2023).
Lennartz, S. T. et al. Direct oceanic emissions unlikely to account for the missing source of atmospheric carbonyl sulfide. Atmos. Chem. Phys. 17, 385–402 (2017).
Ma, J. et al. Inverse modelling of carbonyl sulfide: implementation, evaluation and implications for the global budget. Atmos. Chem. Phys. 21, 3507–3529 (2021).
Remaud, M. et al. Plant gross primary production, plant respiration and carbonyl sulfide emissions over the globe inferred by atmospheric inverse modelling. Atmos. Chem. Phys. 22, 2525–2552 (2022).
Cartwright, M. P. et al. Constraining the budget of atmospheric carbonyl sulfide using a 3-d chemical transport model. Atmos. Chem. Phys. 23, 10035–10056 (2023).
de Vries, A. et al. The contribution of boreal wetlands to the northern hemisphere carbonyl sulfide sink. Geophys. Res. Lett. 52, e2024GL112858 (2025).
Asaf, D. et al. Ecosystem photosynthesis inferred from measurements of carbonyl sulphide flux. Nat. Geosci. 6, 186–190 (2013).
Kohonen, K.-M. et al. Intercomparison of methods to estimate gross primary production based on CO2 and COS flux measurements. Biogeosciences 19, 4067–4088 (2022).
Aslan, T. et al. Thinning turned boreal forest to a temporary carbon source - short term effects of partial harvest on carbon dioxide and water vapor fluxes. Agric. For. Meteorol. 353, 110061 (2024).
Duursma, R. A. et al. Predicting the decline in daily maximum transpiration rate of two pine stands during drought based on constant minimum leaf water potential and plant hydraulic conductance. Tree Physiol. 28, 265–276 (2008).
Gimeno, T. E. et al. Bryophyte gas-exchange dynamics along varying hydration status reveal a significant carbonyl sulphide (cos) sink in the dark and cos source in the light. N. Phytol. 215, 965–976 (2017).
Baker, N. R. & Allison, S. D. Ultraviolet photodegradation facilitates microbial litter decomposition in a mediterranean climate. Ecology 96, 1994–2003 (2015).
King, J. Y., Brandt, L. A. & Adair, E. C. Shedding light on plant litter decomposition: advances, implications and new directions in understanding the role of photodegradation. Biogeochemistry 111, 57–81 (2012).
Zepp, R. G. & Andreae, M. O. Factors affecting the photochemical production of carbonyl sulfide in seawater. Geophys. Res. Lett. 21, 2813–2816 (1994).
Uher, G. & Andreae, M. Photochemical production of carbonyl sulfide in north sea water: a process study. Limnol. Oceanogr. 42, 432–442 (1997).
Kitz, F. et al. Soil cos exchange: a comparison of three european ecosystems. Glob. Biogeochem. Cycles 34, e2019GB006202 (2020).
Rennenberg, H.The Significance of Higher Plants in the Emission of Sulfur Compounds from Terrestrial Ecosystems. In Trace Gas Emissions by Plants (eds. Sharkey, T. D., Holland, E. A. & Mooney, H. A.) Physiological Ecology, 217–260 (Academic Press, San Diego, 1991).
Mammarella, I. et al. Etc l2 archive from hyytiala, 2018–2024 https://hdl.handle.net/11676/oD2Pme94V4Cl6dmxyZDKuCeq (2025).
Likus-Cieślik, J. & Pietrzykowski, M. Vegetation development and nutrients supply of trees in habitats with high sulfur concentration in reclaimed former sulfur mines jeziórko (southern poland). Environ. Sci. Pollut. Res. 24, 20556–20566 (2017).
Giertych, M. J., de Temmerman, L. O. & Rachwal, L. Distribution of elements along the length of scots pine needles in a heavily polluted and a control environment. Tree Physiol. 17, 697–703 (1997).
Zethof, J. H. T., Julich, S., Feger, K.-H. & Julich, D. Legacy effect of 25 years reduced atmospheric sulphur deposition on spruce tree nutrition. J. Plant Nutr. Soil Sci. 187, 834–843 (2024).
Olson, J. S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44, 322–331 (1963).
Kolari, P. et al. Hyytiälä SMEAR II site characteristics https://doi.org/10.5281/zenodo.5909681 (2022).
Aalto, J. et al. Hyytiälä SMEAR II forest year 2020 thinning tree and carbon inventory data https://doi.org/10.5281/zenodo.8138946 (2023).
Kohonen, K.-M. et al. Towards standardized processing of eddy covariance flux measurements of carbonyl sulfide. Atmos. Meas. Tech. 13, 3957–3975 (2020).
Kohonen, K.-M. & Kooijmans, L. Dataset for “Long-term fluxes of carbonyl sulfide and their seasonality and interannual variability in a boreal forest” https://doi.org/10.5281/zenodo.5906705 (2022).
Mammarella, I., Peltola, O., Nordbo, A., Järvi, L. & Rannik, U. Quantifying the uncertainty of eddy covariance fluxes due to the use of different software packages and combinations of processing steps in two contrasting ecosystems. Atmos. Meas. Tech. 9, 4915–4933 (2016).
Aubinet, M. et al. Estimates of the annual net carbon and water exchange of forests: The EUROFLUX methodology. In Advances in Ecological Research (Fitter, A. & Raffaelli, D. eds) Vol. 30, 113–175 (Academic Press, 1999).
Mammarella, I. et al. Relative humidity effect on the high-frequency attenuation of water vapor flux measured by a closed-path eddy covariance system. J. Atmos. Ocean. Technol. 26, 1856 – 1866 (2009).
Rannik, Ü. & Vesala, T. Autoregressive filtering versus linear detrending in estimation of fluxes by the eddy covariance method. Bound.-Layer. Meteorol. 91, 259–280 (1999).
Franz, D. et al. Towards long-term standardised carbon and greenhouse gas observations for monitoring europe’s terrestrial ecosystems: a review. Int. Agrophys. 32, 439–455 (2018).
Heiskanen, J. et al. The integrated carbon observation system in europe. Bull. Am. Meteorol. Soc. 103, E855 – E872 (2022).
Rebmann, C. et al. Icos eddy covariance flux-station site setup: a review. Int. Agrophys. 32, 471–494 (2018).
Virtanen, P. et al. Scipy 1.0: fundamental algorithms for scientific computing in python. Nat. Methods 17, 261–272 (2020).
Granier, A. Une nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres. In Annales des Sciences Forestières Vol. 42, 193–200 (EDP Sciences, 1985).
Oishi, A. C., Oren, R. & Stoy, P. C. Estimating components of forest evapotranspiration: a footprint approach for scaling sap flux measurements. Agric. For. Meteorol. 148, 1719–1732 (2008).
Cermák, J. & Kučera, J. Transpiration of mature stands of spruce (picea abies (l.) karst.) as estimated by the tree-trunk heat balance method. In Forest Hydrology and Watershed Management (eds. Swanson, R, W. P. & Bernier P) 311–317 (Wallingford, UK, 1987).
Cermák, J. & Kučera, J. Scaling up transpiration data between trees, stands and watersheds. Silva Carelica 15, 101–120 (1990).
Dukat, P. et al. Scots pine responses to drought investigated with eddy covariance and sap flow methods. Eur. J. For. Res. 142, 671–690 (2023).
Cristiano, P. M. et al. Evapotranspiration of subtropical forests and tree plantations: a comparative analysis at different temporal and spatial scales. Agric. For. Meteorol. 203, 96–106 (2015).
Rabbel, I., Bogena, H., Neuwirth, B. & Diekkrüger, B. Using sap flow data to parameterize the Feddes water stress model for Norway spruce. Water 10, 279 (2018).
Wilson, K. B., Hanson, P. J., Mulholland, P. J., Baldocchi, D. D. & Wullschleger, S. D. A comparison of methods for determining forest evapotranspiration and its components: sap-flow, soil water budget, eddy covariance and catchment water balance. Agric. For. Meteorol. 106, 153–168 (2001).
Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 3, 309–320 (1987).
Frostig, R., Johnson, M. & Leary, C. Compiling Machine Learning Programs via High-Level Tracing. In SysML 18 https://mlsys.org/Conferences/doc/2018/146.pdf (Stanford, CA, USA, 2018).
Lawrence, D. M. et al. The community land model version 5: Description of new features, benchmarking, and impact of forcing uncertainty. J. Adv. Model. Earth Syst. 11, 4245–4287 (2019).
Kolari, P. et al. Co2 exchange and component co2 fluxes of a boreal scots pine forest. Boreal Environ. Res. 14, 761–783 (2009).
Lasslop, G. et al. On the choice of the driving temperature for eddy-covariance carbon dioxide flux partitioning. Biogeosciences 9, 5243–5259 (2012).
Kolari, P. et al. Field and controlled environment measurements show strong seasonal acclimation in photosynthesis and respiration potential in boreal scots pine. Front. Plant Sci. 5, 717 (2014).
Acknowledgements
The authors are grateful to Sirpa Rantanen, Elmeri Putkinen, and other technical support staff at the Hyytiälä forest station for their expertise and hard work in ensuring the smooth operation and maintenance of the measurement equipment. This study has received financial support from the University of Helsinki via Integrated Carbon Observation System - Hyytiälä (ICOS-HY), the Research Council of Finland project N-PERM (Grant No. 341348), and project ForClimate (Grant No. 347780), and the EU Horizon Europe-Framework Programme for Research and Innovation (Grant No. 101056921-GreenFeedBack).
Author information
Authors and Affiliations
Contributions
A.T., I.M., and T.V. conceived and designed the study. AT wrote the main manuscript and created figures 1-4. A.L., T.A. and K.M.K. collected, processed and finalised the COS eddy covariance data. Y.S. provided the sapflow measurements data. P.K. managed and facilitated the data flow and processed the NEE eddy covariance and environmental datasets. P.D. helped with upscaling the sapflow measurements and writing relevant parts in the manuscript. W.S. ran the differentiable COSSM model, performed analysis and contributed to the section on changes in soil fluxes. K.M.K., K.M., R.D., T.V. and I.M. helped with the conceptual framework of the manuscript and editing of the final version. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thomas, A., Laasonen, A., Kohonen, KM. et al. Examining anomalous summer carbonyl sulfide emissions in a boreal forest after thinning. npj Clim Atmos Sci 9, 1 (2026). https://doi.org/10.1038/s41612-025-01272-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41612-025-01272-w